Biodermogenesi for Striae Distensae: A Combined Clinical and Histopathological Assessment
Batista Castro Zenia1, Benitez Roig Virginia2, Broekhuizen Benitez Javier2, Reig Monzón María Inés3, Busoni Maurizio*,4
1Faculty of Health Sciences, Technical University of Ambato, San Juan de Ambato, Ecuador.
2Helicopteros Sanitarios Hospital, Marbella, Spain.
3AvecBelle Clinic of Aesthetic Medicine, Río Grande, Tierra del Fuego, Argentina,
4Master’s II Degrees in Aesthetic Medicine, University of Camerino, Camerino (MC), Italy
*Corresponding Author: Busoni Maurizio, Master’s II Degrees in Aesthetic Medicine, University of Camerino, Camerino (MC), Italy.
Received: 28 October 2025; Accepted: 04 November 2025; Published: 11 November 2025
Article Information
Citation: Batista Castro Zenia, Benitez Roig Virginia, Broekhuizen Benitez Javier, Reig Monzón María Inés, Busoni Maurizio. Biodermogenesi for Striae Distensae: A Combined Clinical and Histopathological Assessment Fortune Journal of Health Sciences. 8 (2025): 1061-1071.
View / Download Pdf Share at FacebookAbstract
Background and objectives: Striae distensae (SD), particularly their atrophic form striae albae (SA), remains therapeutically challenging. Biodermogenesi is a non-invasive technology combining electromagnetic fields, vacuum, and electrical stimulation (also called V-EMF therapy). We evaluated its clinical and histological effects in chronic SA.
Study design/materials and Methods: We conducted a prospective open-label study in 20 adults with long-standing SA (≥10 years) and Fitzpatrick phototypes III–IV. All patients received 10 sessions of Biodermogenesi (V-EMF therapy) with the Bi-One® LifeTouchTherapy device. Clinical outcomes were assessed via standardized photography, the Global Aesthetic Improvement Scale (GAIS), and patient Likert satisfaction. Digital morphometry (thickness, length, area) was performed using Fiji/ImageJ. In 6 patients, skin punch biopsies were obtained before treatment and 30 days after the final session. Histology included morphometric and semiquantitative assessments of the epidermis, dermal–epidermal junction (DEJ), collagen, vascularity, and fibrosis.
Results: All patients showed visible improvement, including smoother texture and reduced lesion size. Clinical morphometry demonstrated mean reductions of 27% in striae thickness and 34% in area. Histology revealed increased epidermal thickness with reappearance of rete ridges, denser collagen, and enhanced vascularity. Although morphometric changes did not reach statistical significance, semiquantitative analysis supported tissue remodeling. No adverse events occurred. Patient satisfaction correlated with physician GAIS (p=0.037).
Conclusion: Biodermogenesi achieved clinical and histological improvement in chronic SA, including in darker phototypes (III–IV). The therapy appears safe and effective, supporting further randomized studies with longer follow-up.
Keywords
<p>Biodermogenesi; Histology; Non-invasive procedures; Stretch marks, Striae albae; Striae distensae; VEMFtherapy</p>
Article Details
Introduction
Striae distensae (SD), commonly known as stretch marks, are among the most frequent dermatological alterations seen in clinical practice, affecting a wide range of individuals regardless of ethnicity or geography. Their prevalence ranges from 50 % to 90 %, with higher incidence during periods of intense hormonal changes such as adolescence, pregnancy, or prolonged corticosteroid use [1-4]. Although medically benign, these lesions can cause significant cosmetic concerns and are often associated with underlying pathological conditions or complex pregnancies, contributing to emotional distress, especially in young women [5, 6]. Clinically, SD are classified into two stages: the early inflammatory phase or striae rubrae (SR), characterized by red to purple linear lesions with edema and increased vascularity; and the chronic atrophic phase or SA, where lesions appear hypopigmented, atrophic, and less vascularized. Histologically, SR shows inflammatory changes, collagen disorganization, and dermal edema, while SA displays flattened dermal architecture, epidermal thinning, and fragmented collagen bundles [5,7-9]. Etiopathogenesis of SD is multifactorial. Mechanical skin stretching—especially rapid expansion—is combined with hormonal changes, genetic predisposition, and defects in collagen and elastin synthesis or assembly [1,5,7,8,10]. In patients with connective tissue fragility disorders such as Ehlers-Danlos syndrome, early onset of striae without obvious mechanical stress has been observed, suggesting a primary structural dysfunction [11]. Recent studies have further identified dysregulation in the expression of genes involved in dermal remodeling, inflammatory responses, and oxidative stress pathways [5]. The psychological impact of SD has long been underestimated. Multiple studies have demonstrated a strong association between SD and low self-esteem, social anxiety, and even mild depressive symptoms, particularly among young women during adolescence or postpartum. Lesions in exposed areas such as the abdomen, hips, buttocks, or thighs negatively affect body image and often lead to social withdrawal [3,4]. Currently, managing SD remains a major challenge in aesthetic dermatology. The medical literature reports numerous treatment strategies, including topical retinoids [12], carboxytherapy [13], platelet-rich plasma [13], fractional ablative and non-ablative lasers [14],
radiofrequency [15], and microneedling [16]. However, their effectiveness is highly variable, and outcomes are often modest, requiring multiple sessions. A recent comparative review concluded that no therapy has yet achieved the status of gold standard 6. Moreover, the type of SD (rubrae or albae), patient skin phototypes, lesion duration, and anatomical location significantly influence therapeutic response. This clinical heterogeneity underscores the need for innovative techniques that can stimulate dermal regeneration with fewer side effects and better patient tolerability [2], [3,6,14]. In recent years, research in non-invasive regenerative therapies has led to the development of technologies designed to stimulate dermal matrix repair without inducing tissue damage. In this context, the Biodermogenesi technique has gained attention as a method that combines electromagnetic fields, pulsed vacuum, and low-frequency electrical stimulation to promote dermal regeneration without the adverse effects associated with ablative treatments. Recent clinical studies have reported visible improvements in the texture, coloration, and thickness of SD treated with this technique, along with structural changes documented through histological and digital morphometric analysis [17], [18,19]. Therefore, the present study aims to evaluate the effects of Biodermogenesi in the treatment of striae distensae. Specifically, its objectives are to describe the clinical and epidemiological characteristics of affected patients; determine the clinical outcomes of Biodermogenesi therapy based on photographic analysis; assess satisfaction levels reported by patients and physicians; and identify histological changes in the epidermis and dermis resulting from the treatment. This research seeks to contribute scientific evidence supporting the use of safe, non-invasive technologies as effective therapeutic alternatives for a highly prevalent and psychosocially distressing condition.
Methods
An open-label and prospective clinical trial was conducted at the Aesthetic, Cosmetic, and Plastic Surgery Clinic of Helicópteros Sanitarios Hospital in Marbella, Spain. This study was part of the international Biodermogenesi project, all procedures were carried out in accordance with the ethical standards of the institutional research board and the principles set forth in the Declaration of Helsinki [20]. A total of 20 adult patients with varying Fitzpatrick skin phototypes were enrolled. All participants presented with striae distensae of differing anatomical locations, severity grades, and durations. Exclusion criteria included a history of keloid formation, active dermatologic conditions at the treatment site, any malignancy diagnosed within the previous five years, epilepsy, vascular disorders such as varicose veins, phlebitis, or thrombosis, recent use of corticosteroids (topical or systemic within six months), ongoing anticoagulant therapy, diagnosed eating disorders within the past two years, presence of implanted electronic medical devices, and pregnancy or lactation. Written informed consent was obtained from all participants, including authorization for biopsy collection and standardized clinical photography. The procedure was carried out using the Bi-One® LifeTouchTherapy device (Expo Italia Srl, Florence, Italy), a electromedical device for non-invasive therapies. The device delivers electromagnetic fields, vacuum, and electroporation simultaneously. The electromagnetic field is delivered at a variable frequency from 0.5 to 2MHz and with an average power of 4W. The frequency and power are modulated directly by the device based on feedback from an artificial intelligence system, preventing energy overdoses. The electromagnetic field increases the activity of Na+ and K+ ions through electropores of cell membranes [21], promoting both the nourishment and detoxification of skin cells.
Part of the kinetic energy of these ions is transformed into thermal energy, based on the second law of thermodynamics [22]. The artificial intelligence on board the device intervenes second by second, modulating the output, allowing the thermal effect to stabilize between 39 and 40°C, thus activating Van't Hoff's Law, which increases cell regeneration four times more than normal [18]. It should be remembered that the temperature of 40°C must never be exceeded to avoid the risk of damaging the collagen present in the treated tissue [23]. The simultaneous delivery of the vacuum allows skin microcirculation to be reactivated [24,25] and consequently the exchanges with the matrix, while electroporation promotes the absorption of nutrients through the stratum corneum [26].
Clinical Evaluation
The clinical response to Biodermogenesi treatment was evaluated using both qualitative and quantitative criteria, comparing patient outcomes at multiple time points during the therapeutic protocol. All subjects were assessed at baseline (S0) and again 30 days after the final treatment session (S2 + 30). An objective clinical evaluation was performed using the Global Aesthetic Improvement Scale (GAIS), which ranges from 0 to 4. This scale was applied by the primary evaluator through direct observation and standardized photographic comparisons under controlled lighting and positioning. A score of 0 indicated no improvement; 1 corresponded to less than 25 % improvement (mild); 2 indicated 25 – 49 % improvement (moderate); 3 reflected 50 – 74 % improvement [marked]; and 4 represented ≥ 75 % improvements (excellent). Patient-reported outcomes were assessed using a Likert-type scale to capture subjective perception of improvement. A score of 0 indicated no satisfaction; 1 reflected slight satisfaction (< 25 %); 2 indicated moderate satisfaction (25 – 49 %); 3 represented high satisfaction (50 – 74 %); and 4 denoted complete satisfaction (≥ 75 %) 27. Both objective and subjective evaluations were conducted at baseline and following completion of the full Biodermogenesi treatment protocol. The specific clinical parameters assessed included tactile and visual features of the striae. Tactile improvement was evaluated by palpation, focusing on the reduction in depth and dermal fibrosis of the lesions. Visual improvement was determined based on the degree of re-pigmentation and attenuation of the shiny, atrophic appearance typically associated with striae alba. Standardized photographic documentation was obtained at S0 and S2 + 30 using a Sony Cybershot DSC-H9 (Sony, Inc.) under controlled lighting and fixed distance conditions. Frontal and oblique views of the affected areas, as well as contralateral untreated zones, were captured to allow intra-patient comparisons. To objectively quantify changes in striae morphology—specifically thickness, length, and surface area—a digital morphometric analysis was performed using Fiji – ImageJ software (version 2.14.0/1.54f). Representative clinical images (pre- and post-treatment) were selected based on identical scale, lighting, and camera distance.
Measurements were performed as follows:
- • Thickness: A perpendicular line was drawn across the widest portion of each lesion; the mean of three measurements per lesion was calculated.
- • Length: The full linear or curved axis of each striae was traced from origin to endpoint using the segmented line tool.
- • Area: The visible borders of each lesion were outlined using the freehand selection tool, and area was calculated in square millimeters (mm²) following calibration with a digital scale reference.
All measurements were performed in triplicate by a trained evaluator to ensure accuracy and reproducibility.
Histological Evaluation
Skin biopsies (2 mm punch) were obtained from a subsample of patients at two points: prior to the first treatment session (S0) and 30 days after the final session (S2 + 30). Each patient provided paired samples from the treated area (striae) and contralateral healthy skin. The biopsy sites were marked in advance to ensure anatomical consistency. Six specimens were fixed in 10 % neutral buffered formalin, embedded in paraffin, and sectioned at 4–5 μm thickness. Staining protocols included haematoxylin and eosin (H&E) for general morphology and Masson’s trichrome for collagen fibers. Histological slides were reviewed by a histologist with expertise in dermatopathology. Quantitative morphometric analysis was performed using Image-ProPlus software (v10.0.10; Media Cybernetics, Silver Spring, MD, USA) on images acquired under a Leica DMD108 optical microscope. The following parameters were assessed in three repeated measures per section:
- • Mean Epidermal Thickness (MET): Calculated as the area of the epidermis [excluding dermal papillae] divided by the linear length of the granular layer.
- • Flattening Index of the Dermoepidermal Junction (DEJ): Defined as the ratio of the total length of the DEJ [including rete ridges] to the basal length of the granular layer. Values ≤1 indicated complete flattening [28].
To complement the morphometric assessment, a semiquantitative histological analysis was conducted to evaluate dermal remodelling and neocollagenesis. Fifteen representative fields per section (100× magnification) were selected based on tissue preservation and staining quality. The following parameters were scored on a four-point ordinal scale
- • (0 = none; 3 = marked):
- • Collagen fiber density
- • Collagen fiber orientation
- • Presence of neovascularization
- • Residual dermal fibrosis
Scores were averaged across fields per patient and time point. Inter- and intra-observer variability was monitored; discrepancies were resolved by consensus. This semiquantitative evaluation provided a descriptive profile of Biodermogenesi, induced neocollagenesis and tissue remodelling. Data analysis was conducted using SPSS software (version 21.0; IBM Corp., Armonk, NY, USA). Descriptive statistics (mean ± standard deviation) were calculated for all quantitative variables. Pre- and post-treatment comparisons of morphometric data were assessed using the Wilcoxon signed-rank test for non-parametric paired samples. Differences in patient satisfaction and aesthetic scores were analyzed using the chi-square test. A p-value < 0.05 was considered statistically significant.
Results
The sample consisted of 20 adult patients with SD, mostly young women with body weight within normal ranges. The mean age was 36.6 years (± 12.0). The most common skin phototypes were Fitzpatrick type III (55 %) and type IV (20 %). White striae were the most frequently observed clinical form (85 %), with an average duration of 14.8 years (± 10.3). The abdomen (50 %) and thighs (35 %) were the most affected anatomical regions. The main reported causes were pregnancy (40 %) and adolescent growth spurts (35 %). Table 1 summarizes the key demographic and clinical characteristics of the cohort.
Table 1: Epidemiological and Clinical Characteristics of Patients (n = 20).
|
Characteristics |
No. |
% |
|
Age (years) |
||
|
Min. – Max. |
20 – 59 |
|
|
Mean (± SD) |
36.55 ± 12.01 |
|
|
Sex |
||
|
Female |
19 |
95 |
|
Male |
1 |
5 |
|
BMI (Kg/m²) |
||
|
Mean (± SD) |
21.5 (± 3.19) |
|
|
Skin phototype (Fitzpatrick) |
||
|
I |
1 |
5 |
|
II |
3 |
15 |
|
III |
11 |
55 |
|
IV |
4 |
20 |
|
V |
1 |
5 |
|
Duration (years) |
||
|
Min. – Max. |
5 – 40 |
|
|
Mean (± SD) |
14.85 (± 10.25) |
|
|
Type of striae |
||
|
Rubrae |
3 |
15 |
|
Albae |
17 |
85 |
|
Anatomical location |
||
|
Abdomen |
10 |
50 |
|
Thighs |
7 |
35 |
|
Flanks |
1 |
5 |
|
Gluteal region |
1 |
5 |
|
Lumbar |
1 |
5 |
|
Etiology |
||
|
Pregnancy |
8 |
40 |
|
Weight gain |
5 |
25 |
|
Adolescent growth |
7 |
35 |
Qualitative clinical changes
Prior to treatment, striae exhibited a well-defined atrophic morphology, characterized by hypopigmented, linear trajectories with parallel orientation, depressed surfaces, and irregular texture. The lesions showed marked contrast with the surrounding skin due to their whitish coloration and disruption of dermal topography. Affected areas frequently presented with skin laxity, reduced turgor, and superficial dehydration, resulting in a dull, uneven appearance. Following completion of the therapeutic protocol, a progressive qualitative improvement was observed in most patients. Striae showed a visible reduction in depth and extension, with attenuation of the linear pattern and noticeable smoothing of the skin surface. Chromatic contrast was significantly diminished; the lesions showed improved chromatic integration with the surrounding skin tone, adopting a more uniform color that made them less apparent upon clinical examination. Cutaneous texture improved markedly, with restoration of natural luminosity and enhanced surface regularity. Previously depressed regions appeared elevated, contributing to a firmer and more continuous skin contour. Treated areas exhibited a smoother, more hydrated, and structurally refined appearance, resulting in a clearly favorable aesthetic outcome. No clinically relevant adverse effects such as persistent erythema, post-inflammatory hyperpigmentation, or secondary fibrosis were observed.
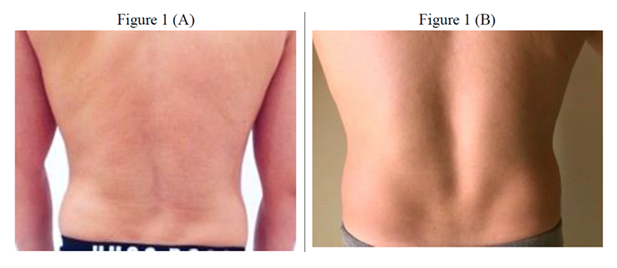
- A) Baseline: Hypopigmented SD characterized by epidermal atrophy, linear dermal depressions, and surface irregularities with a crinkled texture, typical of chronic lesions. Lesions appear well-demarcated with reduced dermal support and collagen fragmentation.
- B) Post-treatment [after 10 sessions]: Notable reduction in striae depth and surface atrophy, with enhanced dermal texture, improved pigment blending with adjacent skin, and partial restoration of cutaneous tensile integrity and elasticity.
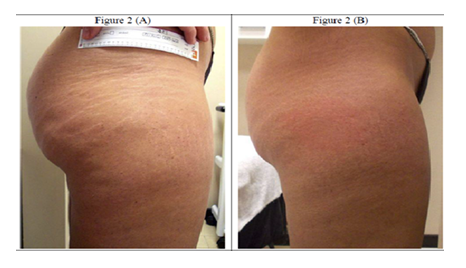
- A) Before the treatments, the stretch marks were evident, white, and deep, with evident skin depletion and collagen fragmentation.
- B) After a cycle of 10 sessions, the stretch marks were filled in, regaining a color similar to that of the adjacent skin tissue. Overall, the tissue was more elastic and compact, suggesting a significant reorganization of the skin.
Quantitative clinical measurements
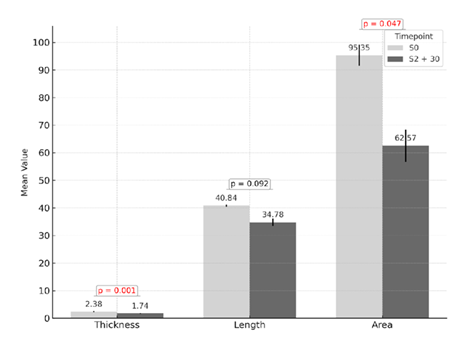
Digital analysis using Fiji–ImageJ software demonstrated a consistent reduction in the dimensional characteristics of striae following treatment. A significant decrease was observed in both epidermal thickness and surface area, with average reductions of approximately 27 % and 34 %, respectively, between baseline measurements [S0] and those taken 30 days after the final session [S2 + 30], as depicted in the comparative analysis presented in Figure 3.
Figure 3: Quantitative analysis of striae parameters before treatment (S0) and 30 days after the final session (S2 + 30). Significant reductions were observed in epidermal thickness (p = 0.001) and surface area (p = 0.047). A non-significant trend toward reduction was noted in striae length (p = 0.092). Values are expressed as means with standard deviation.
It is important to note that the term epidermal thickness used in this context refers to the visual and measurable thickness of the striae as captured through clinical photography and assessed with Fiji–ImageJ software. This measurement reflects macroscopic dimensional changes in the lesion rather than the histological thickness of the epidermis. In contrast, histological evaluation based on biopsy specimens demonstrated a true increase in epidermal thickness, as evidenced by enhanced epithelial stratification and reappearance of rete ridges (see Figure 5). In contrast, the length of the striae showed a downward trend of around 15 %, although this change did not reach statistical significance. These findings support the morphometric improvement induced by the treatment, particularly in parameters related to depth, texture, and the visual integration of striae with the surrounding skin.
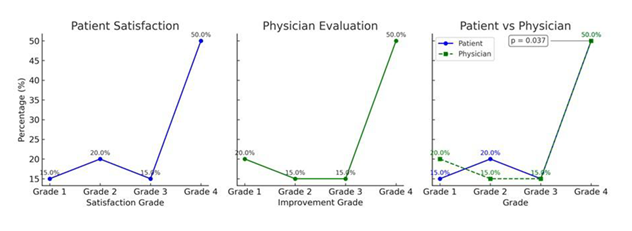
Patient and physician satisfaction assessment
Patient-reported outcomes were collected through a post-treatment satisfaction survey, in which participants rated the perceived improvement of their striae based on visual appearance, texture, and tanning capacity. Most patients reported moderate to high levels of satisfaction, with 85 % of respondents placing their scores in the upper two categories (51–75 or 76–100). Only one participant expressed uncertainty regarding the outcome. Parallel to the patient assessment, a blinded physician evaluator independently rated clinical improvement using a standardized global aesthetic improvement scale. The physician’s evaluations showed strong concordance with patient responses, with a similar distribution of scores. A statistically significant association was found between the two evaluations (p = 0.037), confirming a meaningful correlation between patient perception and clinical assessment. These results are summarized in Figure 4.
Histological results
Qualitative histological findings
In the pre-treatment striae skin samples, the epidermis showed focal thinning, with disruptions in the continuity of the stratum spinosum and partial loss of interpapillary ridges. In several cases, irregularities were identified at the interface between the epidermis and the underlying dermis, with a flattened or poorly defined dermoepidermal junction. In the dermis, collagen bundles were observed with low density, disorganized fibers with irregular trajectories and lacking clear orientation, within a loose extracellular matrix and minimal cellularity. Collagen fibers appear thin, fragmented, or poorly stained, arranged in a lax or discontinuous pattern. Vascularization was minimal, with few or no capillaries identified in the papillary and mid dermis. Areas of residual fibrosis were evident, appearing as dense, eosinophilic, hyalinized deposits with limited organization, often poorly demarcated or poorly integrated into the surrounding matrix. After completing the treatment protocol, the biopsies showed thickening of the epithelium, with an increased number of cell layers in the stratum spinosum and a more defined architectural organization. DEJ displayed a more undulated profile, with marked presence of dermal papillae and rete ridges. In the dermis, collagen fibers appeared denser, more intensely stained with trichrome, compact, and organized in a more parallel fashion. Fibrillar trajectories were more defined and less dispersed compared to the untreated skin. A higher number of capillaries was identified, especially in the papillary and mid dermis, distributed superficially and perivascularly. Residual fibrosis was less evident, with a reduction in dense areas and a relative increase in the laxity of the extracellular matrix. These structural observations are illustrated in Figure 5, which shows representative histological images comparing untreated striae and skin treated after completing the Biodermogenesi protocol.
Figure 5: Histological sections stained with H&E (top) and Masson’s Trichrome (bottom), 100× magnification.
(A) Before treatment: The epidermis is markedly atrophic with flattened rete ridges and reduced basal pigmentation. The dermo-epidermal junction appears smoothed, with diminished anchoring structures. The dermis shows disorganized, fragmented collagen bundles aligned parallel to the surface, scarce elastic fibers, and low vascular density.
(B) After treatment: The epidermis exhibits increased thickness and partial recovery of rete ridges, with more uniform basal pigmentation. The dermo-epidermal junction demonstrates improved interdigitation. The dermis shows denser and more organized collagen with predominantly perpendicular orientation, visible elastic fibers, and moderate neovascularization, indicating dermal remodeling.
Morphometric analysis of Epidermis, DEJ and Dermis
A morphometric evaluation was performed to assess the average epidermal area and the flattening index of the DEJ in paired biopsies obtained from striae-affected skin. Measurements were carried out at two time points: baseline (S0) and thirty days after completing the Biodermogenesi treatment protocol (S2+30). For the epidermal area, the Shapiro-Wilk test indicated a non-normal distribution of the data. Therefore, the Wilcoxon signed-rank test was applied, revealing no statistically significant difference between S0 and S2+30 (W = 5.0, p = 0.625). In the case of the DEJ flattening index, both time points showed normally distributed data. A paired t-test was conducted, and no significant differences were found between S0 and S2+30 (t = –0.71, p = 0.518).
These findings are summarized in Table 2 and are complemented by the semiquantitative dermal evaluation presented in the following section.
Table 2: Morphometric analysis comparing the epidermal area and DEJ flattening index before treatment (S0) and 30 days post-treatment (S2+30). No statistically significant differences were found for either variable.
|
Variable |
S0 (mean) |
S2+30 (means) |
Statistical test |
Statistic |
p-value |
|
Epidermal area |
1.34 |
2.12 |
Wilcoxon |
5 |
0.625 |
|
DEJ flattening index |
1.35 |
1.46 |
Paired t-test |
0.71 |
0.518 |
Histological remodeling was assessed semiquantitatively across four key dermal parameters. Notable improvements were observed in collagen density, fiber orientation, neovascularization, and residual fibrosis after treatment. Collagen appeared more compact and structurally organized in most post-treatment samples, suggesting enhanced dermal integrity. Fiber bundles showed a more parallel and aligned pattern, consistent with functional tissue remodeling. Although this change did not reach statistical significance, the observed trend supports progressive structural reorganization. Neovascularization was more evident in the superficial and mid-dermal layers, indicative of increased metabolic activity and improved microcirculation in the treated skin. Residual fibrosis showed a marked reduction, with a transition from dense, eosinophilic matrix patterns to a looser, more physiological dermal texture, supporting a regenerative shift rather than mere remodeling. Overall, the semiquantitative data reinforces the clinical impression of dermal regeneration induced by Biodermogenesi, providing histological evidence of tissue normalization in previously atrophic striae. A graphical synthesis of these histological trends is provided in Figure 6.
Figure 6: Semiquantitative histological scores for dermal parameters in striae-affected skin before (S0) and after treatment (S2+30). Boxplots display median (Med), Q1, Q3, and individual scores. Significant improvements were found in collagen density (p = 0.031), neovascularization (p = 0.038) and fibrosis (p = 0.031). Fiber orientation was not significant (p = 0.066). Significant values appear in red.
Discussion
Clinical and epidemiological characteristics of patients with striae distensae
The sample analysed in this study consisted of young women with Fitzpatrick skin phototypes III and IV who presented long-standing SA, predominantly located on the abdomen, buttocks, and thighs. This anatomical distribution coincides with current epidemiological data, which identify these areas as the most frequently affected by SD, especially in women of reproductive age and with a history of pregnancy or significant weight fluctuations [1,3,7,11]. The high prevalence of stretch marks in young women has been widely documented in the literature, with figures reaching up to 90 % in specific populations such as pregnant women. Predisposing factors described include family history, rapid weight gain, and the use of topical or systemic corticosteroids; however, the precise role of mechanical stretching and hormonal factors remains controversial [5,7]. Histopathologically, recent studies such as those by Wang et al. have shown that even in the early stages of striae gravidarum, there is marked disruption of the dermal architecture, including the disorganization of elastic fibers and the presence of irregular tropoelastin fibrils—a phenomenon that appears to persist in mature lesions [15,29,30]. These structural findings correlate with the clinical phenotype of SA, characterized by dermal and epidermal atrophy, hypopigmentation, low vascularization, and greater therapeutic resistance [1,2,8]. The inclusion in this study of patients with chronic white stretch marks (≥10 years of evolution) represents a considerable clinical and methodological challenge, since this type of lesion has shown greater resistance to most available therapeutic modalities, including fractional lasers 14, radiofrequency 15, needling 16 or PRP 13. However, this selection also allows us to evaluate the true regenerative potential of emerging techniques such as Biodermogenesi in clinical scenarios where spontaneous tissue regeneration is unlikely [18,19,30,33]. Finally, although most clinical studies on SD have been conducted in patients with phototypes I–III, this work provides new evidence of the safety and efficacy of bioregenerative treatments in intermediate phototypes [III–IV], which are predominant in Latin American populations and present a higher risk of post-inflammatory hyperpigmentation when exposed to thermal therapies [5].
Clinical evaluation of the effect of Biodermogenesi
The clinical results observed after ten treatment sessions with Biodermogenesi showed significant aesthetic improvement of the SA, with recovery of thickness, normalization of pigmentation, and improvement of texture and surface continuity. This evolution was documented through standardized photographic records and blind evaluations using the GAIS scale, showing a statistically significant concordance between the patient’s perception and the physician’s assessment (p = 0.037). These findings are consistent with those described by Veronese et al. [34] and Marafioti et al. [33], who reported visible and relevant improvement in atrophic striae treated with V-EMF [vacuum and electromagnetic field], including recovery of dermal volume and turgor in stretch marks over 20 years old [10,33]. Unlike other therapeutic modalities, which tend to show better results in the early stages of the lesion, such as non-ablative lasers, fractional radiofrequency, or carboxytherapy, Biodermogenesi has demonstrated efficacy in chronic striae, suggesting the presence of a deeper and more sustained regenerative mechanism. In comparative studies, treatment with Biodermogenesi has shown better clinical outcomes than traditional techniques such as fractional CO2 laser or platelet-rich plasma, particularly in terms of safety, tolerability, and long-term outcomes. Laura et al. [18] reported objective clinical improvement without adverse events in phototypes III and IV, results consistent with those obtained in the present study. Furthermore, the proposed pathophysiological mechanisms for Biodermogenesi include synergistic effects derived from the interaction of thermal, piezoelectric, and vacuum stimuli, which promote dermal fibroblast activation, stimulate microcirculation, and generate a pro-regenerative environment that facilitates not only dermal remodeling but also progressive repigmentation of the treated area [10,18,33,34]. The simultaneous action of the electromagnetic field and the vacuum also promotes the extension and alignment of collagen fibers, an element with marked piezoelectric characteristics. The electromagnetic field pushes the ions toward the two opposite ends of the molecule, promoting their extension [31,32], while the vacuum allows the collagen itself to be realigned perpendicular to the stratum corneum [24,25], promoting the restoration of skin volume. The favorable and sustained clinical response observed in the patients treated in this study supports what has been described in the literature and allows us to consider Biodermogenesi as a safe, effective, and well-tolerated alternative in the comprehensive management of SA, even in complex patients with medium to dark phototypes and long-standing lesions [18,33].
Histological changes induced by Biodermogenesi
The post-treatment histological evaluation revealed a significant transformation of the previously atrophic striae. An increase in epidermal thickness was observed, along with the reappearance of dermal papillae and rete ridges. The dermis showed a denser collagen network, with fibers arranged in a more compact and parallel fashion, and a marked increase in both superficial and deep capillaries. These observations are consistent with those reported by Veronese et al., Scarano et al., and Nicoletti et al., who described progressive histological reorganization after Biodermogenesi treatment, including matrix remodeling and vascular reactivation [29,34,35]. In the study by Scarano et al. [35], histological sections obtained after nine sessions showed a thicker epidermis with increased cell layers, more defined basket-weave pattern, and restructured collagen bundles aligned parallel to the skin surface. Additionally, capillary density increased significantly in the papillary and mid dermis, and there was evidence of improved melanocyte distribution without any inflammatory infiltration [10,35]. Nicoletti et al. emphasized that this dermal remodeling reflects a true regenerative effect, supported by histomorphometric analyses. These included measurements of vessel density, epidermal area, and cell proliferation indices, which together demonstrated structural and functional tissue recovery following V-EMF stimulation [29]. In our study, although no statistically significant differences were found in morphometric parameters such as epidermal area or flattening index of the dermoepidermal junction, the semiquantitative and qualitative evaluation of biopsies supports the notion of substantial reorganization of skin architecture. The findings of increased collagen density and vascularity coincide with previous reports and suggest that the regenerative process extends beyond superficial remodeling. Although the morphometric analysis did not show statistically significant differences in epidermal area, this may be due to the slower regenerative kinetics of the outermost layer of the skin compared to the dermis, where more evident structural remodeling was observed. Several authors have reported that epidermal regeneration, although present, tends to occur more gradually and often lags deeper matrix reorganization. Therefore, the absence of significant epidermal thickening does not contradict the regenerative phenomenon observed histologically but rather reflects a temporal difference in the maturation dynamics of the epidermal compartment [9,18,35-37]. These outcomes confirm that Biodermogenesi induces a tissue response that mimics physiological wound healing, involving angiogenesis, fibroblast stimulation, and extracellular matrix reconstruction. Notably, the absence of thermal damage, combined with pleasant sensory feedback and no adverse events, further distinguishes this approach from conventional ablative therapies.
Assessment of clinical satisfaction
A high level of satisfaction was reported by 85 % of the patients, with a statistically significant correlation between their perception and the clinical evaluation carried out by the blinded physician (p = 0.037). This consistency between subjective and objective assessments reinforces the clinical relevance of the improvement perceived by the patients. These results coincide with the study by Scarano et al. [35], in which more than 87 % of patients described the results of V-EMF treatment as very satisfactory. In that study, satisfaction levels were attributed not only to the visible improvement in the striae but also to the safety of the method and the natural appearance of the results. Similar outcomes have been documented by Veronese et al. 34, who reported a high degree of acceptance among patients with striae gravidarum treated with Biodermogenesi technology [18,33]. The degree of satisfaction observed in this study exceeds what has been reported for other techniques such as fractional lasers, microneedling, or PRP, which often present variability in results and adverse events including discomfort during the session, post-inflammatory hyperpigmentation, or longer recovery times [13,14,36,38,39]. In contrast, PRP, which often present variability in results and adverse events including discomfort during the session, post-inflammatory hyperpigmentation, or longer recovery times. In contrast, Biodermogenesi stands out not only for the absence of side effects but also for the progressive and natural results perceived by patients from the first sessions [10,17,19,29,35]. These clinical findings align with the psychological burden documented in several studies on SD, particularly among women. These lesions can significantly impact self-esteem, body image, and interpersonal relationships. The aesthetic recovery observed after Biodermogenesi treatment may thus contribute not only to physical improvement but also to emotional well-being, representing a therapeutic advance with both dermatological and psychosocial implications.
Pathophysiological considerations on the mechanisms of action of Biodermogenesi
Biodermogenesi combines capacitive-modulated electromagnetic fields (V-EMF) with pulsed vacuum, generating biophysical stimulation without producing thermal damage or tissue ablation. Previous studies have shown that this combination of stimuli generates a cascade of regenerative biological responses that includes an increase in microvascular perfusion, which improves tissue oxygenation and activates metabolic signaling pathways mediated by nitric oxide. Fibroblast stimulation through piezoelectric and magneto mechanical mechanisms, inducing the synthesis of collagen types I and III, functional elastin, and other matrix components such as fibronectin and hyaluronic acid. - Redox modulation and mechanical stimulation, which help restore the oxidative balance and favor repair in chronically atrophic tissues. This integrated response appears to replicate physiological tissue regeneration and wound healing mechanisms without the risks or adverse effects associated with ablative devices. The histological evidence of collagen fiber remodeling and vascular reorganization, documented in this and other studies, supports the hypothesis that Biodermogenesi promotes not only dermal restoration but also functional neoformation of high-quality tissue. The combined effect of electromagnetic and mechanical stimulation provided by this technology appears to initiate cellular and structural recovery processes that are typically absent in chronic striae, as reflected in the biological and histological responses documented to date [18,30,34,35].
Strengths, limitations, and future directions of the study
One of the main strengths of this study lies in its design, which combines clinical and histopathological evaluation, allowing a comprehensive understanding of the effects of Biodermogenesi treatment on SD. The inclusion of serial biopsies from six patients and the semiquantitative analysis of key morphological variables, such as epidermal thickness, DEJ architecture, fibrillar density, and vascularization, provides objective and reproducible evidence of the observed regenerative effects. The study also addresses a clinically challenging patient profile: women with long-standing SA (over 10 years of evolution), affecting extensive body areas and presenting with Fitzpatrick skin types III–IV. These factors are commonly associated with poor response to conventional therapies. Demonstrating visible, functional, and structural improvements in such a complex context enhances the clinical relevance of Biodermogenesi as a realistic and effective therapeutic option for patients previously lacking safe and satisfactory alternatives. Despite these contributions, certain limitations should be acknowledged. The limited sample size restricts the generalizability of the findings and constrains the ability to perform robust subgroup analyses. The absence of a control group and the lack of direct comparisons with established techniques such as lasers, PRP, or microneedling reduce the comparative scope of the outcomes. Additionally, although standardized clinical tools such as GAIS were applied, broader functional assessments and validated quality-of-life measures were not included. The study also did not include long-term follow-up to assess the durability of the observed effects, an important consideration in regenerative approaches. While previous reports on Biodermogenesi have documented sustained outcomes up to 12 months of post-treatment, further confirmation in locally treated cohorts would be desirable.
Conclusion
Biodermogenesi represents a non-invasive therapeutic alternative supported by both clinical and histological evidence for the treatment of chronic atrophic striae distensae. Its effectiveness in patients with Fitzpatrick skin types III–IV and lesions over 10 years old broadens its applicability in challenging clinical scenarios. The correlation between observed structural changes and patient-reported improvement supports its comprehensive regenerative impact. This study provides scientific grounds for incorporating Biodermogenesi into advanced therapeutic strategies and encourages further comparative and long-term follow-up research.
Author Contributions
B.C.Z. design the study, M.B. developed the treatment protocols, B.C.Z. and B.R.V. delivered the therapies, B.B.J. and R.M.M.I. evaluated in blind the results, B.C.Z. and M.B. wrote this article.
Funding
This research received no external funding.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study. Written informed consent for publication must be obtained from participating patient who can be identified.
Conflict of Interest
Maurizio Busoni is a member of the Board of Directors of Expo Italia Srl. The other authors declare no conflicts of interest. None of the authors received any financial compensation for conducting this study.
References
- Hague A, Bayat A. Therapeutic targets in the management of striae distensae: A systematic review. J Am Acad Dermatol 77 (2017): 559-568.
- Al-Himdani S, Ud-Din S, Gilmore S, et al. Striae distensae: a comprehensive review and evidence-based evaluation of prophylaxis and treatment. Br J Dermatol 170 (2014): 527-547.
- Yu Y, Wu H, Yin H, et al. Striae gravidarum and different modalities of therapy: a review and update. J Dermatolog Treat 33 (2022): 1243-1251.
- Elsedfy H. Striae distensae in adolescents: A mini review. Acta Biomed 91 (2020): 176-181.
- Schuck DC, de Carvalho CM, Sousa MPJ, et al. Unraveling the molecular and cellular mechanisms of stretch marks. J Cosmet Dermatol 19 (2020): 190-198.
- Forbat E, Al-Niaimi F. Treatment of striae distensae: An evidence-based approach. J Cosmet Laser Ther 21 (2019): 49-57.
- Lokhande AJ, Mysore V. Striae Distensae Treatment Review and Update. Indian Dermatol Online J 10 (2019): 380-395.
- Keen MA. Striae distensae: What’s new at the horizon? BJMP 9 (2016): a919.
- Strnadova K, Sandera V, Dvorankova B, et al. Skin aging: the dermal perspective. Clin Dermatol 37 (2019): 326-335.
- Veronese S, Picelli A, Zoccatelli A, et al. Morphological characterization of two dermal and hypodermal alterations in an adult man: surgical scar vs. stretch mark. J Ultrasound 27 (2024): 857-862.
- Puerto Martínez, Marianela. Caracterización clínica y manejo del Síndrome de Ehlers Danlos. Revista de Ciencias Médicas de Pinar del Río 21 (2017): 124-150.
- Maria C A I, Luiza E B P K, Natalia S C, et al. Transepidermal Retinoic Acid Delivery Using Ablative Fractional Radiofrequency Associated with Acoustic Pressure Ultrasound for Stretch Marks Treatment. Lasers in Surgery and Medicine 45 (2013): 81-88.
- Abeer A Hodeib, Ghada F R Hassan, Marwa N M Ragab, et al. Clinical and immunohistochemical comparative study of the efficacy of carboxytherapy vs platelet-rich plasma in treatment of stretch marks. J Cosmet Dermatol 17 (2018): 1008-1015.
- Güngör S, Sayilgan T, Gökdemir G, et al. Evaluation of an ablative and non-ablative laser procedure in the treatment of striae distensae. Indian J Dermatol Venereol Leprol 80 (2014): 409-412.
- Manuskiatti W, Boonthaweeyuwat E, Varothai S. Treatment of striae distensae with a TriPollar radiofrequency device: a pilot study. J Dermatolog Treat 20 (2009): 359-364.
- Kui Young Park, Hyun Kyu Kim, Sung Eun Kim, et al. Treatment of Striae Distensae Using Needling Therapy: A Pilot Study. Dermatol Surg 38 (2012): 1823-1828.
- Alessandro Roberto, Antonio Cataldo, Maurizio Busoni. A Study on a Non-Invasive Therapy with No Side Effects for the Treatment of Stretch Marks. IJCMCR 54 (2025): 003.
- Laura S, Veronese S, Alberti G, et al. Vacuum and electromagnetic field in synergy for skin rejuvenation: A retrospective study on 217 patients. J Cosmet Dermatol 22 (2023):2989-2995.
- Artigiani A, Cervadoro G, Loggini B, Paolicchi A. Biodermogenesi: la soluzione non invasiva nel trattamento delle smagliature (2012): 1.
- World Medical Association. Declaration of Helsinki – Ethical Principles for Medical Research Involving Human Participants 310 (2013): 2191-2194.
- Singer SJ, Nicolson GL. The fluid mosaic model of the structure of cell membranes. Science 175 (1972): 720-731.
- Perry RH, Green DW. Perry’s Chemical Engineers’ Handbook. 7th ed (1999).
- Kreindel M, Mulholland S. The Basic Science of Radiofrequency-Based Devices. Enhanced Liposuction - New Perspectives and Techniques (2021).
- Meirte J, Moortgat P, Anthonissen M, et al. Short-term effects of vacuum massage on epidermal and dermal thickness and density in burn scars: an experimental study. Burns Trauma 4 (2016): 27.
- Moortgat P, Anthonissen M, Meirte J, et al. The physical and physiological effects of vacuum massage on the different skin layers: a current status of the literature. Burns Trauma 19 (2016): 34.
- Pacini S, Punzi T, Gulisano M, Cecchi F, Vannucchi S, Ruggiero M. Transdermal delivery of heparin using pulsed current iontophoresis. Pharm Res 23 (2006): 114-120
- Norman G. Likert scales, levels of measurement and the "laws" of statistics. Adv Health Sci Educ Theory Pract 15 (2010): 625-632.
- Chapman DM, Ross JB. Objective measurement of three epidermal parameters in psoriasis vulgaris and in dermatopathology in general. Br J Dermatol 119 (1988): 333-343.
- Nicoletti G, Perugini P, Bellino S, et al. Scar Remodeling with the Association of Monopolar Capacitive Radiofrequency, Electric Stimulation, and Negative Pressure. Photomed Laser Surg 35 (2017): 246-258.
- Alberti G, Laura S. Treatment of stretch marks aged more than twenty years with the synergy of electromagnetic field and vacuum. Clinical case studies and subsequent follow-up. Aesthetic Medicine 5: 14-21.
- Nair M, Calahorra Y, Kar-Narayan S, et al. Self-assembly of collagen bundles and enhanced piezoelectricity induced by chemical crosslinking. Nanoscale 11 (2019): 15120-15130.
- Wu Y, Zou J, Tang K, et al. From electricity to vitality: the emerging use of piezoelectric materials in tissue regeneration. Burns Trauma 12 (2024): tkae013
- Marafioti S, Veronese S, Pecorella C, et al. Electromagnetic Fields, Electrical Stimulation, and Vacuum Simultaneously Applied for Major Burn Scars. Bioengineering (Basel) 12 (2025): 179.
- Veronese S, Bacci PA, Garcia Gimenez V, et al. A. V-EMF therapy: A new painless and completely noninvasive treatment for striae gravidarum. J Cosmet Dermatol (2024): 1-8.
- Scarano A, Sbarbati A, Amore R, et al. A New Treatment for Stretch Marks and Skin Ptosis with Electromagnetic Fields and Negative Pressure: A Clinical and Histological Study. J Cutan Aesthet Surg 14 (2021): 222-228.
- El-Ramly, Amany El-Hanafy, Ghada El Maadawi, et al. Histological and quantitative morphometric evaluation of striae distensae treated by long-pulsed 1064-nm Nd: YAG laser. J Egypt Womens Dermatol Soc 12 (2015): 120-128.
- Kurban RS, Bhawan J. Histologic changes in skin associated with aging. J Dermatol Surg Oncol 16 (1990): 908-914.
- Naspolini AP, Boza JC, da Silva VD, et al. Efficacy of Microneedling Versus Fractional Non-ablative Laser to Treat Striae Alba: A Randomized Study. Am J Clin Dermatol 20 (2019): 277-287.
- Gad SE, Neinaa YME, Rizk OK, et al Efficacy of platelet-poor plasma gel in combination with fractional CO2 laser in striae distensae: A clinical, histological, and immunohistochemical study. J Cosmet Dermatol. (2021): 3236-3244.


 Impact Factor: * 6.2
Impact Factor: * 6.2 Acceptance Rate: 76.33%
Acceptance Rate: 76.33%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 