Malignant gastrointestinal neuroectodermal tumor: A rare differential diagnosis of neuroendocrine neoplasm of the Small Intestine - Case reports and Review of the literature
Kvirkvelia1*, M. Jesinghaus2, J. Görlach3, J. Szymanski2, N. Harb2, D.K. Bartsch1
1Department of Visceral-, Thoracic- and Vascular Surgery, University Hospital Gießen and Marburg, Marburg, Germany
2Institute of Pathology, University Hospital Gießen and Marburg, Marburg, Germany
3Institute of Diagnostic and Interventional Radiology, University Hospital Gießen and Marburg, Marburg, Germany
*Corresponding Author: Kvirkvelia Natia, Departements of the University Hospital Marburg, 35043 Marburg, Germany
Received: 21 May 2025; Accepted: 04 June 2025; Published: 17 September 2025
Article Information
Citation: N. Kvirkvelia, M. Jesinghaus, J. Görlach, J. Szymanski, N. Harb, D.K. Bartsch. Malignant gastrointestinal neuroectodermal tumor: A rare differential diagnosis of neuroendocrine neoplasm of the Small Intestine - Case reports and Review of the literature. Journal of Surgery and Research. 8 (2025): 452-459.
View / Download Pdf Share at FacebookAbstract
The malignant gastrointestinal neuroectodermal tumor (GNET) is a rare and very aggressive neoplasm. Due to its rarity, the tumor is often misdiagnosed. We report on two female patients who were operated on under the suspected diagnosis of a neuroendocrine tumor of the small intestine (SI-NET). The patients, aged 37 and 57 years, presented with symptoms of bowel obstruction. In a 37-year-old patient with a chronic bowel obstruction, MRI enterography revealed a tumor in the jejunum with lymph node metastases as well as liver metastases. Laparotomy confirmed the presence of a tumor in the jejunum with lymph node and liver metastases. Segmental resection of the small bowel with lymphadenectomy was performed. The remaining hepatic metastases in the right liver lobe were removed 8 weeks later by right hemihepatectomy. Four months later, the Ga68-FAPI-PET/CT showed significant disease progression. Unfortunately, the patient died 22 months later after initial diagnosis. In the second case, a 57-year-old female presented to our clinic with progressive bowel obstruction for 2 years. The MRI examination revealed a small bowel obstruction due to a tumor mass in the ileum with lymph node metastases suspicious for a SI-NET. Exploratory laparotomy revealed a tumor in the jejunum with peritoneal metastases. A vessel-sparing small bowel resection with lymphadenectomy and partial peritonectomy was performed. The follow-up examinations using MRI and CT six months postoperatively showed no evidence of tumor progression. The final pathology confirmed the diagnosis of a GNET in both cases. Molecular analysis revealed EWSR1 translocations in the tumors of both patients.
Keywords
<p>Malignant gastrointestinal neuroectodermal tumor, GNET, Small intestine, SI-NEN, EWSR1 translocation</p>
Article Details
Introduction
Malignant gastrointestinal neuroectodermal tumor (GNET) is an extremely rare primary neoplasm [1]. This entity is also referred to as clear cell sarcoma-like tumor of the gastrointestinal tract (GCS-like tumor of the gastrointestinal tract) [2]. The most common tumor location is the small intestine (58%), followed by the stomach (16%), colon (11%), ileocecal junction (5%), lower esophagus (5%) and anal canal (5%) [3]. Already in 1985, Alpers and Beckstead described a malignant neuroendocrine tumor of the jejunum with osteoclast-like giant cells [4]. In 2003 Zambrano et al. first defined clear cell sarcoma of the gastrointestinal tract as a distinct entity. They reported six cases of osteoclast-rich tumors of the gastrointestinal tract that resembled clear cell sarcoma of soft tissues and could be clearly distinguished from gastrointestinal stromal tumors (GIST) [5]. In 2012 the term GNET was introduced based on histomorphological and immunohistochemical criteria of 16 cases [6]. The median age at diagnosis for GNET is around 45 years (range: 13–85 years) with an equal gender distribution [7]. At the time of diagnosis, 30% of patients already present with distant metastases and the prognosis is poor with a median survival time of only 18 months [3,8]. To date, approximately 80 cases of small bowel GNETs have been documented in the English literature. The clinical symptoms and imaging findings might mimic those of a small intestine neuroendocrine tumor (SI-NET), a neoplasm with a significantly better prognosis.
Here we report two cases of small bowel GNETs that were operated on under the suspicion of a SI-NET and provide a brief overview of the literature regarding GNET of the small intestine as a differential diagnosis to SI-NET.
Case presentations
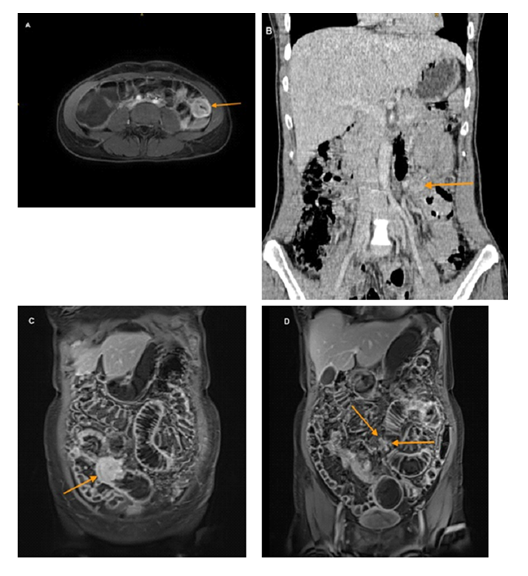
Case 1: A 37-year-old woman presented to our hospital with recurrent abdominal pain of variable localization for 2 years. Physical examination revealed abdominal tenderness without signs of peritonitis. Standard blood analysis showed iron-deficiency anemia with a normal count of white blood cells and platelets. Chromogranin A was within the normal range at 35 ng/ml (normal < 108 ng/ml). The 5-hydroxyindoleacetic acid (5-HIAA) in the urine was also within the normal range at 26.3 µmol/d (normal 10.4 - 47.1 µmol/d). No lesions were found during the upper and lower endoscopies. Magnetic resonance enterography showed a rounded wall thickening of a small intestine loop in the left abdomen with intense contrast enhancement of pathological lymph nodes up to 2 cm in size, extending from the small bowel to the superior mesenteric artery [level 2 according to the Marburg classification, 9], as well as at least 3 liver metastases in the right liver lobe. No mesenteric desmoplasia was visible. (figure 1A and B)
Due to the nonspecific abdominal pain and the radiologically detected small bowel tumor with liver metastases, the suspicion of a metastatic SI-NET was raised. To complement the diagnostic workup for SI-NET, a preoperative DOTATOC-PET/CT was also performed. The Ga68-DOTATOC-PET/CT examination showed only weak somatostatin receptor expression in the tumor and the enlarged lymph nodes, raising some doubts in the diagnosis.
To confirm the dignity of the hepatic mass, a sonographically guided biopsy was performed. Histology revealed liver tissue infiltrated by a nest-forming tumor. Imunohistochemically weak synaptophysin expression, and negativity for cytokeratins (CKMNF 116, CK 18, CK 19) was noted. Chromogranin A also tested negative. Given the positivity for S100, the metastasis of a malignant melanoma was among the possible diagnoses.
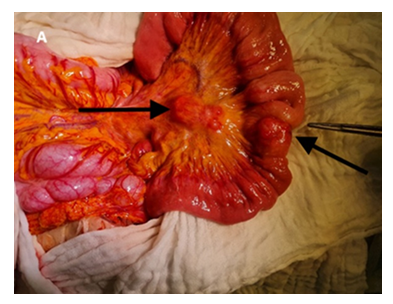
The patient subsequently underwent an exploratory laparotomy. Intraoperatively, a small bowel tumor was found in the jejunum, 120 cm distal of the Treitz ligament, without mesenteric shrinkage, but with mesenteric lymph node metastases extending to the inferior border of the pancreatic neck [level 2 according to the Marburg classification, 9], as well as multiple liver metastases. A segmental resection of the jejunum with retrograde vessel-sparing lymphadenectomy, as well as resection of the liver metastases in segments III and IVb, was performed (figure 2). The liver metastases in the right liver lobe (segments VI, VII, VIII) were left behind to perform a second stage right hemiheaptectomy.
Frozen section described a malignant tumor most likely of epithelial origin, but without precise differentiation. The final histological examination after paraffin embedding revealed a 3 cm small bowel tumor with areas of solid, pseudopapillary, and isolated alveolar growth. The tumor cells exhibited areas of clear cell cytoplasm. Of the 18 resected mesenteric lymph nodes, 10 were found to be tumor infiltrated. The immunohistochemical analysis showed strong positivity for S-100 protein. However, HMB-45, Melan-A, and PanCK were consistently negative. Synaptophysin showed partial and weak expression. The Ki-67 proliferation index was approximately 40% (figure 3) The fusion analysis performed using Next-Generation Sequencing (NGS) identified an EWSR1-CREB1 translocation. In conclusion, the diagnosis of a lymphatic and hepatic metastasized malignant gastrointestinal neuroectodermal tumor (GNET) of the jejunum pT2a, pN1(10/18), pM1, R0 was established.
The postoperative course was uneventful and the patient was discharged at postoperative day 10. The remaining hepatic metastases in the right liver lobe were removed 8 weeks later by right hemihepatectomy. Two months later, new liver metastases were detected in the Ga68-FAPI-PET/CT, which were still progressive one month later in the MRI. Two months later, the GA68-FAP-PET/CT showed significant disease progression with numerous liver metastases, as well as mesenteric and peritoneal manifestations. Despite four courses radiopeptide therapies with 7,0 GBq Y-90 FAP, 7,5 GBq Lu-177 plus 10MBq Ac-225 FAP, 7,3 MBq Ac-225 FAP/ 7,0 GBq Y-90, 9,0 MBq Ac-225 FAP, as well as 4 transarterial embolizations of the liver, the tumor continued to progress. The patient died 22 months after initial diagnosis.
Case 2: A 57-year-old female patient complained of progressively worsening, intermittent abdominal pain over 2 years. Within the last 3 weeks prior to admission, she also experienced episodes of bowel obstruction and recurrent vomiting. At initial presentation physical examination revealed a massively distended and tender abdomen without signs of peritonitis. Standard blood analysis showed no anemia with a hemoglobin level of 13.3 g/l. Chromogranin A was within the normal range at 48.0 µg/l (normal < 102 µg/l). The 5-hydroxyindoleacetic acid (5-HIAA) in the urine was also within the normal range at 5.6 mg/g Krea (normal 1.3-6.9 mg/g Krea). CEA and CA 19-9 were also within the normal range. Magnetic resonance enterography had already been performed on an outpatient basis. The imaging showed signs of a small bowel obstruction due to a rounded small bowel lesion in the middle part of the ileum. The enlarged lymph nodes, up to 1 cm in size, extended to the segmental branches of the mesenteric vessels [level 1 according to the Marburg classification, 9]. No mesenteric shrinkage was visible, but peritoneal carcinomatosis was suggested because of multiple peritoneal nodes up to 5mm in size. Liver metastases were not visible. The MRI diagnosis was small bowel tumor with manifest bowel obstruction, lymph node and peritoneal metastases, possibly a SI-NET (Figure 1C and D)
Because of the bowel obstruction the patient underwent an exploratory laparotomy without further imaging such as Ga68-DOTATOC-PET/CT. Intraoperatively, extensive peritoneal metastases were observed with multiple tumor deposits up to 3 mm in size in the whole peritoneum and intestinal mesentery summing up to a peritoneal carcinomatosis index (PCI) according to Sugarbaker [10] of 18. The highly obstructive tumor was located in the jejunum, 160 cm distal to the Treitz ligament. Enlarged, mesenteric lymph nodes were found up to the origin of the superior mesenteric artery, but without mesenteric desmoplasia. A segmental small bowel resection with vessel-sparing lymphadenectomy and partial peritonectomy was performed, which resulted in a R2 resection.
On frozen section analysis, the peritoneal deposits were classified as a malignant, the potential diagnoses included a diffusely growing carcinoma, a neuroendocrine tumor or a mesenchymal neoplasm, respectively.
After paraffin embedding, a malignant tumor was observed in the small intestine and peritoneum, consisting of a predominantly monomorphic cell population with small, hyperchromatic, oval nuclei and a narrow eosinophilic cytoplasmic rim. The primary tumor exhibited a spindle-shaped growth pattern with clear cell cytoplasm, while the tumor tissue in the peritoneum displayed a diffuse infiltrative cell pattern that differed from the primary tumor. The mitotic activity of the neoplasm was overall low, with a maximum of 6 mitoses per 10 high power fields (HPF). Of the 16 examined locoregional lymph nodes, 5 showed capsular-invasive metastases.
Immunohistochemically the tumor cells exhibited strong positivity for SOX10 and S-100. Melan-A and HMB-45 were negative, as were the strains for broad-spectrum cytokeratins (CK18, CKpan), Calretinin, Inhibin, Hormone receptors, E-cadherin and GATA 3. Synaptophysin exhibited a heterogeneous, partially strong expression. (Figure 3) Chromogranin A was negative. The Ki-67 proliferation index was up to 20%. Using Next-Generation Sequencing (NGS) an EWSR1-ATF1 fusion was detected. Thus, finally the diagnosis of a malignant gastrointestinal neuroectodermal tumor (GNET) pT2b, pN1(5/16), pM1(PER), R0 (local) was made.
The postoperative course was uneventful and the patient was discharged after 10 days. The interdisciplinary tumor conference recommended palliative chemotherapy only in the event of tumor progression. The follow-up examinations using MRI of the abdomen and CT of the thorax six months and 12 months postoperatively showed no definitive tumor formations, indicating no evidence of tumor progression.
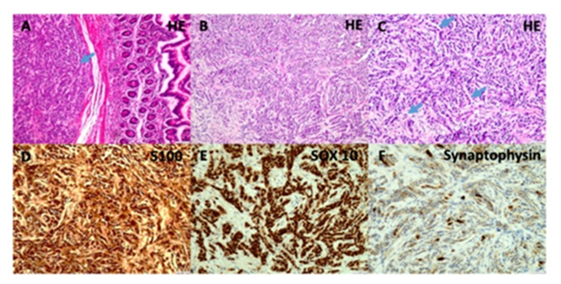
Figure 3: A: Histologic appearance of a primary GNET presenting as a submucosal mass beneath non-dysplastic small intestinal mucosa. The tumor shows solid growth with sheets and nests of oval to spindled mesenchymal cells featuring clear or eosinophilic cytoplasm, prominent nucleoli, and rare mitotic figures (HE, magnification ×10)
B-C: Peritoneal metastases from the same neoplasm demonstrating an alternating growth pattern, including dissociative tumor architecture with trabecular arrangements and infiltrating single tumor cells. Despite the invasive pattern, the cytomorphological features remain low-grade. (B magnification ×10, C magnification ×20)
D-F: Immunohistochemistry of the peritoneal metastases reveals strong nuclear expression of S100 (D magnification ×20) and SOX10 (E magnification ×20), along with heterogeneous cytoplasmic expression of Synaptophysin (F magnification ×20).
Discussion
Between 1998 and 2024, 81 cases of small bowel GNETs of the small bowel with relevant clinical data have been reported in the English literature (table 1). More than 60% of patients were younger than 45 years at the time of diagnosis, as was one of our patients. Genders are equally affected in the literature, although both our patients were female [8].
The clinical symptoms include abdominal pain, nausea, obstruction, abdominal distension, bloating, ascites and abdominal mass [3,6,11]. Other nonspecific symptoms include anorexia, weight loss, weakness, anemia and fever [6]. Our two patients were diagnosed because of progressive symptoms of bowel obstruction.
The CT and/or MRI morphological signs of GNETs include irregular masses, thickening of the intestinal wall often associated with bowel obstruction, enlarged lymph nodes and liver metastases as in our two patients [11-14]. These radiological signs are quite similar to those of SI-NET, although these tumors are fequently associated with mesenteric desmoplasia and shrinkage of the mesentery [9]. In contrast to SI-NET, mesenteric desmoplasia associated with GNET is not yet described. This was also the case in the presented two patients, although both had advanced GNET disease. The Ga68-DOTATOC-PET/CT in GNET seems to show heterogeneous expression, but mainly negative or weak somatostatin receptor positivity as in one of our patients, wheras the fast majority of SI-NET show strong positive results [15]. Thus, if a patient with a small bowel tumor and mesenteric lymph node metastases has a normal serum chromogranin A, no radiological signs of a mesenteric desmoplasia and none or only weak somatostatin receptor expression on the Ga68-DOTATOC-PET/CT, than a GNET has to be kept in mind. In addition, as in our two patients about 30% of small bowel GNETs are located in the jejunum. This is in contrast to SI-NENs, which are located in over 90% in the ileum [16-18].
Our systematic review of the literature revealed that 38% (30/78) of patients already had distant metastases at the time of diagnosis (table 1), which is lower than for SI-NEN with around 60% [19,20]. These were present in either the liver or the peritoneum, as in our patients. Bone or lung metastases seem to be rare in GNET in contrast to Si-NET (table 1).
The only chance for cure for small bowel GNET is the resection of the primary tumor with an oncologic, organ-sparing lymphadenectomy as also proposed for SI-NEN [16]. Since the majority of these tumors are located in the jejunum or proximal ileum, this means a segmental small bowel resection, as reported in the literature (table 1) and performed in our two patients. The extent of lymphadenectomy is not defined for GNET. Since these tumors are intraoperatively difficult to distinguish from SI-NET by frozen section, as in our two patients, we propose a systematic bowel-sparing lymphadenectomy up to the inferior border of the pancreas with the dissection of at least 8 lymph nodes as recommended for SI-NEN [9,15]. If ever possible R0 resection of the primary tumor and all metastases should be accomplished, even with two-stage hepatectomy, to prolong survival. This strategy was followed by one of the presented patients, but she died 22 months after diagnosis due diffuse liver metastases.
The rarity and histological similarities of GNET with other neoplasms can easily lead to a misdiagnosis. The histological appearance of a GNET is heterogeneous, making intraoperative frozen section analysis very challenging for diagnosis. The diagnosis GNET could not be made on frozen section in the presented two patients, the diagnosis remained quite vague. In the fast majority of patients, the diagnosis GNET can only be established postoperatively after immunohistochemical analysis. The typical immunohistochemical markers of a GNET are positivity for S100 and SOX10 proteins, along with negativity for melanocytic markers. Neuroendocrine markers are variable positive. Around 50% of cases exhibit positive staining for synaptophysin and neuron-specific enolase [21]. Of the 25 cases in the literature that included the evaluation of the expression of at least one neuroendocrine marker (including chromogranin-A, synaptophysin, neuron-specific enolase and CD56) immunohistochemically, 19 (76%) were found to express at least one of these markers [8]. The molecular hallmark of the GNET is the gene fusion translocations involving EWSR1, usually EWSR1-CREB1 or EWSR1-ATF1 [6,22], as in our two patients.
Several differential diagnoses can be considered, including NET, GIST, clear cell sarcoma and primary or metastatic melanoma [6,8]. A SI-NET was primarily considered as a differential diagnosis in both of our cases. Although GNET might express neuroendocrine markers, especially synaptophysin and neuron-specific enolase, the EWSR1 rearrangement clearly differentiates it from a neuroendocrine tumor [1]. Metastatic clear cell sarcoma of tendons and aponeuroses is the most important differential diagnosis of GNET [13]. Clear cell sarcoma (CCS) is a rare soft tissue sarcoma and was first described in 1965. The main characteristic of clear cell sarcoma (CCS) is the expression of melanin pigments and melanocytic markers. Both tumors share positivity for S100 protein and SOX10 and exhibit similar genetic abnormalities, including EWSR1 fusions. Lack of melanocytic-associated markers expression is helpful for differentiating GNET [3]. GIST can be also a differential diagnosis, as it may also exhibit epithelioid and spindle cell morphology. The negativity for CD34, CD117 and DOG1, however, supports the diagnosis of GNET [3]. Melanoma, another differential diagnosis, typically occur in older patients, who may have a synchronous or a history of primary cutaneous or acral melanoma, are usually positive for MB-45 or Melan-A and do not have EWSR1 rearrangements [8].
Due to its extreme rarity, prognostic information for GNET is very limited, standardized staging protocols are lacking and there is no consensus on treatment, despite the attempt for curative resection, if ever possible [3]. The presented two patients and 38% (30/78) of reported patients with GNET had stage IV disease at the time of diagnosis, which could not be completely resected (table 1). Because of the rarity of the disease, there are as yet no standardized palliative treatment regimens. Su et al. showed the therapeutic effect of apatinib against this malignancy [23]. Su et al. presented a case with a tumor stage of IV and poor prognosis. The young patient was initially treated with traditional chemotherapy, including gemcitabine plus paclitaxel/gemcitabine plus vinorelbine, which showed progressive disease. Apatinib was administered as a fifth-line therapy. It was given with temozolomide. The overall survival of the young patient from the initiation of treatment with apatinib was 33 months [23]. Apatinib mesylate (hereafter referred to as Apatinib) is a tyrosine kinase inhibitor (TKI), that selectively targets the vascular endothelial growth factor receptor 2 (VEGFR-2) and has shown promising effects in prolonging progression-free survival (PFS) for a variety of advanced sarcomas after failure of standard multimodal therapy [24].
Subbiah et al. presented a case of a metastatic GNET, which showed a near-complete response to crizotinib and pazopanib for 1.5 years [25]. EWSR1-ATF and EWSR1-CREB1 are both believed to upregulate the mesenchymal-epithelial-transition pathway through the melanocyte transciption factor (MITF), and thus, may create a vulnerability to MET inhibitors [26].
The GNET is highly prone to local recurrence and has a poor prognosis [8]. The time to distant recurrence varied between 2 weeks and 109 months [1]. The median survival was 32 months in one patient series [6]. In another patient series by Chang et al. 5 (33%) patients were alive with tumor recurrence or metastasis, whereas 8 (53%) were alive with no evidence of disease after a median follow-up of 25 months [3]. Our literature review showed that follow-up information was available for 61 patients. The median disease-free survival was 15 months (range 1-161 months).
In conclusion, GNET is an extremly rare tumor of the small bowel which might mimick the clinical symptoms, the radiological appearance and the histological frozen section features of SI-NEN. However, GNET has unique clinical and histopathological features, including absence of mesenteric desmoplasia and presence of EWSR1-ATF1/CREB1 fusions, which establish the diagnosis. Since the prognosis is otherwise dismal, an oncologic organ-sparing small bowel resection with systematic lymphadenectomy and metastasectomy with the goal of a R0 resection should be performed, whenever possible.
|
Parameter |
|
|
Age years, median (range) |
39 (12-70) |
|
Female gender |
37/81 (46%) |
|
Localisation jejunum |
22/81 (27%) |
|
Localisation ileum |
28/81 (35%) |
|
Unknown (small Intestine) |
31/81 (38%) |
|
Distant metastases at diagnosis |
30/78 (38%) |
|
EWSR1 Gen Fusions |
65/ 81 (80%) |
|
EWSR1- ATF1 |
25/81 (31%) |
|
EWS R1-CREB1 |
9/81 (11%) |
|
EWSR1 rearrangements |
31/81(38%) |
|
Unknown |
16/81 (20%) |
|
Curative treatment intention |
53/67 (79%) |
|
Palliative treatment intention |
14 /67 (21%) |
|
Operation |
78/78 (100%) |
|
SB-resection plus systematic LAD |
51/ 78 (65%) |
|
SB resection |
18/78 (23%) |
|
Right hemicolectomy |
5/78(6%) |
|
Resection of distant metastases |
4/78 (5%) |
|
Median follow-up (range), months |
21 (1-161) |
|
Median survival (range), months |
15 (1-161) |
|
Disease status |
|
|
NED |
28/81 (35%) |
|
AWD |
13/81 (16%) |
|
DOD |
19/81 (23%) |
|
DFS |
1/81 (1%) |
|
Unknown |
20/81 (25%) |
SB - small bowel, LAD - lymphadenectomy, NED - no evidence of disease, AWD - alive with disease DOD - dead of disease, DFS - disease-free survival
Table 1: Characteristics of malignant gastrointestinal neuroectodermal tumor in the small bowel – summary of 81 cases (including the present 2 cases) reported in the world literature [1,3,5,7,11,13,14,22,23,25,27-69].
Conflicts of interest
The authors declare no conflict of interest.
References
- Mishra P, Biswas D. Malignant gastrointestinal neuroectodermal tumor A case-based review of literature. Journal of Cancer Research and Therapeutics 18 (2022): 885-897.
- Morani AC, Ramani NS, Yedururi S. Malignant Gastrointestinal Neuroectodermal Tumor: A New Kid on the Block? J Comput Assist Tomogr 46 (2022): 676-681.
- Chang B, Ling Y, Wen WG. Malignant Gastrointestinal Neuroectodermal Tumor: Clinicopathologic, Immunohistochemical, and Molecular Analysis of 19 Cases. Am J Surg Pathol 44 (2020): 456-466.
- Alpers CE, Beckstead JH. Malignant neuroendocrine tumor of the jejunum with osteoclast-like giant cells. Enzyme histochemistry distinguishes tumor cells from giant cells. Am J Surg Pathol 9 (1985): 57-64.
- Zambrano E, Miguel M. An osteoclast-rich tumor of the gastrointestinal tract with features resembling clear cell sarcoma of soft parts: reports of 6 cases of a GIST simulator. Int J Surg Pathol 11 (2003): 75-81.
- Stockman DL, Miettinen M, Suster S. Malignant gastrointestinal neuroectodermal tumor: clinicopathologic, immunohistochemical, ultrastructural, and molecular analysis of 16 cases with a reappraisal of clear cell sarcoma-like tumors of the gastrointestinal tract. Am J Surg Pathol 36 (2012): 857-868.
- Francesco E, D`Amico, Ruffolo C, et al. Clear cell sarcoma of the ileum: report of a case and review of the literature. Int J Surg Pathol 20 (2012): 401-406.
- Wang J, Khin T. Clear cell sarcoma-like tumor of the gastrointestinal tract: an evolving entity. Arch Pathol Lab Med 139 (2015): 407-412.
- Bartsch DK, Windel S, Kanngießer V, et al. VesselSparing Lymphadenectomy Should Be Performed in Small Intestine Neuroendocrine Neoplasms. Cancers (Basel) 14 (2022): 3610.
- Jacquet P, Sugarbaker PH. Clinical research methodologies in diagnosis and staging of patients with peritoneal carcinomatosis. Cancer Treat Res 82 (1996): 359-374.
- Mohammed JA, Jumana AA. Malignant gastrointestinal neuroectodermal tumor: a case report and review of the literature. Diagn Pathol 12 (2017): 29.
- Gahanbani A, Boyle D, Elton C. Gastrointestinal clear cell sarcoma-like tumour of the ascending colon. Ann R Coll Surg Engl 98 (2016): e37-39.
- Gokce A, Faruk EK. Clear Cell Sarcoma-Like Tumor of the Gastrointestinal Tract. J Gastrointest Cancer 50 (2019): 651-656.
- Sasaki M, Tanaka M. Malignant gastrointestinal neuroectodermal tumor presenting with small intestinal obstruction: A case report. DEN Open 2 (2022): e119.
- Lamarca A, Bartsch DK, Caplyn M. European Neuroendocrine Tumor Society (ENETS) 2024 guidance paper for the management of well-differentiated small intestine neuroendocrine tumours. J Neuroendocrinol 36 (2024): e13423.
- Bartsch DK, Maurer E, Holzer K. Neoroendokrine Neoplasien des Jejunum und Ileums (SI-NEN). Springer Medizin (2022): Viszeral und Allgemeinchirurgie.
- Keck KJ, Maxwell JE, Utria AF. The Distal Predilection of Small Bowel Neuroendocrine Tumors. Ann Surg Oncol 25 (2018): 3207-3213.
- Zeuß D, Marth T. Tumoren des Dünndarms. Springer, Berlin, Heidelberg (2016): 1-27.
- Norlen O, Stalberg P, Öberg K. Long-term results of surgery for small intestinal neuroendocrine tumors at a tertiary referral center. World J Surg 36 (2012): 1419-1431.
- Watzka FM, Fottner C, Miederer M. Surgical Treatment of NEN of Small Bowel: A Retrospective Analysis. World J Surg 40 (2016): 749-758.
- Doyle Leona A, Hornick Jason L. Mesenchymal Tumors of the Gastrointestinal Tract Other than GIST. Surg Pathol Clin 6 (2013): 425-473.
- Cristina RA, Khedoudja N. EWS-CREB1: a recurrent variant fusion in clear cell sarcoma--association with gastrointestinal location and absence of melanocytic differentiation. Clin Cancer Res 12 (2006): 5356-5362.
- Dan S, Hujuan Y, Ming Z. Malignant gastrointestinal neuroectodermal tumor: a case report and literature review. Annals of Medicine & Surgery 85 (2023): 6196-6201.
- Xie L, Guo W, Wang Y. Apatinib for advanced sarcoma: results from multiple institutions’ off-label use in China. BMC Cancer 6 (2018): 396.
- Subbiah V, Holmes O, Gowen K. Activity of c-Met/ALK Inhibitor Crizotinib and Multi-Kinase VEGF Inhibitor Pazopanib in Metastatic Gastrointestinal Neuroectodermal Tumor Harboring EWSR1-CREB1 Fusion. Oncology 91 (2016): 348-353.
- Davis IJ, McFadden AW, Zhang Y. Identification of the receptor tyrosine kinase c-Met and its ligand, hepatocyte growth factor, as therapeutic targets in clear cell sarcoma. Cancer Res 70 (2010): 639-645.
- Abdulkader I, Cameselle TJ, Alava E. Intestinal clear cell sarcoma with melanocytic differentiation and EWS [corrected] rearrangement: report of a case. Int J Surg Pathol 16 (2008): 189-193.
- Aditi D, Sreenija Y. Malignant Gastrointestinal Neuroectodermal Tumour- Case Report with Review of Literature. Journal of Gastrointestinal Cancer 52 (2021): 124.
- Balkaransingh P, Saad AS, Govil SC. Clear cell sarcoma of the gastrointestinal tract presenting as a second malignant neoplasm following neuroblastoma in infancy. Pediatr Blood Cancer 58 (2012): 481.
- Camilla EC, Luca N. Clear cell sarcoma of the ileum: report of a case and review of literature. Virchows Arch 451 (2007): 839-845.
- Christopher WT, Wendy C. Malignant gastrointestinal neuroectodermal tumour (GNET): Neural mesenchymal tumours of the gastrointestinal tract with striking histology and EWSR1 gene rearrangement. Pathology (2019): 324-327.
- Girish V, Adam Q. Clear cell sarcoma of the small bowel: a potential pitfall. Case report. APMIS 113 (2005): 716-719.
- Green C, Spangolo DV, Röbbins PD. Clear cell sarcoma of the gastrointestinal tract and malignant gastrointestinal neuroectodermal tumour: distinct or related entities? A review. Pathology 50 (2018): 490-498.
- Harshavardhini S, Saishalini CN, Pavithra V. Malignant gastrointestinal neuroectodermal tumor-A case report. Indian J Pathol Microbiol 64(2021): 373-375.
- Huang G, Chen Q, Zhong L. Primary malignant gastrointestinal neuroectodermal tumor occurring in the ileum with intra-abdominal granulomatous nodules: A case report and review of the literature. Oncol Lett 17 (2019): 3899-3909.
- Huang WP, Li LM, Gao JB. Postoperative multiple metastasis of clear cell sarcoma-like tumor of the gastrointestinal tract in adolescent: A case report. World J Clin Cases 10 (2022): 6175-6183.
- Jia Y, Yan Y, Hebbard P. Malignant Gastrointestinal Neuroectodermal Tumor (GNET) Mimicking Small Bowel Lymphoma: A Case Report. Cureus 16 (2024): e59105.
- Kaori T, Hideki O. Clear-cell sarcoma of the small intestine detected by FDG-PET/CT during comprehensive examination of an inflammatory reaction. J Med Invest 56 (2009): 70-75.
- Keduovinuo K, Shraddha P. Gastrointestinal Neuroectodermal Tumor: a Diagnostic Dilemma. Indian J Surg 79 (2017): 166-168.
- Kervarrec T, Lecointre C, Kerdraon R. Gastro-intestinal neuroectodermal tumor (GNET): A case report of a small intestine tumor with hepatic metastases. Ann Pathol 35 (2015): 506-510.
- Khin T, Ian J. Clear Cell Sarcoma-Like Tumor of the Gastrointestinal Tract, Presenting as a Second Malignancy after Childhood Hepatoblastoma. Case Rep Med 12 (2014): 17.
- Konstantinos L, Alexandros P. Clear cell sarcoma of the jejunum: a case report. World J Surg Onco 25 (2013): 11-17.
- Donner LR, Trompler RA, Dobin S. Clear cell sarcoma of the ileum: the crucial role of cytogenetics for the diagnosis. Am J Surg Pathol 22 (1998): 121-124.
- Laura G, John H. Visceral clear cell sarcoma of soft tissue with confirmation by EWS-ATF1 fusion detection. Ultrastruct Pathol 30 (2006): 111-118.
- Lorenzo T, Khalil Z. Primary clear cell sarcoma of the ileum: an uncommon and misleading site. Virchows Archiv 9 (2005): 772-777.
- Mădălina B, Miruna C, Mariana A. The Malignant Gastrointestinal Neuroectodermal Tumor (GNET): A Distinct Entity and the Challenging Differential Diagnosis with Mesenchymal, Lymphoid, and Melanic Tumors: A Case Report and Brief Review of the Literature. Diagnostics (Basel) 13 (2023): 1131.
- Manel N, Bahaeddine L. Small Intestine Gastrointestinal Clear Cell Sarcoma: A Case Report and Review of the Literature. J Investig Med High Impact Case Rep 8 (2024): 16.
- Mee J, Sun HC. Primary gastrointestinal clear cell sarcoma: report of 2 cases, one case associated with IgG4related sclerosing disease, and review of literature. Ann Diagn Pathol 13 (2009): 30-35.
- Michela L, Khin T. Clear Cell Sarcoma-like Tumor of the Gastrointestinal Tract: Clinical Outcome and Pathologic Features of a Molecularly Characterized Tertiary Center Case Series. Anticancer Res 38 (2018): 1479-1483.
- Nicolaus F, Maria AT, Luisa M. Clear cell sarcoma-like tumor with osteoclast-like giant cells in the small bowel: further evidence for a new tumor entity. Int J Surg Pathol 13 (2004): 313-318.
- Pamela LL, Carol MA. Gastrointestinal melanoma or clear cell sarcoma? Molecular evaluation of 7 cases previously diagnosed as malignant melanoma. Am J Surg Pathol 32 (2008): 858-866.
- Patrick S, Walid DS. Intra-abdominal clear-cell sarcoma: a report of 3 cases, including 1 case with unusual morphological features, and review of the literature. Int J Surg Pathol 20 (2012): 378-385.
- Przemysław W, Andrzej W. Malignant gastrointestinal neuroectodermal tumor (clear cell sarcoma-like tumor of the gastrointestinal tract) of the small intestine in a 12-year-old boy. Dev Period Med 22 (2018): 358-363.
- Achten R, M DR. An unusual case of clear cell sarcoma arising in the jejunum highlights the diagnostic value of molecular genetic techniques in establishing a correct diagnosis. Histopathology 46 (2005): 472-474.
- Raskin AG, Pozharisski KM, Iyevleva AG. Unusual Clinical Presentation of Gastrointestinal Clear Cell Sarcoma. Gastrointest Tumors 2 (2015): 83-88.
- Rotaru V, Chitoran E, Mitroiu MN. Intestinal Clear Cell Sarcoma-A Case Presentation of an Extremely Rare Tumor and Literature Review. Medicina (Kaunas) 60 (2024): 847.
- Shalaby A, Ramesh B, Deshpande P. Malignant Gastrointestinal Neuroectodermal Tumor of Small Intestine Showing DOG1 Expression: A Case Report and Review of Literature. Int J Surg Pathol 32 (2024): 374-379.
- So YP, Jung WS. Clear Cell Sarcoma-Like Tumor of the Gastrointestinal Tract with Peritoneal Metastasis in a Young Adult: A Case Report with Literature Review. J Korean Soc Radiol 84 (2023): 1169-1175.
- Su H, Wen-Sheng L, Wen-Hao R. Multiple clear-cell sarcomas of small intestine with parotid gland metastasis: A case report. World J Gastroenterol 23 (2017): 2258-2265.
- Suarez-Vilela D, Izquierdo Francisco M, Tojo-Ramallo S. Malignant gastrointestinal neuroectodermal tumor showing overlapped immunophenotype with synovial sarcoma: CD99 and SOX10 antibodies are useful in differential diagnosis. Am J Surg Pathol 36 (2012): 1905-1908.
- Surbhi K, Seema R. Malignant Gastrointestinal Neuroectodermal Tumor: a Unique Rare Neoplasm. Indian J Surg Oncol 8 (2017): 630-633.
- Takuhisa O, Yasumitsu H. A long-term survivor of clear cell sarcoma-like tumor of the gastrointestinal tract with liver metastasis: a case report. Surg Case Rep 6 (2020): 260.
- Taylor K, Eliane Cortez. A Case Series of Metastatic Malignant Gastrointestinal Neuroectodermal Tumors and Comprehensive Genomic Profiling Analysis of 20 Cases. Curr Oncol 29 (2022): 1279-1297.
- Yagi T, Nagata S, Yamamoto T. Malignant gastrointestinal neuroectodermal tumor with BRAF mutation and a history of malignant melanoma: A case report. Mol Clin Oncol 14 (2021): 23.
- Yang JC, Chou AJ, Oeffinger KC. Clear cell sarcoma of the gastrointestinal tract after very low-dose therapeutic radiation therapy: a case report. J Pediatr Surg 47 (2012): 1943-1945.
- Yegen G, Güllüoglu M, Mete Ö. Clear cell sarcoma-like tumor of the gastrointestinal tract: a case report and review of the literature. Int J Surg Pathol 23 (2015): 61-67.
- Zhao M, Zhao TW, Ma J. Clinicopathologic and molecular characteristics of malignant gastrointestinal neuroectodermal tumors. Zhonghua Bing Li Xue Za Zhi 46 (2017): 750-755.
- Zhihua Z, Dandan Z. Primary malignant neuroectodermal tumor of the ileum with predominantly uncommon pseudopapillary architecture. Int J Clin Exp Pathol 7 (2014): 8967-8971.
- Zhu P, Zhang T, Bi K. Primary Clear Cell Sarcoma of the Ileum: A Case Report With Next-Generation Sequencing Analysis. Int J Surg Pathol 29 (2021): 677-684.


 Impact Factor: * 4.2
Impact Factor: * 4.2 Acceptance Rate: 72.62%
Acceptance Rate: 72.62%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 