Prognostic Factors and Survival of Patients with Primitive Lung Cancer in Douala and Yaounde
Hugo Mbatchou1,2, Jean Paul Engbang1,3,*, Laurent-Mireille Mangamba Endale3,4, Liliane Amina Nga1, Emmanuel Nyankiye5, Bruno Tengang3, Etienne Okobalemba Atenguena6,7, Anne-Marthe Maison1,2, Esther Bell Dina1,2, Walter Eric Yone Perfura6,8, Charles Onana Mbele2, Marcelin Ngowe Ngowe1,6
1Faculty of Medicine and Pharmaceutical Sciences, the University of Douala, Douala-Cameroon
2Douala General Hospital, Douala-Cameroon
3Douala Laquintinie Hospital, Douala-Cameroun
4Faculty of Health Sciences, the University of Buea, Buea-Cameroon
5Akwa Pneumology Practice, Douala-Cameroon
6Faculty of Medicine and Biomedical Sciences, The University of Yaounde I, Yaoundé-Cameroon
7Yaounde General Hospital, Yaounde-Cameroon
8Yaounde Jamot Hospital, Yaounde-Cameroon
*Corresponding author: Jean Paul Engbang, Faculty of Medicine and Pharmaceutical Sciences, The University of Douala, Douala-Cameroun
Received: 08 June 2021; Accepted: 21 June 2021; Published: 26 July 2021
Article Information
Citation: Hugo Mbatchou, Jean Paul Engbang, Laurent-Mireille Mangamba Endale, Liliane Amina Nga, Marcelin Ngowe Ngowe. Prognostic Factors and Survival of Patients with Primitive Lung Cancer in Douala and Yaounde. Fortune Journal of Health Sciences 4 (2021): 412-424.
View / Download Pdf Share at FacebookAbstract
Background: Primary lung cancer (PLC) represents a real public health problem. It is one of the main types of cancer in the world in terms of incidence and also the main cause of death from cancer. In Africa primary bronchial cancer appears to be a rare entity.
Objective: To study the prognostic factors and survival of patients with PLC in Douala and Yaoundé. Patients and method: We performed an analytical, longitudinal (retrospective) study over a period from January 2010 to December 2019 including all records of patients in whom the diagnosis of PBC was histologically proven. The data collected were recorded and analyzed by SPSS version 25. Survival was determined by the Kaplan Meier method and the search for prognostic factors was carried out using the Cox proportional hazards model. A value less than 0.05 were considered significant.
Results: A total of 94 patients were included in the study. The sex ratio was 1.24. The median age at cancer diagnosis was 62 years old. More than half of the population (61 cases; 64.9%) resided in urban areas; 51 (54.3%) were exposed to tobacco, 14 (14.9%) had occupational exposure and 88.1% of women were exposed to biomass smoke. The average smoking index was 29.24 ± 26.27 pack-years and the average duration of exposure was 30.17 ± 4.36 years. The histological types encountered were in order of decreasing frequency: adenocarcinoma (48 cases; 51.1%), squamous cell carcinoma (33 cases; 35.1%), small cell lung cancer (8 cases; 8.5 %) and undifferentiated large cell carcinoma (5 cases 5.3%); in the majority of cases the cancer was diagnosed at stage IV for non-small cell lung cancer (62 out of 86 cases) and at the localized stage for small cell lung cancer (6 out of 8). Of the 94 cases collected, 56 (59.6%) had adhered to anti-tumor treatment. The median overall survival was 4 months with 95% CI [1.52-6.79]. The overa
Keywords
<p>Lung cancer - prognostic factors - survival - Yaoundé - Douala - Cameroon</p>
Article Details
1. Introduction
According to the World Health Organization (WHO) the general term "cancer" applies to a large group of diseases that can affect any part of the body; one of the hallmarks of cancer is the rapid proliferation of abnormal cells which, beyond their usual boundaries, can invade adjacent parts of the body and then swarm into other organs [1]. Primary lung cancer (PLC) is a neoplasm that develops at the expense of cells in the bronchial lining. The main risk factor is smoking [2–6]. Bonchopulmonary cancer (BPC) represents a real public health problem because it is one of the five main types of cancer in the world in terms of incidence with around 2.1 million new cases diagnosed in 2018 and also the main cause of death by cancer with 1.8 million deaths in the same year [7]. In Canada, PLC accounts for 26% of cancer deaths, with nearly 28,600 new cases diagnosed in 2017 [8]. The Canadian Cancer Society (CCS) estimates that 29,300 new cases of PBC were expected to be diagnosed in 2019, ranking it among the most diagnosed cancers in this Canada [9]. In Brazil, the National Cancer Institute estimated the incidence rate of BPC at 8.7% in men and 6.2% in women in 2018 [10]. In France, BPC is considered the fourth most diagnosed cancer with 49,109 new cases diagnosed in 2017 with a clear male predominance having an average age at diagnosis of 65 ± 10 years [11]. In India, the number of new cases in 2016 was 67,000, of which 72.7% were male, making it the second most common cancer affecting men [12]. In Africa, primary bronchial cancer appears to be a rare entity for two reasons: the small technical platform reducing diagnostic means and its clinical nature mimicking pulmonary tuberculosis [13,14]. Bray et al estimated the incidence rate of BPC in West Africa at 3.6 per 100,000 and in Central Africa at 6.1 per 100,000 in 2018 [15]. According to Gonzague et al, one of the specificities of cancer management in Africa is the delay in diagnosis, so 80% of patients are seen at an already advanced stage with a generally poor prognosis [16]. Souilah et al found in Algeria in 2012 a 1-year survival rate of 40% [17]; in the same Ihadadene et al in Algeria concerning locally advanced or metastatic BPC had found a survival rate at 1 year of 47.3%, at 2 years of 30.1% and at 3 years of 12.9% [18]. In Cameroon, Metchedjin et al. were interested in the problem of pleuropulmonary cancers in Douala and it emerged that the rare cases of pleuropulmonary cancers encountered posed many problems, the most important of which were diagnostic delay, lack of financial means, inadequate histological results and difficulty in offering treatment [19]; This is why we decided to do a study on the prognostic factors and survival of patients with BPC in Douala and Yaounde.
2. Patients and Methods
This was a retrospective analytical study, conducted from January 2009 to December 2018, in the pulmonology, oncology and radiotherapy departments of five medical institutions (Douala General Hospital, Akwa Pneumology Practice; Bonanjo Respiratory Disease Center, Yaounde General hospital and Yaounde Jamot Hospital,). Histological proven PLC patient records were included and followed during this period in these structures. Patients with other large lung conditions and histopathological confirmed non-malignant tumours were excluded. The various socio-demographic, clinical, paraclinical, therapeutic and evolutionary data were taken from the registers of those cited departments of these hospitals. Patients were contacted during their follow-up visits to the hospital and those who could not report for review in the hospital were contacted via telephone. Deaths of subjects were confirmed via contact with their families and relatives. These variables were recorded and processed using SPPS version 25 software. The different associations between the variables were studied using the χ2 test or Fisher's exact test. We plotted the survival curves and determined the probabilities of survival using the Kaplan-Meier method. The comparison of the different survival curves was made using the Log-Rank test (p <0.05). Variables that were statistically associated with the 5% cut-off were introduced into the Cox regression model for multivariate analysis, allowing us to identify prognostic factors associated with survival.
This work received an ethical clearance from the Ethics Committee of the University of Douala, who granted us ethical clearance No 2182 CEI-UDo/02/2020 / T, to conduct our study in strict compliance with the ethics
3. Results
3.1 General characteristics of the study population
A total of 94 patients were included in the study. The sex ratio was 1.24.
The median age at cancer diagnosis was 62 years old
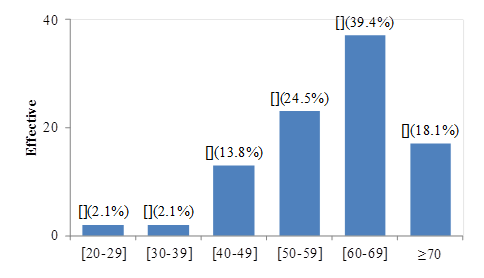
Figure 1: Distribution by age at diagnosis
More than half of the population (61 cases; 64.9%) resided in urban areas; 51 (54.3%) were exposed to tobacco, 14 (14.9%) had occupational exposure and 88.1% of women were exposed to biomass smoke. The average smoking index was 29.24 ± 26.27 pack-years and the average duration of exposure was 30.17 ± 4.36 years.
The histological types encountered were in order of decreasing frequency: adenocarcinoma (48 cases; 51.1%), squamous cell carcinoma (33 cases; 35.1%), small cell lung cancer (SCLC) (8 cases; 8.5 %) and undifferentiated large cell carcinoma (5 cases 5.3%); in the majority of cases the cancer was diagnosed at stage IV for non-small cell lung cancer (62 out of 86 cases) and at the localized stage for small cell lung cancer (6 out of 8). Of the 94 cases collected, 56 (59.6%) had adhered to anti-tumor treatment.
3.2 Survival
As shown in figure 2, The median overall survival was 4 months with a confidence interval (95% CI) of (1.52-6.79). The three-year overall survival rate was 8.7%. The 6, 12 and 24-month survival rates were 50%, 17.4% and 12% respectively.
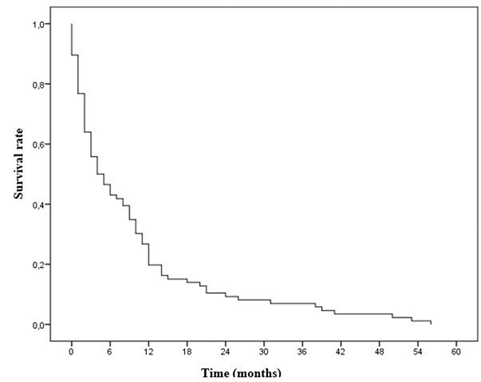
Figure 2: Overall survival curve of the study population
3.3 Pronostic factors
Univariate study: In univariate analysis, some factors were found to be significant. The prognostic factors were not found to be age greater than or equal to 60 years, the smoking index ≥ 20PA, the Performance Status (according to the WHO) between stages 2 and 4 and the sites of pulmonary and cerebral metastasis (Table 1).
Table1: Univariate analysis of prognostic factors
|
Vital status at 6 months |
HR (IC95%) |
p-value |
|||||
|
Deaths |
Alive |
||||||
|
Variable |
n |
% |
n |
% |
|||
|
Age |
[20-40] |
1 |
1,8% |
3 |
8,1% |
Réf |
|
|
[40-60] |
16 |
28,1% |
20 |
54,1% |
2,61(0,79-8,63) |
0,114 |
|
|
≥60 |
40 |
70,2% |
14 |
37,8% |
3,58(1,09-11,71) |
0,035* |
|
|
Gender |
Female |
24 |
42,1% |
18 |
48,6% |
Réf |
|
|
Male |
33 |
57,9% |
19 |
51,4% |
1,11(0,73-1,67) |
0,611 |
|
|
Presence of medical history |
No |
36 |
67,9% |
20 |
54,1% |
Réf |
|
|
Yes |
17 |
32,1% |
17 |
45,9% |
0,66(0,43-1,01) |
0,061 |
|
|
Tobacco exposure |
No-smoker |
25 |
43,9% |
18 |
48,6% |
Réf |
|
|
Active smoker |
16 |
28,1% |
6 |
16,2% |
1,61(0,95-2,73) |
0,074 |
|
|
Passive smoker |
5 |
8,8% |
4 |
10,8% |
1,22(0,59-2,51) |
0,592 |
|
|
Ex-smoker |
11 |
19,3% |
9 |
24,3% |
1,06(0,62-1,82) |
0,814 |
|
|
Somking index |
<20 PA |
5 |
26,3% |
6 |
46,2% |
Réf |
|
|
≥20 PA |
14 |
73,7% |
7 |
53,8% |
2,54(1,08-5,95) |
0,031* |
|
|
Consultation delay (months) |
<6 |
35 |
64,8% |
18 |
60,0% |
Réf |
|
|
≥6 |
19 |
35,2% |
12 |
40,0% |
1,04(0,66-1,64) |
0,847 |
|
|
Diagnostic delay (months) |
<30 |
26 |
57,8% |
18 |
66,7% |
Réf |
|
|
≥30 |
19 |
42,2% |
9 |
33,3% |
1,01(0,62-1,64) |
0,947 |
|
|
Discovery mode |
Asymptomatic |
0 |
0,0% |
1 |
2,7% |
Réf |
|
|
Symptomatic |
57 |
100,0% |
36 |
97,3% |
- |
0,999 |
|
|
BMI |
<18,5 |
6 |
20,7% |
2 |
10,0% |
Réf |
|
|
≥18,5 |
23 |
79,3% |
18 |
90,0% |
0,64(0,29-1,40) |
0,271 |
|
|
Performance Status (according to WHO) |
[0-1] |
11 |
20,4% |
17 |
53,1% |
Réf |
|
|
[2-4] |
43 |
79,6% |
15 |
46,9% |
1,67(1,05-2,66) |
0,028* |
|
|
Histological type |
Adenocarcinoma |
28 |
49,1% |
20 |
54,1% |
Réf |
|
|
Squamous cell carcinoma |
21 |
36,8% |
12 |
32,4% |
0,99(0,63-1,55) |
0,985 |
|
|
Undifferebtiateed large cell carcinoma |
4 |
7,0% |
1 |
2,7% |
1,99(0,78-5,08) |
0,149 |
|
|
CBPC |
4 |
7,0% |
4 |
10,8% |
0,70(0,33-1,49) |
0,358 |
|
|
Degree of differentiation |
Grade 1 |
3 |
11,5% |
3 |
23,1% |
Réf |
|
|
Grade 2 |
8 |
30,8% |
1 |
7,7% |
1,93(0,67-5,55) |
0,217 |
|
|
Grade 3 |
8 |
30,8% |
6 |
46,2% |
1,17(0,45-3,08) |
0,739 |
|
|
Grade 4 |
7 |
26,9% |
3 |
23,1% |
1,20(0,42-3,44) |
0,722 |
|
|
Metastasis sites |
None |
9 |
16,4% |
15 |
42,9% |
Réf |
|
|
Pleura |
16 |
29,1% |
10 |
28,6% |
1,55(0,88-2,75) |
0,126 |
|
|
Bone |
10 |
18,2% |
5 |
14,3% |
1,62(0,83-3,13) |
0,152 |
|
|
Lung |
6 |
10,9% |
1 |
2,9% |
2,88(1,21-6,85) |
0,016* |
|
|
Liver |
7 |
12,7% |
4 |
11,4% |
1,16(0,56-2,41) |
0,679 |
|
|
Brain |
4 |
7,3% |
0 |
0,0% |
4,74(1,58-14,23) |
0,005* |
|
|
Adrenals |
3 |
5,5% |
0 |
0,0% |
2,66(0,78-9,07) |
0,117 |
|
|
Stage of cancer |
Stage II |
0 |
0,0% |
1 |
3,4% |
Réf |
|
|
Stage III |
9 |
17,3% |
9 |
31,0% |
1,08(0,14-8,18) |
0,938 |
|
|
Stage IV |
43 |
82,7% |
19 |
65,5% |
1,45(0,20-10,59) |
0,709 |
|
|
Tumor treatment adherence |
No |
25 |
43,9% |
12 |
32,4% |
Réf |
|
|
Yes |
32 |
56,1% |
25 |
67,6% |
0,67(0,44-1,02) |
0,065 |
|
|
Treatment delay |
<10 |
7 |
23,3% |
7 |
31,8% |
Réf |
|
Multivariate study: All variables that had a significant p-value in the uni-variate analysis were introduced into the cox model for multivariate analysis; which allowed us to estimate the Hazard Ratio of each modality of each of the variables. From this resulted the table above and allowed us to conclude that the poor prognostic factor is the presence of brain metastases (Table 2).
Table 2: Multivariate analysis of prognostic factors
|
Factors |
HR (IC95%) |
p-value |
|
|
Age |
[20-40] |
Réf |
|
|
≥60 |
0,62(0,11-3,45) |
0,593 |
|
|
Tobacco index |
<20 PA |
Réf |
|
|
≥20 PA |
2,92(0,92-9,24) |
0,068 |
|
|
Metastasis sites |
Aucun |
Réf |
|
|
Poumons |
1,56(0,46-5,24) |
0,472 |
|
|
Cerveau |
80,01(4,14-1544) |
0,004* |
|
|
Performance Status (WHO) |
[0-1] |
Réf |
|
|
[2-4] |
0,95(0,29-3,18) |
0,953 |
4. Discussion
The male sex was more represented with 55.3% of cases than the female sex with 44.7% of cases, i.e. a sex ratio of 1.24. This was close to the results obtained by Metchedjin et al who found in a study carried out in Douala-Cameroon in 2007 a sex ratio of 1.1 [19]. These results are lower than the majority of studies in which the difference is a little more important, for example Diallo et al in Mali in 2005 and Mejri et al in Tunisia in 2015, respectively found a sex ratio of 3 and 2.7 [20 , 21]. This could be explained by the increase over the years in smoking among women who seem to be more sensitive to its carcinological action [22, 23]. The mean age of the patients was 60.16 years. This value approached that found in Senegal by Niang et al in 2007, Diallo et al in 2005 who had obtained respective mean ages of 59.2 years and 54.58 years [6, 20]. However, this average is lower than that found by Cadelis et al in Guadeloupe-France in 2009, which was 65 years [24]. This could be explained by the fact that Guadeloupe is a region with low tobacco consumption [24]. Regarding age groups, in our series, BPC was rare before the age of 40 (4.2%) and began to have a considerable increase from the age of 40 to reach its peak between 60 and 69 years; these results corroborated with data from the World Cancer Report [25]. Most of our study population resided in urban areas. This would emanate from the fact that the population in urban areas is more apt to smoke than that in rural areas, these facts have been observed by the Global Adult Tobacco Survey which found in 2013 in Cameroon that the seniority of tobacco among individuals in urban areas is much higher than that of individuals in rural areas [26]. This could also be explained by the fact that air pollution is more in urban areas [27]. We could also mention the fact that populations in rural areas do not always have easy access to specialized health structures in urban areas either because of the distance or because of the low socio-economic level which does not allow them to have access to health facilities (access to specialized health structures).
In our series, 42 patients or 44.7% were smokers. This frequency was lower than those found by Ihadadene et al in Algeria in 2015 and Niang et al in Senegal in 2007, which were 83% and 87.5% respectively [6,18]. This difference could be explained by the fact that the female sex, which represents the largest proportion of non-smokers, was more representative in our sample (42 women against 52 men) compared to the other studies (Ihadadene et al: 34 women against 290 men; Niang et al: 8 women against 64 men) [6,18]. The average smoking index was 29.24 pack-years. The average exposure time was 30.17 years. This result is superimposable on that of Cadelis et al in Guadeloupe-France in 2009 who found an average exposure period of 30 years [24]. The prevalence of smoking in Cameroon in 2014, which was 17.5%, seems to be close to that found in Guadeloupe in 2014, which was 11.9% [22, 28]. Exposure to biomass concerned 88.1% of women, indeed this result seems to corroborate the observations made by Koning et al who had found that fuels derived from biomass are the main and often the only source of household energy for cooking and heating in almost half of the world's population; exposure to large quantities of these fumes presents a health risk of the same order as exposure to tobacco smoke and of which the most affected are women who cook in rural areas in developing countries [29]. The average consultation time was 5.86 months. More than half of the patients (63.1%; 53 cases) had consulted within less than six months. This consultation period is close to that found by Alaoui et al in Morocco in 2013 which was 4.6 months but longer than that of Cadelis et al in Guadeloupe-France in 2009 which was 24 days [24, 30]. This long consultation period under our skies would be justified by the trivialization of warning signs, the low socio-economic level and the lack of accessibility to specialized health structures by poor populations. We found that in the vast majority of patients, the diagnosis was made when they already had Performance Status at stage 1 (23.4%) and stage 2 (36.2%); This result is similar to that found by Niang et al, in which stages 1 and 2 were predominant and could be explained by the fact that the majority of the population only consults when symptoms cause discomfort in their daily activities.
The histologic types encountered were adenocarcinoma (51.1%), squamous cell carcinoma (35.1%), SCLC (8.5%) and carcinoma large cell undifferentiated (5.3%); this same order was found in the study by Mejri et al in Tunisia in 2014 where adenocarcinoma predominated with 41%, followed by squamous cell carcinoma with 17% and finally SCLC with 15% [21]. But Diallo et al in 2005 had instead found that squamous cell carcinoma was the most common in their population with 42.9% of cases followed by adenocarcinoma [20]. This difference would result from the fact of the current modification of the composition of tobacco; in fact, currently smoked tobacco is actually light blond tobacco with a lower yield of nicotine but a high yield of nitrosamine, a carcinogen which experimentally causes glandular tumors [31]. This could also be explained by the fact that women being more likely to develop adenocarcinomas were represented with a considerable number [32]. The diagnosis was more made at stages III (19.1%) and IV (66%); this result was close to that of Kallel et al in whom stages III and IV were the most represented with respectively 26% and 68.2% of cases [33]. These results come from the fact that most patients consult only when the symptoms cause discomfort in their daily activities and therefore at an already advanced stage of the disease.
The mean treatment time was 53.19 days. This long therapeutic period is explained by the fact that on the one hand the term cancer in our context is still synonymous with witchcraft, so patients generally go first to traditional practitioners after the announcement of the diagnosis and on the other hand, the low socio-economic level does not make healthcare accessible to all. In our series, 59.6% of patients had adhered to anti-tumor treatment and 40.4% to symptomatic treatment; 51 patients (91.1%) had received chemotherapy alone, the chemotherapy-radiotherapy combination was used in 7.1% of cases (4 patients) and 1 patient (1.8%) underwent surgical resection associated with radiotherapy. These results did not agree with all the stages of the disease obtained in our results, this would demonstrate the difficulty that specialists have in starting an adequate treatment after the diagnosis in our country due to the lack of infrastructure (radiotherapy was not available only in one of our five study sites) and at the low socio-economic level of the population which makes anti-tumor therapies inaccessible to all
The median overall survival was 4 months. The three-year overall survival rate was 8.7%. The six, twelve and twenty-four month survival rates were 50%, 17.4% and 12%respectively. The six-month and twelve-month survival rates were comparable to the results of Kwas et al in Tunisia in 2017, which were respectively between 50 and 74% and between 9 and 25% [34]. The overall survival rate at thirty-six months was lower than that found by Ihadadene in Algeria in 2015 which was 12.9% as well as the survival rate at twelve and twenty-four months which were respectively 47.3% and 30.1% [18]. The median overall survival was lower than the results of Grivaux et al in France in 2009 and those of Souilah et al in Algeria in 2009, which were respectively 7 months and 9.5 months [17, 35]. These differences between the survival rates and medians could be explained by the size of our sample, which was smaller than the other studies (324 cases for Ihadadene et al in Algeria, 5,447 cases for Grivaux et al in France and 497 cases for Souilah et al in Algeria [17,18,35]). In the search for factors associated with the vital prognosis of our patients, in univariate analysis the factors that influenced the mortality at 6 months were: age greater than or equal to 60 years, the smoking index ≥ 20PA, the Performance Status (according to the WHO) between stages 2 and 4, the presence of pulmonary and cerebral metastases. This result was similar to that of Grivaux et al who had found factors identical to ours with in addition the socio-professional category, the site of the sample, the mode of diagnosis, the histological nature of the BPC (CBPC versus NSCLC) [35]. This surplus could be explained by the size of the sample, which was significantly larger than that of our study (5447 patients versus 94 patients). After introduction into the Cox regression model and calculation of the adjusted Hazard Ratio (HR) of deaths, we retained a poor prognostic factor which was: the presence of cerebral metastases. We have found in several studies that the sex of patients is classically described as having an impact on the vital prognosis, but this did not appear as a discriminating factor in ours, whether in univariate or multivariate analyzes. Several meta-analyzes consider the Performance Status (which we found in our univariate analysis) as having a powerful independent prognostic value which is extremely reproducible [36, 37].
5. Limitations of the study
Due to the retrospective collection we are aware of an insufficiency of data provided by medical files, in particular with regard to the smoking index and the duration of exposure to tobacco in smoking patients, the body mass indices, the balance sheets, extension and follow-up of patients. -The immunohistochemical data were almost absent and there was a lack of precision on the anatomopathological results. All these are because of the lack of equipment necessary to carry out these investigations in our pathology laboratories.
6. Conclusion
Bronchopulmonary cancer remains an important problem in public health, in particular because of characteristic elements such as the sex ratio which is 1.24 in favor of men, the average age at the time of diagnosis of 60.16 years, the average consultation time of 5.86 months, the average smoking index of 29.24 PA with an average duration of exposure of 30.17 years and 88.1% of women exposed to biomass smoke. Very low survival, with a median overall survival of 4 months and overall survival rates at six, twelve, twenty-four and thirty-six months respectively of 50%; 17.4%; 12% and 8.7%. The poor prognostic factor identified here is the presence of brain metastases.
7. References
- Organisation mondiale de la santé, Bureau régional de l’Europe. Programmes nationaux de lutte contre le cancer: politiques et principes gestionnaires: résumé d’orientation. Genève: Organisation mondiale de la santé; 2002.
- Benjamin P. KB Pneumologie. 2ème édition. Paris: Vernazobres-Grego; (2011):444.
- Rivera C, Rivera S, Fabre E, Pricopi C, Pimpec-Barthes F, Riquet M et al. Le tabac et ses conséquences dans le cadre du traitement du cancer bronchique. Rev Pneumol Clin 72 (2016):136-41.
- Bigay-Gamé L. Les cancers du poumon de moins de 40 ans. Rev Mal Respir Actual 9 (2017):84-8.
- Delva F, Brochard P, Pairon J. Les facteurs de risque professionnels des cancers bronchopulmonaires Quelle surveillance médicale après exposition à des cancérogènes pulmonaires professionnels. Rev Mal Respir Actual 9 (2017):94-9.
- Niang A, Bonnichon A, Ba-Fall K, Dussart C, Camara P, Vaylet F et al. Le cancer bronchique au sénégal. Med Trop 67 (2007):651-656.
- Centre international de recherche sur le cancer. Dernières données mondiales sur le cancer: le fardeau du cancer atteint 18,1 millions de nouveaux cas et 9,6 millions de décès par cancer en 2018. Genève; (2018):3.
- Melosky B, Cheema P, Agulnik J, Albadine R, Xu Z, Liu G et al. Canadian perspectives: update on inhibition of ALK-positive tumours in advanced non-small-cell lung cancer. Curr Oncol 25 (2018).
- Canadian Cancer Society. Canadian Cancer Statistics 2019.
- Santos M. Estimativa 2018: Incidência de Câncer no Brasil. Rev Bras Cancerol 64 (2018):119-20.
- Knoepfli A, Vaillant P, Billon Y, Zysman M, Menard O, Tiotiu A et al. Influence de l’âge des patients sur le délai de prise en charge du cancer bronchique. Bull Cancer 106 (2019):421-30.
- Dhillon P, Mathur P, Nandakumar A, Fitzmaurice C, Kataki A, Sathishkumar K et al. The burden of cancers and their variations across the states of India: the Global Burden of Disease Study 1990–2016. Lancet Oncol 19 (2018):1289-306.
- Bayo S, Parkin D, Koumaré A, Diallo A, Ba T, Soumaré S et al. Cancer in Mali, 1987–1988. Int J Cancer. 45 (1999):679-84.
- Refeno V, Nomeharisoa R, Tiana N, Nasandratriniavo A, Louis H, Rafaramino F et al. Aspects cliniques des cancers bronchopulmonaires primitifs au service d’oncologie du CHUA-HUJRA Antananarivo. Pan Afr Med J22 (2015).
- Bray F, Ferlay J, Soerjomataram I, Siegel R, Torre L, Jemal A et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin68 (2018):394-424
- Gutiérre D. Palliatifs sans frontieres cameroun. 29.
- Souilah S, Elaalia F, Khenouf K, Yahiaoui R, Taright S, Amrane R et al. Étude de survie du cancer bronchique(2012):1.
- Ihadadene D, Jaafar M, Ketfi A, Gharnaout M. Devenir des patients atteints de cancer bronchique (KC-B) localement avancé ou métastatique. Rev Mal Respir 33 (2016):90.
- Metchedjin A, Episseyo J, Tchetcheu C, Nyankiye E. Problématique des cancers pleuropulmonaires à Douala, Cameroun. Elsevier Masson SAS (2007):1.
- Diallo S, Kaptue Y, Sissoko F, M’baye O, Gomez P. Problématique du cancer bronchique dans le service de pneumologie du Point G Bamako Mali. [Thèse de médecine]. Bamako: Université de Bamako; N°422: (2005):111.
- Mejri I, Ben Saad S, Daghfous H, Ben Khelifa M, Tritar F. Le cancer pulmonaire primitif en Tunisie: du diagnostic au traitement. Rev Mal Respir 32 (2015):131.
- Awono P. Rapport sur la mise en œuvre de la convention cadre de l’OMS pour la lutte antitabac (2014): 26.
- Bunel V, Mazières J. Le cancer bronchique féminin. Rev Mal Respir Actual 6 (2014):92-5.
- Cadelis G, Kaddah S, Bhakkan B, Quellery M, Deloumeaux J. Epidémiologie et incidence du cancer bronchique primitif dans une région à faible consommation tabagique: la Guadeloupe. Données 2008–2009 du registre des cancers. Rev Mal Respir 30 (2013):537-48.
- Stewart B, Wild C. World cancer report. Lyon (2014): 632.
- Institut Nationale de la Statistique. Enquête mondiale sur le tabagisme chez les adultes, rapport princopal. Yaoundé (2013):154.
- Pelassy P. Etude comparative de la pollution atmospherique particulaire entre un site urbain et un site rural de la region de Yaounde (Cameroun). Atmospheric Environ 13 (1979):1329-36.
- Raphaël A, Jean-Baptiste R, Nguyen-Thanh V. Baromètre santé DOM 2014, Tabagisme et usage d’e-cigarette. 14 (2014).
- Koning H, Smith K, Last J. Combustion de biomasse et sante. Bulletin de l'OMS 63(1985):215-232.
- Alaoui-Yazidi A, Amro L, Sajiai H. Profil épidémiologique, clinique, anatomopathologique et thérapeutique du cancer bronchique au Maroc (expérience Marrakech). Afr J Cancer 5 (2013):88-93.
- Trédaniel J. Évolution épidémiologique du cancer du poumon en France et dans le monde. Rev Mal Respir Actual 10 (2018):182-5.
- Assma B, Nicolas M. Le cancer du poumon chez la femme est-il différent? Rev Med 8 (2012):1108-11.
- Kallel N, Yangui, Msaad S, Feki W, Ayedi H, et al. Facteurs pronostiques des cancers bronchiques primitifs. Rev Mal Respir 34 (2017): 80-81.
- Kwas H, Guermazi E, Khattab A, Hrizi C, Zendah I, Ghédira H et al. Facteurs pronostiques du cancer bronchique non à petites cellules au stade avancé. Rev Pneumol Clin 73 (2017):180-7.
- Grivaux M, Zureik M, Marsal L, Asselain B, Peureux M, Chavaillon J et al. Survie à cinq ans des cancers bronchiques primitifs dans les centres hospitaliers généraux. Rev Mal Respir 26 (2009):37-44.
- Gester F, Paulus A, Sibille A, Corhay J, Duysinx B, Louis R. Facteurs pronostiques du cancer pulmonaire non à petites cellules. Rev Med Liège 71 (2016):34-39.
- Paesmans M. Facteurs pronostiques du cancer bronchique. Rev Mal Respir 22 (2005):76-80.


 Impact Factor: * 6.2
Impact Factor: * 6.2 Acceptance Rate: 76.33%
Acceptance Rate: 76.33%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 