Redefining Adequate Surgical Resection Margins for Oral Squamous Cell Carcinoma: Our Institutional Experience in 5 Consecutive Years
Irene Urquiza-Fornovi*, Mario Santás-Alegret, Ana Ramos-Zayas, Irene Ruiz-Martín, María Mejía-Nieto, Ramón Gutiérrez-Díaz, Gregorio Sánchez-Aniceto
Department of Oral and Maxillofacial Surgery, “12 de Octubre” University Hospital, Institute for Biomedical Research (i+12), Universidad Complutense, Madrid 28041, Spain.
*Corresponding author: Irene Urquiza-Fornovi, Department of Oral and Maxillofacial Surgery, "12 de Octubre" University Hospital, Institute for Biomedical Research (i+12), Universidad Complutense, Madrid 28041, Spain
Received: 18 July 2021; Accepted: 26 July 2021; Published: 24 August 2021
Article Information
Citation: Irene Urquiza-Fornovi, Mario Santás-Alegret, Ana Ramos-Zayas, Irene Ruiz-MartÃn, MarÃa MejÃa-Nieto, Ramón Gutiérrez-DÃaz, Gregorio Sánchez-Aniceto. Redefining Adequate Surgical Resection Margins for Oral Squamous Cell Carcinoma: Our Institutional Experience in 5 Consecutive Years. Journal of Surgery and Research 4 (2021): 422-440.
View / Download Pdf Share at FacebookAbstract
Background: Resection margin status is an important predictor of prognosis in patients with surgically treated OSCC. We introduced the concept of “adequate” versus "inadequate” resection margins.
Methods: A sample of 87 consecutive patients who underwent surgical treatment for OSCC between 2014 and 2019 were retrospectively examined. Patient demographics, tumour characteristics, adjuvant therapy, recurrence status and patient survival (overall -OS- and disease-free -DFS) were evaluated. According to pathological findings, margins were considered clear (≥5 mm), close (1-5 mm) or involved (<1 mm). Using statistical analysis, a binomial cut-off point was established at 3 mm, and patients were classified into two groups according to primary tumour resection margins: “adequate” (margins ≥3 mm) and “inadequate” (margins <3 mm).
Results: Clear surgical margins (≥5 mm) were reported in 72% tumour specimens, close in 12% and involved in 16%. Applying the 3 mm cutt-off, 21% patients were considered to have “inadequate” and 79% “adequate” resections. Adjuvant therapy was provided in 60% of cases, in accordance with Clinical Practice Guidelines. OS rate was 63% and DFS rate 64%. OS was significantly lower (p <0.05, HR 2.24) in the “inadequate” resection group (44%) versus the “adequate” group (75%). Tumour recurrence was observed in 25% in the “adequate” resection group versus 44% in the “inadequate” group (p >0.05).
Conclusions: An adequate surgical margin for OSCC could be defined at our institution by ≥3 mm, a close margin (≤2.99 mm) being an adverse risk factor in OSCC survival although further studies are needed to analyse the impact in terms of cancer recurrence rates.
Keywords
<p>Oral squamous cell carcinoma, Surgical margin status, Close margins, Positive margins, Survival analysis, Radiotherapy</p>
Article Details
1. Introduction
OSCC is the sixth most common malignancy worldwide and it has increased in recent years in Western countries [1,2]. Almost half of the patients develop early disease (stages I or II). Advanced stage disease (stages III or IV) endangers local recurrence (LR) and distant metastasis, especially in the first two years after diagnosis [2]. Surgical treatment with or without adjuvant therapies remains the main option for oral cancer. The primary goal of surgery for OSCC is the complete excision of the primary tumour with no residual cancer cells left behind. Lymph node dissection is performed based on the presence or occult regional metastases [3,4]. The importance of obtaining tumour-free margins when treating OSCC has been known for decades. Achieving clear margins during surgical resection is thought to reduce LR and improve prognosis [4]. Resection margin status is one of the most important predictors of prognosis in oncologic patients and it represents the only factor potentially controllable by the surgeon [1,2,5,6]. A microscopically positive surgical margin is associated with a higher risk for LR and a poor clinical outcome. Specifically, pathological margins are more significantly predictive of LR than clinical margins [7]. Margin status is decisive in determining which patients receive postoperative adjuvant therapy or re-resection; also considering other poor prognostic factors such as advanced T and N stages, extracapsular nodal spread (ECS), depth of invasion (DOI) and lymphovascular and perineural invasion (LVI, PNI) [2,5]. An inadequate surgical margin, including the clear but close margin, is generally considered an indication for adjunctive radiotherapy (RT) [6]. However, there is controversy regarding the adjuvant treatment for patients with close surgical margins who are at intermediate risk. Definition of clear, close or involved margins and their association with LR, DFS, and OS remains as a controversial debate in the literature, and it is often a subject of discussion. National Comprehensive Cancer Network [8] guidelines define a clear margin as an invasive tumour that is at least 5 millimetres (mm) from the resected margin. A close margin is defined as an invasive tumour that is located between 1 and 4.9 mm from the resected margin. An invasive tumour less than 1 mm from the margin of resection constitutes a positive margin. National Comprehensive Cancer Network [8] guidelines define a clear margin as an invasive tumour that is at least 5 millimetres (mm) from resection. A close margin is considered when distance from the specimen is between 1 and 4.9 mm. Positive margins are described as being less than 1 mm from the resected carcinoma. However, it is unclear what precise cut-off point at a close margin determines the risk of LR compared to a positive microscopic margin. Using a unique definition of close margin for every subsite of head and neck is probably inappropriate, since every district has different characteristics in terms of lymphatic drainage, vascularization, and presence of biologic barriers. This seems to be related to the complexity and three-dimension particularities of the maxillofacial anatomic region. A 5 mm distance is not always easy to achieve when the tumour invades noble structures. Therefore, several authors [3,6] have wondered whether a smaller margin leads to worse recurrence and/or survival rates. Some authors [9-11] have suggested that 3 mm of surrounding healthy tissue is sufficient to be considered a free margin in OSCC, since tumours with margins < 3mm had a similar impact on the incidence of local recurrence as involved margins. The purpose of this study is to analyse the impact of margin status on recurrence (local, regional, locoregional and distant) and OS, using the concept of “adequate” resection margins. Secondly, the possible risk factors that define poor outcomes in OSCC are identified. In this research statistical analysis of different cut-off points resulted significant for a margin cutpoint of 3 mm in the sample studied. So we decided to establish this measure for our subsequent binomial classification of margin status.
2. Materials and Methods
A retrospective series of 87 consecutive patients who underwent primary surgery for newly diagnosed OSCC was examined. The study identified cases between January 2014 and December 2018 from a single institution in Madrid, Spain. Patients fulfilling the following criteria were included: (1) primary SCC of the oral cavity in any stage (I-IV); (2) treated by primary surgery, including neck sentinel node biopsy or elective/radical neck dissection +/- adjuvant therapy; (3) detailed pathological examination of the resection and neck specimens with deep and mucosal margin size recorded in mm; and (4) at least 1.5 years follow-up. The exclusion criteria were: (1) previously treated OSCC and (2) synchronous pri-mary tumours. In the time period previously indicated, 121 patients with OSCC were operated in our Maxillofacial Department. A total of 34 subjects were excluded from our sample because they did not meet all the inclusion criteria. From our 87 sample, DOI was only documented in 78 cases. Adjuvant therapy was prescribed postoperatively depending on the presence of pathological features following EHNS-ESMO-ESTRO Clinical Practice Guidelines [12]. All treatment-related decisions were made after discussion in our Multidisciplinary Head and Neck Cancer Committee taking place weekly. We analysed patient demographics (gender, age), tumour characteristics (primary site, T stage, tumour grade, DOI, nodal status (N0/N+), ECS, LVI, PNI, resection margin status in mm), adjuvant therapy and the incidence of recurrence. Recurrences were classified in different subtypes according to AJCC Cancer Staging Manual, 8th edition [8]: local (relapse disease at the primary tumour site), regional (cervical recurrence), locoregional (recurrence disease in both previous sites at the time of diagnosis) and distant metastasis. DFS was considered as the time between date after primary treatment (surgery +/- adjuvant therapy) and the date of clinico-radiological confirmation of disease relapse. This information was obtained from electronic medical records registered in Health Care Information System (HCIS, DXC.technology®, Madrid Healthcare System). Pathological stage and site were recorded as defined by the Union for International Cancer Control/American Joint Committee on Cancer TNM classification. Death certificate information was tracked through HCIS, HORUS (Horus Hardware®, Community of Madrid) and some cases by direct telephone call with relatives of the patient studied. Deceases were divided in two groups: death of disease (DOD) and non-DOD. According to pathological findings, margins were considered clear (≥5 mm), close (1-5 mm) or involved (<1 mm). For the purpose of our study, two further distinctive groups of patients were then defined according to their primary tumour resection status: “inadequate” resections (margins <3 mm) and “adequate” (≥3 mm). All surgical specimens were obtained from the tumour bed. They were refrigerated to -20º C temperature and posteriorly cut frozen with a microtome on paraffin sections. Intraoperative examination of the frozen sections (FS) was conducted in order to assess margin proximity. Previous authors had also described this “patient-based approach” technique [13,14]. Descriptive statistics were calculated for the variables of interest overall by median, interquartile range (IQR), absolute and relative frequency, according to the nature of the variables. Next, Kaplan-Meier survival curves were created followed by the log-rank test to compare differences in the survival time distribution across the following factors. Cox proportional hazard regression modelling was conducted to examine the strength of association between the covariates and survival time. The proportionality of the covariates was then tested by adding an interaction term between time and the variable of interest. If the interaction term resulted in a p-value less than 0.05, the interaction term was kept in the model to incorporate the non-proportionality. In the final model only variables significant at a p <0.10 were eligible for inclusion. The results were represented by Hazard Ratio (HR) and 95% confidence interval (CI). All analyses were conducted using Stata/SE 16 (StataCorp, College Station, TX).
3. Results
3.1 Patient and tumour characteristics
Patient characteristics are summarized in Table 1. A total of 87 subjects met inclusion criteria. The gender distribution was 49 men (56%) and 38 women (44%). The median age at diagnosis of OSCC was 67 years (range 34-92). Divided into three age groups: seven (8%) were younger than 50 years, 33 (38%) were between 50 and 65 years and 47 (54%) were older than 65 years. Following the American Society of Anaesthesiologists Classification (ASA) [12], the most common group was ASA II with 45 patients (52%). Median follow-up among all cases was three years and 54 days (minimum one year and six months; maximum six years and two months). The primary tumour was located on the tongue in 34 (39%) subjects, on the floor of the mouth in 18 (21%), on the alveolar ridge in 17 (20%), on the retromolar trigone in nine (10%), on the buccal mucosa in eight (9%) and on the hard palate in one (1%) case. Following the TNM classification of the Eighth Edition of the American Joint Committee on Cancer [8], advanced stages (III, IV) were more frequent (59, 68%) than early stages (I, II) (28, 32%). Stage pT4 was the most common (35; 40%). Locally advanced stages pT3/4 (51; 59%) were more prevalent than pT1/T2 (36; 41%). Fifty-one patients were pN0 (59%) and 36 (41%) were pN+.
|
Characteristics (N = 87) |
|
|
Sex (male/female) |
49 (56%) /38 (44%) |
|
Age, years, median (range) |
67 (34 – 92) |
|
ASA |
|
|
I |
3 (3%) |
|
II |
45 (52%) |
|
III |
35 (40%) |
|
IV |
4 (4%) |
|
Primary tumour site |
|
|
Tongue |
34 (39%) |
|
Floor of mouth |
18 (21%) |
|
Alveolar ridge |
17 (20%) |
|
Buccal mucosa |
8 (9%) |
|
Hard palate |
1 (1%) |
|
T-classification |
|
|
pT1 |
22 (25%) |
|
pT2 |
13 (15%) |
|
pT3 |
17 (18%) |
|
pT4 |
35 (40%) |
|
N-classification |
|
|
pN0 |
51 (59%) |
|
pN1 |
13 (15%) |
|
pN2 |
15 (17%) |
|
pN3 |
8 (9%) |
|
Clinical stage |
|
|
Early I / II |
28 (32%) |
|
Advanced III / IV |
59 (68%) |
|
Other pathological findings |
|
|
ECS |
13 (15%) |
|
PNI |
11 (13%) |
|
LVI |
4 (5%) |
|
DOI |
|
|
≤5mm |
33 (38%) |
|
>5mm |
45 (52%) |
|
Not documented |
9 (10%) |
|
Adjuvant therapy |
|
|
Yes |
57 (66%) |
|
No |
30 (34%) |
|
Follow-up period, months, median (range) |
38 (18 – 74) |
ASA, American Society of Anesthesiologists; pT, pathological T stage; pN, pathological nodal status; ECS, extracapsular nodal spread; PNI, perineural invasion; LVI, lymphovascular invasion; DOI, depth of invasion; mm, millimetres.
Table 1: Clinical and pathological characteristics of the patients.
As for DOI (documented in 78 cases), in 33 patients (38%) it was ≤5 mm and in 45 (52%) it was >5 mm. In the group of subjects with DOI ≤5 mm, 82% (27) were early T1/T2 stages while 84% (38) with DOI >5 mm corresponded to T3/T4 stages. In terms of nodal status, 73% (24) of cases with DOI ≤5 mm were N0. Conversely, 49% (22) with DOI >5 mm were N+. A total of 22 (67%) patients with DOI ≤5 mm were diagnosed with early stage (I/II) while 40 (89%) with DOI >5 mm had advanced stage disease (III/IV).
The distribution of patients according to DOI and the relationship between DOI, recurrence and survival is summarized in Table 2.
|
Recurrence |
Survival |
|||||||
|
Non-recurrence |
Local |
Regional |
Locoregional |
Distant |
Alive |
DOD |
Non-DOD |
|
|
DOI ≤5 mm |
27 (82%) |
4 (12%) |
1 (3%) |
1 (3%) |
- |
28 (85%) |
3 (9%) |
2 (6%) |
|
(N=33) |
||||||||
|
DOI >5 mm |
26 (58%) |
5 (11%) |
2 (4%) |
11 (24%) |
1 (2%) |
27 (60%) |
16 (36%) |
2 (4%) |
|
(N=45) |
||||||||
DOI, depth of invasion; DOD, death of disease; mm, millimeters.
Table 2: Distribution of patients according to their tumour DOI, recurrence and survival rates.
As seen in Table 2, patients were divided according to DOI ≤5 mm or DOI >5 mm:
- Among patients with DOI ≤5 mm, four developed LR (one case was stage I; one was stage III; and two were stage IVA). One subject had regional recurrence (stage IVA) and another had both locoregional and distant recurrence (stage III). In terms of survival, all living patients (28 cases) had DFS.
- In the group of subjects with DOI >5 mm, five had LR (one case was stage II, one was stage III and three cases were stage IVA). Two patients developed regional recurrence (one was stage III, and the other was stage IVA). Locoregional recurrence was recorded in 11 cases (three cases were stage III and eight were stage IV). Four patients had both locoregional and distant recurrences. Only one patient had distant recurrence only (stage IV). Regarding the survival of 27 subjects with DOI >5 mm, DFS was 100% (two cases were stage I; three cases were stage II; seven cases were stage III; and 15 cases were stage IV).
3.2 Resection margin status, descriptive analysis
3.2.1 Involved vs. close vs. Clear margin status: 1. In the group of patients with involved margin resections (14, 16%), nine were mucosal (64%) and five were deep (36%), of which two corresponded to bone margins.
Recurrence was observed in seven subjects (50%): one with local recurrence (14%), one had regional recurrence only (14%) and two cases (29%) had both locoregional and distant recurrence. Three patients had only distant metastasis (43%). In terms of survival, six (43%) cases died during follow-up, all of them were DOD (one patient was stage III, three were stage IVA and two were stage IVB). OS was 57% (eight patients), of which four were stage I, one was stage II and three were stage IV. DFS was recorded in seven cases (88%).
- In the category of subjects with close margin resections (10, 11%), seven were mucosal (70%) and three were deep (30%). Six cases (60%) in this group received adjuvant therapy (RT). Recurrence was registered in 30% of patients. Two of them corresponded to local and one to locoregional recurrence. In terms of survival, seven patients (70%) died. Four of these cases (57%) were DOD (two were stage III and two were stage IVA). Three subjects (43%) were non-DOD (one died of SARS Cov-2 disease, one secondary to a metastatic bladder cancer and another died due to a metastatic lung cancer). OS was 30% (three cases), all of them had DFS (one patient was stage IVB; one was stage II; and another case was stage I). According to our redefined resection status, the tumours were subdivided into:
- Subjects with close margins at 1-2.99 mm distance from tumour (close-inadequate) were four (40%). Two were mucosal (50%) and two were deep (50%). All of them received adjuvant therapy. Tumour recurrence was found in two patients (50%), which was local. All died, three cases (75%) were DOD and one case (25%) was non-DOD.
- Patients with close margins at 3-4.99 mm distance from tumour (close-adequate) were six (60%). Five were mucosal (67%) and one deep (33%). Three subjects in this group received adjuvant therapy (50%). In the non-adjuvant therapy group, one case was diagnosed with T1N0 oral tongue cancer, one with T2 retromolar trigone and one was stage IVB. The latter patient was recommended to receive adjuvant treatment, but declined it.
- From the group of patients with clear margin resections (63, 72%), 37 (59%) received adjuvant treatment (32 cases had RT and five, CT/RT).
In terms of recurrence, 16 subjects (25%) had recurrences. Seven (44%) cases corresponded to local recurrences, one (6%) case had only regional recurrence, seven (44%) had locoregional recurrence and one (6%) case had metastases. In the group of subjects with locoregional recurrence, three patients also had distant metastases. During follow-up period, 14 patients died (22%). Twelve (86%) were DOD (one was stage III disease and 11 were stage IVA disease). Two (14%) were non-DOD. OS was observed in 49 patients (78%), of which 46 (94%) were DFS.
3.2.2 Adequate vs. Inadequate margin status: “Adequate” resections were observed in 69 patients (79%) and “inadequate” resections were found in 18 (21%) cases.
- In the group of patients with “adequate” resections (69), 17 subjects presented tumour recurrence (25%): local recurrence was observed in seven cases (41%), regional relapse in one case (6%) and locoregional in eight (47%). In the locoregional recurrence group, three patients presented distant metastases as well. Isolated distant recurrence was observed in one subject (6%). In this group, 22% of patients had local or locoregional recurrences. A total of 17 (25%) cases with “adequate” resection margins died. Thirteen (77%) were DOD and four (24%) non-DOD. All non-DOD causes were related to a second primary tumour: one case developed lymphoma, two cases were diagnosed with lung cancer and one case with bladder cancer. Survival was observed in 52 (OS 75%) patients, with 49 of them being disease-free survivors (DFS 56%).
- Subjects with “inadequate” resections were 18 in total. The types of margins involved were mucosal (11; 61%) and deep (7; 39%). Two patients with “inadequate” deep margin resections were bone. Only four (22%) cases with “inadequate” margins underwent second surgery to extend the primary surgical resections; three had “inadequate” mucosal margins and one had an “inadequate” deep margin. Thirteen patients (72%) received adjuvant treatment, 10 cases received RT and three received CT-RT. Recurrence was observed in eight (44%) subjects. Two cases (25%) had local recurrence only; one (13%) had regional recurrence; four (50%) had locoregional recurrence, of which two had also distant metastases; and three (37%) had distant recurrence only. Of the 13 patients who received adjuvant, eight cases (61%) had subsequent recurrence. In this group, 39% of patients had either local or locoregional recurrences. In terms of OS, eight patients (44%) were alive after follow-up period. Seven (39%) of them were DFS. Ten subjects (55%) with “inadequate” margin resections died; nine (90%) were DOD and one (10%) non-DOD. Of the living patients with "inadequate" margins, four were stage I, one was stage II and three were stage IV.
3.We also analysed advanced ASA stages (III, IV) as a possible independent risk factor for “adequate”/” inadequate” margin status in terms of survival in univariate analysis. Results showed that advanced ASA stages are a risk factor for mortality when the margin is “adequate” with HR 4.2 (CI 1.36-12.91) and p=0.012. Age was also analysed as potential risk factor for margin status, not finding differences statistically significant.
3.3 Adjuvant therapy
Out of 87 patients, 52 (60%) received postoperative RT to complete the oncological treatment strategy. Eight of them (9%) also received CT. Among the adjuvant group, 29 were alive after follow-up period (OS was 33%) of which 28 were considered as DFS (32%).
- All subjects with involved margin resections (nine cases) received adjuvant therapy. Six received RT alone and three received CTRT.
- In the group of patients with close margin resections:
- All patients with close-inadequate margins (four cases) received adjuvant therapy (RT only).
- Two patients (33%) with close-adequate margin resections received adjuvant therapy (RT alone) according to stage status.
- Of the group of subjects with clear margins resections, 37 (59%) received adjuvant RT (five subjects also received CT).
In one case, a clinical watchful waiting treatment was decided after surgery due to the stage status (pT1N0, floor-of-mouth location). Another patient in this group refused adjuvant therapy.
The relationship between margin status, recurrence and survival is shown in Table 3.
|
Recurrence |
Survival |
|||||||
|
Non-recurrence |
Local |
Regional |
Locoregional |
Distant |
OS |
DFS |
DOD |
|
|
Total N=87 |
61 (70%) |
10 (11%) |
2 (2%) |
12 (14%) |
2 (2%) |
60 (69%) |
56 (64%) |
22 (25%) |
|
Inadequate (N=18) |
9 (50%) |
3 (17%) |
1 (6%) |
4 (22%) |
1 (6%) |
8 (44%) |
7 (39%) |
9 (50%) |
|
Involved (<1 mm) N=14 |
7 (50%) |
1 (7%) |
1 (7%) |
4 (29%) |
1 (7%) |
8 (57%) |
7 (50%) |
6 (43%) |
|
Close-inadequate (1-2.99 mm) N=4 |
2 (50%) |
2 (50%) |
- |
- |
- |
- |
- |
3 (75%) |
|
Adequate (N=69) |
52 (75%) |
7 (10%) |
1 (1%) |
8 (12%) |
1 (1%) |
52 (75%) |
49 (71%) |
13 (19%) |
|
Close-adequate (3-4.99 mm) N=6 |
5 (83%) |
- |
- |
1 (17%) |
- |
3 (50%) |
3 (50%) |
1 (17%) |
|
Clear (≥5 mm) N=63 |
47 (75%) |
7 (11%) |
1 (2%) |
7 (11%) |
1 (2%) |
49 (78%) |
46 (73%) |
12 (19%) |
OS, overall survival; DFS, disease-free survival; DOD, dead of disease; mm, millimeters.
Table 3: Tumour recurrence and patient survival in relation with resection margin status.
3.3 Recurrence
Recurrence was observed in 26 patients (29%). The mean local, regional, locoregional and distant recurrence were 38%, 8%, 46% and 8% respectively. The median time to recurrence was two years and 10 months. The rate of recurrence rate after the reported involved margins was 50% (seven cases), 30% (three cases) in close margins and 25% (16 cases) in clear margins. In the group of patients with “adequate” resection margins the recurrence rate was 25% (17 cases) versus 61% (11 cases) in the group of patients with “inadequate” resection margins. Dividing patients into the two groups “inadequate” <3 mm (involved + close 1-2.99 mm) and “adequate” ≥3 mm (close 3-4.99 mm + clear) we found differences in recurrence, but not statistically significant in our sample size (p 0.11; HR 1.98; 95% CI 0.88-4.45) (Figure 1). We also analyszed other cut-off points, without finding statistical differences (cut-off point at 5 mm: p 0.25, HR 1.61; cut-off point at 1 mm: p 0.18, HR 1.86).
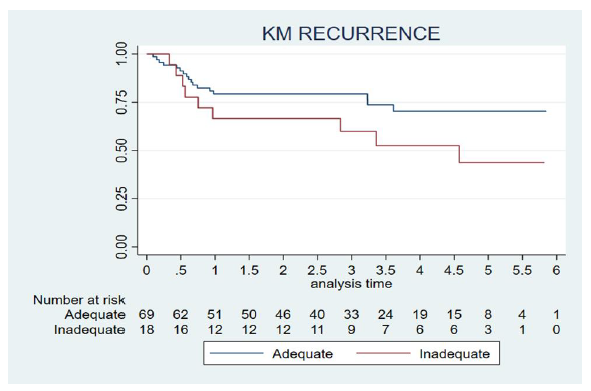
Figure 1: Tumour recurrence according to resection margin status. Patients were divided into two groups: “inadequate” <3 mm (involved + close 1-2.99 mm) and “adequate”: ≥3 (close 3-4.99 mm + clear). The abscissa axis shows time (years) to relapse for each group.
- In the category of patients with “adequate” margin resections, although all factors showed a significant association with OS in univariate analysis, only DOI and ECS showed a significant negative impact on recurrence when analyszed in a multivariate Cox proportional hazards regression model.
- Among subjects with “inadequate” margin resections, we only found LVI as another statistically significant risk factor (p 0.007; HR 16.49; 95% CI, 1.03-263.75). Poor differentiated status (p 0.3) and adjuvant therapy (p 0.0159) did not show statistical significance. Risk factors other than margins showing a significant impact on recurrence were stages III+IV, pT 3+4, N+, DOI >5 mm and ECS. Although all these factors showed a significant association with OS in univariate analysis, only stages III+IV, DOI and ECS showed a significant negative impact on recurrence when analyszed in a multivariate Cox proportional hazards regression model. Analysis of age groups showed no significance as potential risk factor in terms of recurrence. Other risk factors for recurrence between the "adequate" and "inadequate" margin groups are shown in Table 4.
|
Adequate margins (n=69) |
Inadequate margins (n=18) |
|
|
pT 3+4 |
p1 0.0014; HR 7.95; 95% CI, 1.78-35.39 |
p2 0.001; HR 7.43; 95% CI, 2.20-25.01 |
|
N+ |
p 0.0017; HR 4.79; 95% CI, 1.74-13.18 |
p 0.0099; HR 3.61; 95% CI, 1.21-10.75 |
|
Stages III+IV |
p 0.0008; HR 11.72; 95% CI, 1.53-89.73 |
p 0.00; HR 17.80; 95% CI, 2.39-132.32 |
|
DOI >5 mm |
p 0.0087; HR 5.32; 95% CI, 1.19-13.74 |
p 0.02; HR 2.82; 95% CI, 1.10-7.22 |
|
ECS |
p 0.0083; HR 5.25; 95% CI, 1.74-15.81 |
p 0.0061; HR 3.672; 95% CI, 1.57-8.50 |
|
PNI+ |
p 0.036; HR 3.17; 95% CI, 1.01-9.95 |
- |
pT, pathological T stage; N+, positive nodal status; DOI, depth of invasion; ECS, extracapsular nodal spread; PNI+, perineural invasion; HR, hazard ratio; CI, confidence interval; 1Wilcoxon p-value for continuous variables; Chi-square p-value for categorical variables in the group with adequate margins; 2Wilcoxon p-value for continuous variables; Chi-square p-value for categorical variables in the inadequate margins group; (A) First panel describes the items statistically significant for recurrence in patients with adequate/inadequate margins: pT3-T4 stages, positive nodal status (N+), advanced stages (III/IV), DOI >5 mm, ECS and PNI. (B) Second panel shows the statistical results as p <0.05, Hazard Ratio (HR) and Confidence Interval (CI) for “adequate” margins. (C) Third panel describes the statistical results for “inadequate” margins.
Table 4: Comparison of risk factors for recurrence between the “adequate” and “inadequate” margin resection groups
3.4 Survival
OS was 69% (60 cases); 89% in early tumour stages (25 cases) and 59% in advanced stages (35 cases). OS after the reported involved margins was 57%, 30% in close margins and 78% in clear margins. In terms of nodal status, eight (16%) pN0 subjects died. In contrast, death occurred in 18 (50%) pN+ cases. In the group of patients with “adequate” margins OS was 75%. In the category with “inadequate” margins it was 44%. Among the living patients the DFS was 93% (56 cases). When dividing patients into two groups “inadequate” and “adequate”, we found statistical differences in OS (p=0.037) with a HR of 2.24; 95% CI, 1.02-4.91. “Inadequate” margins (<3 mm) have twice the risk of death than “adequate” margins (≥3 mm) (Figure 2).
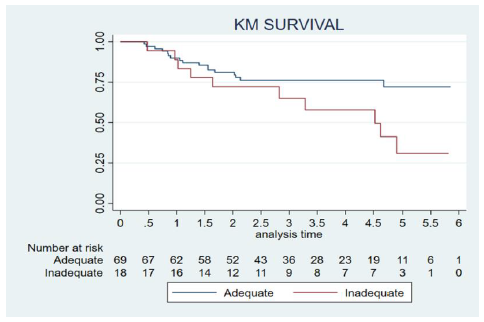
Figure 2: Patient survival rates according to margin resections status. Patients are divided into two groups: “inadequate” <3 mm (involved + close 1-2.99 mm) and “adequate”: ≥3 (close 3-4.99 mm + clear). The abscissa shows the time (years) to decease for each group.
Among the group of patients with “adequate” margin resections, risk factors for mortality were male sex (p 0.01; HR 0.18; 95% CI, 0.041-0.79), pT3-4 (p 0.044; HR 3,013; 95% CI, 0.975-9.312), N+ (p 0.0073; HR 3.48; 95% CI, 1.31-9.22), DOI >5 mm (p 0.02; HR 4.95; 95% CI, 1.10-22.25), LVI+ (p 0.0495; HR 4.43; 95% CI, 0.99-19.64) and ECS (p 0.0463; HR 2.98; 95% CI, 0.96-9.26). Although all factors mentioned in the table showed a significant association with OS, in univariate analysis, only male sex and N+, showed a significant negative impact on survival when analysed in a multivariate Cox proportional hazards regression model.
- In the group of subjects with “inadequate” margin resections, risk factors for mortality were deep margin (p 0.0178; HR 4.72; 95% CI, 1.30- 17.13), LVI+ (p 0.00) and adjuvant therapy (p. 0.034). All these factors showed a significant association with OS in univariate analysis. However, only deep margin and LVI showed a significant negative impact on survival when analysed in a multivariate Cox proportional hazards regression model. There were risk factors other than margins that showed a significant impact on OS (Table 5) in the univariate analysis. Only DOI and ECS showed a significant negative impact on survival when analysed in a multivariate Cox proportional hazards regression model. ASA Classification of patients showed differences statistically significant in terms of survival comparing ASA I/II with ASA III/IV. The results obtained in Cox regression model showed a HR 2.4 with a 95% CI 1.10-5.25 and p=0.027. The age groups showed no statistical differences in terms of OS.
|
pT 3+4 |
p1 0.002; HR 5.24; 95% CI, 1.80-15.23 |
|
Stages III+IV |
p 0.0025; HR 5.2; 95% CI, 1.58-17.58 |
|
N+ |
p 0.0025; HR 5.2; 95% CI, 1.58-17.58 |
|
Deep margin |
p 0.00; HR 0.21; 95% CI, 0.07-0.66 |
|
DOI >5 mm |
p 0.0076; HR 3.57; 95% CI, 1.31-9.67 |
|
ECS |
p 0.0129; HR 2.865; 95% CI, 1.20-6.82 |
|
PNI+ |
p 0.0011; HR 3.85; 95% CI, 1.61-9.22 |
|
LVI+ |
p 0.0068; HR 4.59; 95% CI, 1.36-15.46 |
|
ASA III/IV |
p 0.027; HR 2.41; 95% CI, 1.10-5.27 |
pT, pathological T stage; N+, positive nodal status; DOI, depth of invasion; mm, millimetres; ECS, extracapsular nodal spread; PNI+, perineural invasion; LVI, lymphovascular invasion; ASA, American Society of Anesthesiologists; HR, hazard ratio; CI, confidence interval; 1Wilcoxon p-value for continuous variables; Chi-square p-value for categorical variables in the group with adequate margins (A) First panel describes the items statistically significant for OS: pT3-T4 Stages, advanced stages (III/IV), N+, deep margin, DOI >5 mm, ECS, PNI, LVI and ASA III-IV. (B) Second panel shows the statistical results as p <0.05, Hazard Ratio (HR) and Confidence Interval (CI).
Table 5: Risk factors apart from margins with significant impact in overall survival (OS).
4. Discussion
4.1 Background
According to the current guidelines [8], surgical resection of OSCC is indispensable for curative treatment. On this basis, a clear margin status remains a global concern for head and neck surgeons since decades. Previous literature [13-19] reflects a tendency towards developing wider oral cavity resections in order to achieve better local control of the tumour. Nevertheless, the surgeon should always be aware of the balance between surgical excisions and the resulting local morbidity, functional as well as aesthetic [4]. Surgical margins can be considered as “adequate” depending on the surgeon’s skills, anatomical subsite, biologic behaviour, proximity to important structures and previous treatments [6,17,20]. Arpan et al. [18,21] showed that following the concept of field of cancerization, tumour-free margins may bear genetic mutations which result in development of a recurrence. This requires to extent the resection if severe dysplasia is encountered in order to assure complete removal of the tumour. Recent data suggest that a 5 mm margin may be redundant [6,9,14,17,19], and a redefinition of close and clear margins could be needed. In this study we proposed a further distinctive classification as an attempt to simplify the conventional terms of clear, close and involved margins. We divided them into only two groups, “adequate” and “inadequate” with a cut-off point in 3 mm.
4.2 Margin status and survival in OSCC
Previous literature correlating margin status with OS and DFS of surgically treated OSCC patients have shown considerably disparity of results [5,9,22-25]. This is probably because OS is a rather crude measure of margin status than locoregional recurrence, because survival rates in OSCC are determined by multiple factors [4]. However, our findings suggested that a margin of less than 3 mm doubles the risk of death with a HR 2.24 (p 0.037). In this group of patients the DFS was 64%. This supported our initial hypothesis of setting a lower cut-off point to achieve better clinical outcomes. We also analysed another cut-off point at 5 mm, as in clinical guidelines and described by most authors in their studies [4,5,18,20,22,24], obtaining similar results, with HR 2.55 and p 0.015. In a previous study from our institution [25] we analysed the resection margins of 82 patients undergoing surgical treatment for primary OSCC. An arbitrary intraoperative (macroscopic) safety resection margin of 1 cm was established. In multivariate analysis, no statistically significant difference in OS was found between patients with close margins >3 mm and those with margins >5 mm. However, only patients diagnosed with oral tongue cancer were included. In 2006, Binahmed et al. [9] studied cohort of OSCC patients treated with surgery +/- RT and 5-year survival. Their results showed that patients with involved and close margins (<2 mm) had a similar incidence of treatment failure. This is consistent with results obtained by other authors [4,6]. Nason et al. [6] in 2009 also established that each 1-mm increase in clear surgical margin decreased the risk of death at 5 years by 8%. In our work we found that OS and DFS rates are similar for both involved and close-inadequate margin groups (1-2.99 mm), so we considered analysing them as a single subtype called “inadequate”. Wong et al. [31] in 2012 reported a cut-off point of 1.6 mm as a prognostic indicator for disease-specific survival. However, these data would be confounded by the inclusion of oropharyngeal and oral cavity SCC in the same cohort. On the contrary, the results published in 2019 by Cariati et al. [5] suggested that surgical margins are not directly related to OS and that other factors might significantly influence patient outcomes. They hypothesized that aggressive adjuvant treatment of patients with close surgical margins could help to obtain an OS pattern similar to that of patients with negative margins.
4.3 OSCC according to margin status
In our study group, adjuvant treatment was prescribed postoperatively following EHNS-ESMO-ESTRO Clinical Practice Guidelines [12] and all clinical cases were previously discussed in a multidisciplinary Tumour Board meeting. Controversies on this topic have been found in literature due to the heterogeneity of the previous studies. Stathoupoulos et al. [30] found that histopathologic evidence of tumour cells within a distance <5 mm from the surgical margins does not necessarily seem to offer a safe indicator for further treatment (RT). Welinder et al. [32] found no statistically significant differences in DFS between the surgery-plus-RT group and the surgery-only group. Therefore, they advocated a “watch-and-wait” approach before initiating postoperative RT for patients with close surgical margins. In the same line, Jang et al. [29] described that additional postoperative adjuvant RT did not increase the LR in early-stage oral cancer with close surgical margin <5 mm. For these authors, adjuvant radiotherapy in this group could increase morbidity and its oncologic benefit is uncertain. Binahmed et al. [9] included early and advanced OSCC stages in their study, finding no impact on local or regional recurrence or survival. This agrees with the results of our multivariate analysis, in which adjuvant therapy was not an independent risk factor for either OS or DFS. In a systematic review by Brown et al. [31,33] in 2010, recurrence rates were similar after adjuvant RT in all stages of OSCC compared to surgery alone. They also found that OS was lower for patients who underwent postoperative RT concluding that prospective randomized trials or changes to future protocols were needed. In contrast, in 2018 results published by Fridman et al. [24,26] showed that patients with stage I to II OSCC and positive/close margins have poor long-term outcomes, suggesting that perhaps for this population adjuvant treatment may be associated with improved survival.
4.4 Margin status and recurrence in OSCC
Previous literature [6,17,18,24,26,27,29,34,35] have described different findings regarding OSCC recurrence and margin status. For some authors [24,26,27,29], a cut-off point at ≤5 mm suggests a twofold risk of recurrence rates. In these cases, some articles such as Fridman et al. [26] have only considered patients with stages T1-T2. Barry et al. [27] described that for stage I/II OSCC, the size of the resection margin did not seem to influence local control. Accordingly, Jang et al. [29] concluded that a close surgical margin (<5 mm) did not significantly increase LR in clinical stage I oral cancers, whereas it significantly increased LR in clinical stage II to IVA oral cancers. A meta-analysis by Anderson et al. [36], found statistical significance in terms of LR in patients with 5 mm pathological margin resections. However, they excluded studies where cases had received adjuvant therapy, suggesting that their results may only be applicable to low- or moderate risk tumours. Yamada et al. [18] tested with a multivariate analysis in 2016 the impact of free margin width on the incidence of LR and suggested that a tumour with a free margin of 4 mm or less was associated with an increased risk of LR. In our study 30% of patients had tumour recurrence during the follow-up period. In contrast to some previous authors [26,27], we considered not only stages I/II but also advanced stages of disease. Recurrence rates were compared in our case considering a cut-off point at 3 mm. In the group of patients with “adequate” margin resections the recurrence rate was 25% while it rose to 61% in the category of “inadequate” margins. In particular, we are aware that margin status is more related to local or locoregional recurrence while regional and distant metastasis are more related to initial T and N stage [8]. Despite the differences between the two groups, we found no statistical significance in the analysis performed. We consider expanding the sample group in the future to avoid possible biases. As stated Yamada et al. [18] in their article, increasing the cut-off distance improves the sensitivity of the test at the expense of specificity, which could lead to an increasing number of patients being identified as high-risk who are actuality low-risk, potentially leading to overtreatment and unnecessary toxic effects. Other authors [6,17,18,34,35] have studied other cut-off points lower than ours and obtained statistically significant results in terms of recurrence; for example, 2.2 mm in the case of Zanoni et al. [35] and 1 mm in the work published by Buckajian et al. [34] and Tasche et al. [17]. Nason et al. [6] described that recurrence-free survival was significantly worse for patients with ≤2 mm margin resections. They suggested that mucosal margins tend to be exaggerated. Interestingly, their findings showed that recurrences most often involved the deep resection margin, highlighting the importance of a three-dimensional resection. In our sample, deep margins were an independent risk factor for both recurrence and survival. Similar results were reported by other authors such as Prateek et al. [4] and Barry et al. [27].
4.5 Other predictors involved in OSCC outcomes
Present study is consistent with some authors [4] who described other independent risk factors compromising OS in OSCC, such as pT3-T4, advanced stages, node-positive status, deep margins, DOI, ECS, PNI, LVI and ASA III/IV (HR 2.4). DOI was confirmed as a strong statistically significant risk factor for tumor survival while margin status was not specifically investigated in this sense: relatively small sample, majority of patients presenting with tumors in advanced stages (68%). Although a positive deep margin showed statistically significance as isolated risk factor for tumor survival, an “inadequate” deep margin was not the most commonly found in our series (38,8%) and most probably all of them presented a DOI> 5mm. Unfortunately we have been able to collect this data (DOI) just from 78 out of our 87 patients. Our results agree with previous studies and it is in line with current guidelines [8], where different DOI cut-off points determine different T-stages. Resection margin requirements for early and advanced tumours may be different. As we illustrated in Table 5., advanced stages (III/IV) have a negative impact in survival compared to early stages. Previous authors [27] have only studied T1/T2 stages for outcome analysis and margin status. In these early stages, resection margins are more determinant in terms of recurrence and also potentially controllable by the surgeon. In our sample there were only 40% of early stages, so we considered to analyse T/T2 as well as T3/T4 stages in order to increase the sample size. Mishra et al. [22] hypothesized that most of early stage tumours are not associated with other adverse clinical and pathological factors and that positive margins may be the only poor prognostic factor which determining the need for adjuvant therapy in these cases. Jang et al. [29] found that close and positive margins were associated with a significant worse LR rate in advanced stages. Besides, subsites of oral cavity cancer show different behavior and the adequate resection margin is not easy to achieve in some cases due to the proximity of noble structures, such as hard palate or retromolar trigone. We know that our sample is very heterogeneous in this respect as we analysed all the OSCC anatomical subsites. However, our statistical analysis showed no differences with this item in terms of recurrence or survival. It is also important to determine whether if intraoperative samples are obtained from the primary specimen or directly from the tumour bed. Mateus Szewczyk et al. [1] demonstrated that positive fresh frozen margins, regardless of resection to the R0 stage, could be a powerful adverse factor determining an aggressive nature of the tumour. This feature should be taken into account in adjuvant treatment planning. Buchakjian et al. [34] evaluated the benefits of sampling from the main margin specimen and also found other independent risk factors for OS rates, such as age. In our sample, the age groups did not show statistically significant differences when analysed in terms of survival and recurrence. Fifty-six percent of the patients were male and 43% were female. Divided into three age groups, 8% were younger than 50 years, 38% were between 50–65-years and 54% were older than 65. Contrary to our findings, previous authors considered age as an independent risk factor [26,34]. In the article published by Girardi et al. [16] in 2017, they found significantly higher rates of free margins in female patients of all ages. In our study, gender was not an independent factor for margin status, as described by other authors [6]. In 2020, Capote-Moreno et al. [37] conducted a retrospective observational study to evaluate the epidemiology and risk factors in a cohort of OSCC patients. They found that the diagnosis of OSCC is more frequent at older ages and described that sex differences in distribution had decreased over time. They suggested that further studies are needed to improve knowledge about genetics and tumour behaviour in oral cancer. Comorbid diseases should be considered as an independent prognosis factor for OS in head and neck carcinoma (HNC), as Schimansky et al. [38] described in their multicentre cohort study in 2019. In our study we collected data about the ASA stage, leading to the similar conclusion that advanced ASA stages were risk factors compromising OS rate in patients with OSCC.
4.6 Strength and limitations of the study
This article has some limitations. One is that it is retrospective in nature and limited to five years follow-up. This may lead to selection bias and confound the analysis of the results. The data was collected from a single institution and constitute a relatively small sample. Cause-specific mortality data was not always available, so self-report from telephone calls to the patients’ relatives was used. Our list of confounding variables was extensive and adjusted in our regression models, but residual confounding by unmeasured factors could also be a possibility. As primary strength, our study attempts to clarify the definition of an adequate margin status. In addition, LR and OS are studied by preforming a multivariate analysis with other possible risk factors. SCCs from other head and neck locations (oropharynx, larynx, skin) were not subject of this study according to their individual treatment schemes. All T-stages are included in order to gain a broader understanding not only of early stage disease, but also of advanced stages. The different subsites in oral cavity SCC were studied as they could be considered as an independent prognostic factor due to their different biologic behaviours. Patients who had previously received RT or CT were excluded from this study in an attempt to avoid further confounding factors.
5. Conclusions
Margin status is a strong prognostic indicator in terms of OS for OSCC in the sample studied. We suggest a microscopic cut-off point at 3 mm to ensure a tumour-free resection for majority of OSCC treated at our institution. There is no single definition for a clinically adequate resection margin. A simplified binomial classification distinguishing between adequate and inadequate margins is recommended in a generalised way to assess the best treatment options. Further prospective studies are needed to analyse recurrence rates in inadequate margins and their outcomes after adjuvant therapy.
Author contributions:
Conceptualization, I.U.-F., M.S.-A., A.R.-Z., I.R.-M. and G.S.-A.; methodology, I.R.-M. and G.S.-A.; software, I.U.-F., M.S.-A. and A.R.-Z.; validation, I.U.-F., I.R.-M., M.M.-N., R.G.-D. and G.S.-A.; formal analysis, I.U.-F., M.S.-A. and A.R.-Z.; investigation, I.U.-F., M.S.-A. and A.R.-Z.; resources, G.S.-A. and I.R.-M.; data curation, I.U.-F., M.S.-A., A.R.-Z. and G.S.-A.; writing-original draft preparation, I.U.-F.; writing-review and editing, I.U.-F., M.S.-A., A.R.-Z., I.R.-M., M.M.-N.; R.G.-D. and G.S.-A.; visualization, I.U.-F.; supervision, M.M.-N., R.G.-D., I.R.-M. and G.S.-A.; project administration, I.U.-F., M.M.-N., R.G.-D. and G.S.-A.; All authors have read and agreed to the published version of the manuscript.
Funding:
This research received no external funding.
Institutional review board statement:
The study was conducted according to the guidelines of the Declaration of Helsinki. Confidentiality and privacy of personal data were protected following current EU regulation 2016/679. Local ethical committees’ review of the protocol deemed that formal approval was not required owing to the retrospective, observational and anonymous nature of this study.
Informed Consent Statement:
Patients from the research did not received an specific informed consent for participation due to the retrospective, observational and anonymous nature of the study. Nevertheless, prior to surgical intervention they were all given written informed consent for surgery treatment.
Data Availability Statement:
The data that support the findings of this study are available in Excel® Database (Microsoft® Office Professional Plus 2010, ©2010 Microsoft Corporation. Version 14.0.4760.1000. Product Id 02260-018-0000106-48756.
Conflicts of Interest:
The authors of this manuscript have no financial or personal relationships with other people or organizations that could inappropriately influence our work.
Abbreviations:
The following abbreviations are used in this manuscript:
AJCC American Joint Committee of Cancer
ASA American Association of Anesthesiologists
CT Chemotherapy
CI Confidence Interval
DOI Depth of Invasion
DOD Death of Disease
DFS Disease-Free Survival
ECS Extracapsular Nodal Spread
FS Frozen Sections
HR Hazard Ratio
IQR Interquartile Range
LR Local Recurrence
LVI Lymphovascular
mm Millimetres
OSCC Oral Squamous Cell Carcinoma
OS Overall Survival
PNI Perineural Invasion
KM Kaplan Meier
RT Radiotherapy
References
- Szewczyk M, Golusinski W, Pazdrowski J, et al. Positive fresh frozen section margins as an adverse independent prognostic factor for local recurrence in oral cancer patients. Laryngoscope 128 (2018): 1093-1098.
- Dillon JK, Brown CB, McDonald TM, et al. How does the close surgical margin impact recurrence and survival when treating oral squamous cell Carcinoma? J Oral Maxillofac Surg 73 (2015): 1182-1188.
- Kain JJ, Birkeland AC, Udayakumar N, et al. Surgical margins in oral cavity squamous cell carcinoma: Current practices and future directions. Laryngoscope 130 (2020): 128-138.
- Jain PV, Sharan R, Manikantan K, et al. Redefining adequate margins in oral squamous cell carcinoma: outcomes from close and positive margins. Eur Arch Oto-Rhino-Laryngology 277 (2020): 1155-1165.
- Cariati P, Cabello-Serrano A, Mosalve-Iglesias F, et al. What is the real prognostic value of close margins in oral oncology? Curr Probl Cancer 43 (2019): 1-9.
- Nason RW, Binahmed A, Pathak KA, et al. What is the adequate margin of surgical resection in oral cancer? Oral Surgery, Oral MED ORAL PATHOL ORAL RADIOL ENDODONTOLOGY 107 (2009): 625-629.
- Park H. Surgical margins for the extirpation of oral cancer. J Korean Assoc Oral Maxillofac Surg 42 (2016): 325-326.
- Ridge JA, William ML, Patel SG, et al. Lip and Oral Cavity. In AJCC Cancer Staging Manual, 8th ed; Amin, MB Edge, SB, eds. Springer International Publishing: American Joint Comission of Cancer, Switzerland 7 (2017): 79-95.
- Binahmed A, Nason RW, Abdoh AA. The clinical significance of the positive surgical margin in oral cancer. Oral Oncol 43 (2007): 780-784.
- Chandu A, Adams G, Smith CH. Factors affecting survival in patients with oral cancer: an Australian perspective. Int J Oral Maxillofac Surg 5 (2005): 514-520.
- Dik EA, Willems SM, Ipenburg, NA, et al. Resection of early oral squamous cell carcinoma with positive or close margins: relevance of adjuvant treatment in relation to local recurrence: margins of 3 mm as safe as 5 mm. Oral Oncol 6 (2014): 611-615.
- Machiels JP, René LC, Golusinski W, et al. Squamous cell carcinoma of the oral cavity, larynx, oropharynx and hypopharynx: EHNS-ESMO-ESTRO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 31 (2020): 1462-1475.
- Pathak KA, Nason RW, Penner C, et al. Impact of use of frozen section assessment of operative margins on survival in oral cancer. Oral surg oral med oral pathol oral radiol endod 107 (2009): 235-239.
- Bulbul MG, Zenga J, Tarabichi O, et al. Margin practices in oral cavity cancer resections: survey of american head and neck society members. Laryngoscope 131 (2020): 782-787.
- Doyle DJ, Goyal A, Bansal P, et al. American Society of Anesthesiologists Classification. ASA Classification System Physical Status.
- Girardi FM, Zanella VG, Kroef RG. Correlation between clinical and pathological data and surgical margins in patients with squamous cell carcinoma of the oral cavity. Braz J Otorhinolaryngol 79 (2013): 190-195
- Tasche KK, Buchakjian MR, Pagedar NA, et al. Definition of “close margin” in oral cancer surgery and association of margin distance with local recurrence rate. JAMA Otolaryngol - Head Neck Surg. 143 (2017): 1166-1172.
- Yamada S, Kurita H, Shimane T, et al. Estimation of the width of free margin with a significant impact on local recurrence in surgical resection of oral squamous cell carcinoma. Int J Oral Maxillofac Surg 45 (2016): 147-152.
- Alicandri-Ciufelli M, Bonali M, Piccinini A, et al. Surgical margins in head and neck squamous cell carcinoma: What is “close”? Eur Arch Oto-Rhino-Laryngology 270 (2013): 2603-2609.
- Robbin TK, Triantafyllou A, Suárez C, et al. Surgical margins in head and neck cancer: Intra- and postoperative considerations. Auris Nasus Larynx 46 (2019): 10-17.
- Shah AK, Ashram VJ. Postoperative pathologic assessment of surgical margins in oral cancer: A contemporary review types of resection margins concepts of “positive” or “negative” resection margin, “adequate” margin of resection, “revised” or “supplemental” margin. J Oral Maxillofac Pathol 2 (2020): 2018-2021.
- Mishra A, Malik A, Datta S, et al. Defining optimum surgical margins in buccoalveolar squamous cell carcinoma. Eur J Surg Oncol 45 (2019): 1033-1038.
- Batsakis JG. Surgical excision margins: a pathologist’s perspective. Adv Anat Pathol 6 (1999): 140-148.
- Sutton DN, Brown JS, Rogers SN, et al. The prognostic implications of the surgical margin in oral squamous cell carcinoma. Int J Oral Maxillofac Surg. 32 (2003): 30-34.
- Vazquez-Martinez, C.; Gutierrez-Diaz, R.; Sanchez-Aniceto, G. Rethinking about surgical margins? A new proposal to redefine clear margin concept. Int J Oral Maxillofac Surg 48 (2019): 71-72.
- Fridman E, Na’ara S, Agarwal J, et al. The role of adjuvant treatment in early-stage oral cavity squamous cell carcinoma: An international collaborative study. Cancer 124 (2018): 2948-2955.
- Barry CP, Ahmed F, Rogers SN, et al. Influence of surgical margins on local recurrence in T1/T2 oral squamous cell carcinoma. Head Neck 37 (2015): 1176-1180.
- Varvares MA, Poti S, Kenyon B, et al. Surgical margins and primary site resection in achieving local control in oral cancer resections. Laryngoscope 125 (2015): 2298-2307.
- Jang JY, Choi N, Ko YH, et al. Differential impact of close surgical margin on local recurrence according to primary tumor size in oral squamous cell carcinoma. Ann Surg Oncol 24 (2017): 1698-1706.
- Stathopoulos P, Smith WP. Close resection margins do not influence local recurrence in patients with oral squamous cell carcinoma: A prospective cohort study. J Oral Maxillofac Surg 76 (2018): 873-876.
- Wong LS, McMahon J, Devine J, et al. Influence of close resection margins on local recurrence and disease-specific survival in oral and oropharyngeal carcinoma. Br J Oral Maxillofac Surg 50 (2012): 102-108.
- Welinder BK, Lawaetz M, Dines LM, et al. No difference in disease-free survival after oral cancer resection with close tumor margins in patients with and without postoperative radiotherapy. Ear, Nose Throat J 97 (2018): 314-322.
- Brown JS, Shaw RJ, Bekiroglu F, et al. Systematic review of the current evidence in the use of postoperative radiotherapy for oral squamous cell carcinoma. Br J Oral Maxillofac Surg 50 (2012): 481-489
- Buchakjian MR, Ginader T, Tasche KK, et al. Independent predictors of prognosis based on oral cavity squamous cell carcinoma surgical margins. Otolaryngol neck Surg Of J Am Acad Otolaryngol Neck Surg 159 (2018): 675-682.
- Zanoni DK, Migliacci JC, Xu B, et al. A proposal to redefine close surgical margins in squamous cell carcinoma of the oral tongue. JAMA Otolaryngol - Head Neck Surg 143 (2017): 555-560.
- Anderson CR, Sisson K, Moncrieff M. A meta-analysis of margin size and local recurrence in oral squamous cell carcinoma. Oral Oncol 51 (2015): 464-946.
- Capote-Moreno A, Brabyn P, Muñoz-Guerra M.F, et al. Oral squamous cell carcinoma: epidemiological study and risk factor assessment based on a 39-year series. Int J Oral Maxillofac Surg 49 (2020): 1525-1534.
- Schimansky S, Lang S, Beynon R, et al. Association between comorbidity and survival in head and neck cancer: Results from head and neck 5000. Head Neck 41 (2019): 1053-1062.


 Impact Factor: * 4.2
Impact Factor: * 4.2 Acceptance Rate: 72.62%
Acceptance Rate: 72.62%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 