Use of Artificial Intelligence (AI) for analyzing images of the peritoneal space to evaluate peritoneal carcinomatosis
Rafael Seitenfus1,2*, Eduardo Dipp De Barros1, Paulo Roberto Walter Ferreira2, Rafael De Jesus Rehm3
1Department of Surgical Oncology, Hospital Santa Rita- Complexo Hospitalar Santa Casa de Misericórdia de Porto Alegre, Porto Alegre-RS, Brazil
2Graduate Program of Pathology, Federal University of Health Sciences of Porto Alegre (UFCSPA)
3Graduate Program in Engineering, Innovation and Entrepreneurship, Faculdade de Desenvolvimento do RioGrande do Sul (FADERGS), Porto Alegre-RS, Brazil
4Escola de Tecnologia da Informação, Centro Universitário UniSenac, Porto Alegre-RS, Brazil.
*Corresponding Author: Rafael Seitenfus, Department of Surgical Oncology, Hospital Santa Rita- Complexo Hospitalar Santa Casa de Misericórdia de Porto Alegre, Porto Alegre-RS, Brazil
Received: 26 December 2024; Accepted: 03 January 2024; Published: 30 September 2025
Article Information
Citation: Rafael Seitenfus, Eduardo Dipp de Barros, Paulo Roberto Walter Ferreira, Rafael De Jesus Rehm. Use of Artificial Intelligence (AI) for analyzing images of the peritoneal space to evaluate peritoneal carcinomatosis. Journal of Surgery and Research. 8 (2025): 477-480.
View / Download Pdf Share at FacebookAbstract
In recent years, the use of artificial intelligence as a supportive tool in therapeutic decision-making has been increasingly explored. Concurrently, advancements in the management of peritoneal carcinomatosis have introduced new challenges. Multimodal therapies, the exploration of novel methods for delivering intraperitoneal therapeutic agents, and new drugs present significant challenges for tools that evaluate the behavior of peritoneal metastases. This short communication discusses the use of an innovative tool that enhances the detection and monitoring of peritoneal metastatic nodules. Our initial experience underscores the need for image standardization and highlights the difficulties faced in the first cases evaluated. The numerical data obtained through oncologic risk pixel analysis and the technological evolution are discussed and presented in this report, which includes the first images evaluated by this peritoneal assessment tool.
Keywords
<p>Artificial intelligence (AI), deep learning, diagnostics, Peritoneal metastasis, carcinomatosis</p>
Article Details
Introduction
The treatment of peritoneal carcinomatosis has evolved significantly over the last 20 years [1,2]. The understanding that local control of peritoneal metastases through the combination of surgery and intraperitoneal chemotherapy can directly impact the patient's journey has introduced new challenges to the evaluation of peritoneal carcinomatosis. The tool routinely used to measure the response of solid tumours is RECIST (Response Evaluation Criteria in Solid Tumours). First published in 2000 [3], it has adapted to modern needs when assessing tumour behaviour, such as evaluating metabolic response [4]. It has also helped to interpret the role of new treatments, serving as the reference for determining tumour response and the parameters used for phase II and phase III trials.
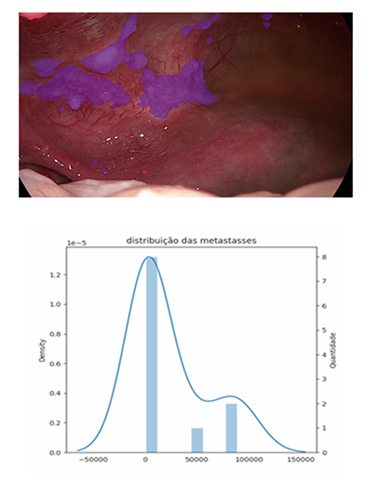
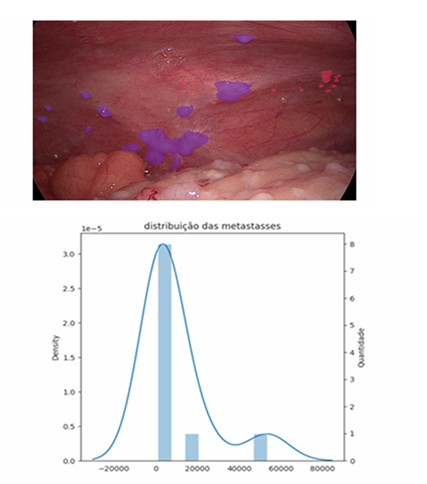
Peritoneal carcinomatosis has always posed a challenge in its ability to be measured in terms of extent, behaviour, and spread. RECIST identifies several limitations of the method as a tool for measuring carcinomatosis, including disease smaller than 10 mm, cystic/mucinous lesions, and areas with locoregional treatment [5-7]. All of these characteristics determine that peritoneal carcinomatosis is a manifestation. Peritoneal carcinomatosis is considered non-measurable by RECIST, making the tool difficult to apply for evaluating peritoneal treatments in this condition. A prospective study including cancer patients with different aetiologies and lesions that could be monitored with RECIST 1.1 identified low rates of measurable lesions in ovarian and gastric tumours, mainly due to the presence of peritoneal carcinomatosis [8]. Colon tumours have a good rate of lesions assessable by RECIST 1.1 due to the presence of liver metastases, but this is limited when peritoneal carcinomatosis is present. Thus, studies seeking to assess peritoneal responses in precision oncology-analysis trials lack an adequate tool to monitor peritoneal response. Different forms of direct measurement of peritoneal carcinomatosis have been described over the years, such as the Lyon Staging System, the Japanese Research Society for Gastric Cancer carcinomatosis staging (JRSGS), the Dutch Simplified carcinomatosis assessment, the Fagoty Score, and the Peritoneal Cancer Index (PCI) [9,10]. All assess two main characteristics: nodule size and distribution within the peritoneal space. All were developed to aid in the proper selection of patients for surgical procedures and were not designed with the intention of monitoring peritoneal response to different treatments at different times. However, all have proven effective in selecting patients for surgical resection and determining the prognosis of carcinomatoses of different aetiologies. Currently, the score developed in 1996 by Dr Pierre Jacquet and Paul H Sugarbaker [11] is the most widely used tool for measuring the distribution of peritoneal carcinomatosis around the world, the so-called Peritoneal Carcinomatosis Index (PCI). Today, the PCI has been consolidated as a tool for assessing carcinomatosis for therapeutic decision-making; it has been used as one of the peritoneal response parameters that can be used to evaluate treatments of the peritoneal space [12-14]. The PCI has become the most widely used peritoneal assessment tool, likely because it is easy to learn and has a 90% correspondence between surgeons [15]. When we assess peritoneal response and the correlation between RECIST and PCI, we are unable to identify a trend or correspondence between the two scores [13]. Thus, the PCI still proves superior to RECIST in assessing peritoneal response and aiding in therapeutic decision making [15]. However, the subjectivity of the assessment, as well as the large gradient contained in the PCI assessment score (scores 2 = 0.5cm to 5cm) [11], does not provide accuracy in measuring the response of peritoneal nodules to treatments of the peritoneal space. Furthermore, there exists difficulty subdividing the groups currently classified by the index. This characteristic, in addition to potentially generating underestimated or even overestimated scores regarding tumour distribution and volume, is dependent on the operator and their familiarity with the scoring instrument, allowing for inaccuracy when assessing peritoneal response. The evolution in the treatment of peritoneal carcinomatosis has brought new challenges to traditional assessment tools such as the PCI [2]. The use of the peritoneal space for the application of therapeutic agents, no longer in a single application but in recurrent applications, has led to the need for reassessment of the peritoneal space throughout the course of treatment. The understanding of bridge or downstage treatments as a modern approach to peritoneal disease [16] will be increasingly explored in the management of peritoneal carcinomatosis. The accuracy in identifying and classifying these subgroups, currently grouped by current tools, becomes important in the more precise selection of patients with peritoneal metastases. Thus, the tools for measuring carcinomatosis aim to not only establish the possibility of resectability or prognosis, as the PCI was initially used, but also assess the effective response of peritoneal metastases to different forms of treatment. This new dynamic in the approach to peritoneal metastases brings with it different challenges, pointing to the need for new alternatives for assessment within the peritoneal space. When we assess peritoneal response and the correlation between RECIST and PCI, we are unable to identify a trend or correspondence between the two scores [13]. Thus, the PCI still proves superior to RECIST in assessing peritoneal response and aiding therapeutic decision making, even with its limitations. Currently, the only score constructed with the objective of directly assessing the response of peritoneal metastases to intraperitoneal treatment is the Peritoneal Regression Score (PGRS) proposed by Solass et al. [17]. It uses three samples of 4 mm in different high-risk peritoneal areas to assess the presence or absence of viable neoplastic cells in the sample, as well as the degree of fibrosis. Thus, a peritoneal response score scaled from four different grades is obtained, allowing monitoring of the peritoneal response to different forms of treatment over time. Recently, Janina Baake et al. [18] demonstrated in a retrospective analysis the existence of a direct relationship between PGRS and the prognosis of patients with peritoneal carcinomatosis treated with intraperitoneal chemotherapy. This could become a tool for selecting patients who are candidates for more aggressive treatments, such as cytoreductive surgery. These findings reinforce the need for precision tools in peritoneal assessment, aiding in the identification of specific groups of patients who may benefit from intraperitoneal treatments. The future prospects of using new modalities of intraperitoneal therapy, such as organoids and nanoparticles [2], will demand even more precision from peritoneal assessment tools. The use of artificial intelligence (AI) as an auxiliary tool in cancer diagnosis has been explored with enthusiasm in recent years [19-23]. Faced with this reality, we started using a tool to assess peritoneal metastases during video laparoscopy. This tool uses, in an unprecedented way, AI to recognise, measure, and monitor the images captured of peritoneal metastases and risk areas. Image processing by AI can recognise peritoneal nodules and provide direct information on the physical characteristics of these nodules. The first analyses of peritoneal images (figures 1-2) using this tool for data extraction were performed in a prospective study that, at the time of writing, is still in progress with the approval of the ethics committee of the Complexo Santa Casa de Porto Alegre (CEP-77421423.0.0000.5335). The first cases analysed by the AI carcinomatosis recognition tool introduce some parameters and challenges discussed below.The capturing of images directly from the towers usually generates low-quality files, leading to the loss of important data. This determines the need for specific equipment for capturing and storing high-resolution files. The need for an implantable peritoneal anchor to serve in the image as a reference centre for image analysis seems to be paramount for making comparisons at different times and with overlapping images. It allows the creation of a fixed reference and parameters for the peritoneal anatomical location (LAP) of different nodules in the region under analysis. The standardisation of an image area to be analysed must be discussed among peritoneal treatment specialists. Even if several image acquisitions are used, four peritoneal regions seem ideal, and the determination of the area around the peritoneal anchor is decisive for standardisation and comparison of the same patient or different patients. Fixing the standard-analysis area allows for the exploration of a concept called the oncological risk pixel (ORP). Thus, the pixel obtained in the captured image will have constant characteristics and can be explored utilising different analysis methodologies. The ORP represents the pixel identified by the AI as possibly related to the neoplasm in that image. This whole process of ORP recognition and segmentation of image nodules performed by a series of mask analysis and clustering methodologies, both manual and automated, can determine a series of new mathematical variables. Those that can already be individually identified in each image today include the total number of nodules, the percentage of nodules smaller than 1cm, the total number of ORP in the image, the total area of the nodules, the average area of the nodules, the median of the nodules, and the individual area of each nodule. The sum of the parameters acquired from four images of the peritoneal space is required in order to get a more accurate map of the individual's peritoneal metastases. However, possibilities brought about by this new technology are unsettling and need to be validated in future studies to understand its full potential.
- • Number of metástases
- Possible carcinomas: 2
- Carcinomas: 11
- • Area/Pixels
- Pixels with oncological risk: 4670 px
- Pixels with carcinomas: 254051 px
- Total affected pixels: 258721 px
- Largest metastasis: 83344 px
- Smallest metastasis: 810 px
- • Number of metástases
- Possible carcinomas: 12
- Carcinomas: 10
- • Area/Pixels
- Pixels with oncological risk: 8384 px
- Pixels with carcinomas: 98073 px
- Total affected pixels: 106457 px
- Largest metastasis: 53458 px
- Smallest metastasis: 955 px
References
- Foster JM. The contemporary management of peritoneal metastasis : A journey from the cold past of treatment futility to a warm present and a bright future 73 (2023): 49-71.
- Guiral DC. Primary and metastatic peritoneal surface malignancies 1 (2003): 13
- Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment 12 (2000): 92.
- Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours : Revised RECIST guideline ( version 1 . 1 ). Eur J Cancer 45 (2008): 228-247.
- Roensholdt S, Detlefsen S, Mortensen MB. Response Evaluation in Patients with Peritoneal Metastasis Treated with Pressurized IntraPeritoneal Aerosol Chemotherapy (PIPAC) (2023).
- Mazzei MA, Khader L, Cirigliano A, et al. Accuracy of MDCT in the preoperative definition of Peritoneal Cancer Index (PCI) in patients with advanced ovarian cancer who underwent peritonectomy and hyperthermic intraperitoneal chemotherapy (HIPEC) (2013).
- Li P, Trial C, Simone M De, et al. Pressurized Intraperitoneal Aerosol Chemotherapy 8 (2011): 1-11.
- Mansour N, Heinrich K, Zhang D, et al. Patient eligibility for trials with imaging response assessment at the time of molecular tumor board presentation 12 (2024): 1-8.
- Gilly FN, Cotte E, Brigand C, et al. Quantitative prognostic indices in peritoneal carcinomatosis 32 (2006): 597-601.
- Fagotti A, Ferrandina G, Fanfani F, et al. A Laparoscopy-Based Score To Predict Surgical Outcome in Patients With Advanced Ovarian Carcinoma: A pilot study 13 (2006): 1156-1161.
- Jacquet P, Sugarbaker PH. Clinical research methodologies in diagnosis and staging of patients with peritoneal carcinomatosis 23 (1996).
- Verheij FS, Bakkers C, Eden WJ Van, et al. Comparison of the Peritoneal Cancer Index and Dutch region count as tools to stage patients with peritoneal metastases of colorectal cancer 12 (2020): 1153-1161.
- Luise F, Veronica R, Würzinger L, et al. The importance of the Peritoneal Cancer Index ( PCI ) to predict surgical outcome after neoadjuvant chemotherapy in advanced ovarian cancer. Arch Gynecol Obstet 306 (2022): 1665-1672.
- Swellengrebel HAM, Zoetmulder FAN, Smeenk RM. Quantitative intra-operative assessment of peritoneal carcinomatosis e A comparison of three prognostic tools. Eur J Surg Oncol 35 (2009): 1078-1084.
- Elias D, Souadka A, Fayard F, et al. Variation in the peritoneal cancer index scores between surgeons and according to when they are determined (before or after cytoreductive surgery). Eur J Surg Oncol 38 (2012): 503-508.
- Sgarbura O, Villeneuve L, Alyami M, et al. European Journal of Surgical Oncology Current practice of pressurized intraperitoneal aerosol chemotherapy (PIPAC): Still standardized or on the verge of diversi fi cation ? Juan Jos 5 (2020): 4-11.
- Solass W, Sempoux C, Detlefsen S, et al. Peritoneal sampling and histological assessment of therapeutic response in peritoneal metastasis: proposal of the Peritoneal Regression Grading Score (PRGS). Pleura and Peritoneum 1 (2016): 99-107.
- Baake J, Nadiradze G, Archid R, et al. Peritoneal regression grading score ( PRGS ): fi rst evidence for independent predictive and prognostic significance. Pleura and Peritoneum 8 (2023): 55-63.
- Bhinder B, Gilvary C, Madhukar NS. Elemento O. HHS Public Access 11 (2021): 900-915.
- Kann BH, Hosny A, Brigham MG, et al. Artificial Intelligence for Clinical Oncology 39 (2022): 916-927.
- Bera K, Schalper KA, Rimm DL, et al. diagnosis and precision oncology 16 (2019): 703-715.
- Hosny A, Parmar C, Quackenbush J, et al. Artificial intelligence in radiology. Nat Rev Cancer 18 (2018): 500-510.
- Majumder A, Sen D. Artificial intelligence in cancer diagnostics and therapy: Current perspectives. Indian J Cancer 58 (2021): 481-492.


 Impact Factor: * 4.2
Impact Factor: * 4.2 Acceptance Rate: 72.62%
Acceptance Rate: 72.62%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 