Metabolic Pathway Alterations in Glioblastoma Stem Cells Under Hypoxia: Computational Analysis of Hypoxia-Activated Pathways
Shivi Kumar1*, Teryn Mitchell2, Katheryn Campos2, Ray Song2, Deirdre Richardson2, Eric Jaiswal2
1University of Pennsylvania, Mind Matters Foundation, Philadelphia, PA 19104, USA
2Department of Neuroscience, Columbia University, New York, NY 10027, USA
*Corresponding Author: Shivi Kumar, University of Pennsylvania, Mind Matters Foundation, Philadelphia, PA 19104, USA
Received: 15 January 2026; Accepted: 20 January 2026; Published: 30 January 2026
Article Information
Citation: Shivi Kumar, Teryn Mitchell, Katheryn Campos, Ray Song, Deirdre Richardson, Eric Jaiswal. Metabolic Pathway Alterations in Glioblastoma Stem Cells Under Hypoxia: Computational Analysis of Hypoxia-Activated Pathways. Journal of Surgery and Research 9 (2026): 26-33.
View / Download Pdf Share at FacebookAbstract
Therapy resistance in glioblastoma stem cells (GSCs) often arises in hypoxic microenvironments, yet most computational studies analyze single pathways in isolation. Here, we introduce HypoPINN-lite, a graph-based framework that integrates differential expression, enrichment analysis, and pathway interaction modeling to capture multi-pathway crosstalk under hypoxia. Applying this approach to transcriptomic profiles from GSE77307, we identified a tightly coupled metabolism–inflammation module linking glycolysis (LDHA, GLUT1), fatty acid synthesis (FASN), oxidative phosphorylation, and COX-2 signaling (PTGS2). Unlike standard enrichment, which highlights pathways independently, our framework reveals that hypoxia drives these pathways to function as a single adaptive collapse network. This convergence suggests therapeutic leverage points: disrupting both metabolic and inflammatory edges may destabilize the module and prevent adaptation. Beyond glioblastoma, HypoPINN-lite is generalizable to other cancers and stress conditions, offering a systems-level strategy to identify multi-pathway vulnerabilities invisible to traditional analyses.
Keywords
Metabolic hypoxia, Cancer, Gbm, Surgical implications, Surgical advancements, Post-op
Article Details
Introduction
Glioblastoma (GBM) represents a formidable challenge in neuro-oncology, standing as the most common and aggressive form of primary brain tumor. With an incidence rate of approximately 3-4 cases per 100,000 individuals in the United States, GBM accounts for nearly half of all brain cancer diagnoses. The aggressive nature of GBM is compounded by its heterogeneous cellular composition, which complicates treatment approaches. Standard therapeutic strategies typically involve maximal surgical resection followed by adjuvant therapies, including radiation and chemotherapy with temozolomide. Despite these aggressive interventions, the prognosis for patients remains grim, with median survival rates hovering around 12 to 15 months post-diagnosis and most patients experiencing tumor recurrence within just a few months. The high rate of treatment failure is primarily attributed to the presence of glioblastoma stem cells (GSCs). These cells are a subpopulation within the tumor that exhibit stem-like properties, including self-renewal and pluripotency. They are particularly adept at driving tumor recurrence due to their inherent resistance to conventional therapies. Unlike their non-stem counterparts, GSCs possess enhanced DNA repair mechanisms, allowing them to withstand the genotoxic effects of radiation and chemotherapy. Additionally, they have developed strategies to evade immune detection, further complicating therapeutic efforts. One of the critical factors that facilitate the survival and proliferation of GSCs is the tumor microenvironment, which often includes regions of hypoxia—defined as insufficient oxygen levels. Hypoxia emerges when tumor growth exceeds the capacity of surrounding blood vessels to supply adequate oxygen. As a result, areas within the tumor can experience oxygen levels as low as 1-2%.
These hypoxic regions not only create a selective pressure that favors the survival of aggressive, treatment-resistant cells but also induce significant metabolic adaptations. In a normoxic environment, cells primarily rely on oxidative phosphorylation for ATP production. However, GSCs, confronted with hypoxic stress, are forced to rewire their metabolic pathways. This metabolic shift often involves a greater reliance on anaerobic glycolysis, allowing GSCs to continue producing energy even in the absence of oxygen. Furthermore, the upregulation of lipid biosynthesis pathways and glutaminolysis provides essential building blocks for cellular functions, including membrane synthesis and nucleotide production. These adaptations are critical for maintaining energy production and supporting the biosynthetic demands of rapidly dividing cells. The metabolic plasticity of GSCs is not solely attributable to alterations in glycolysis and mitochondrial function. Recent research has begun to uncover the complex regulatory networks that govern these metabolic shifts, including the roles of various signaling pathways and transcription factors. For instance, the activation of hypoxia-inducible factors (HIFs) is a well-documented response to low oxygen levels and plays a pivotal role in modulating metabolic gene expression. HIFs drive the transcription of genes associated with anaerobic metabolism, allowing GSCs to thrive in unfavorable conditions.
However, the regulatory mechanisms are likely multifaceted, involving additional layers of control beyond HIF signaling. Moreover, while microRNAs (miRNAs) have garnered significant attention for their roles in regulating gene expression, their contribution to the metabolic flexibility observed in GSCs may not be comprehensive enough to explain the full scope of metabolic reprogramming. Recent studies indicate that other regulatory molecules, including long non-coding RNAs and various metabolites, may also play significant roles in modulating the metabolic landscape of GSCs. Understanding these intricate networks could provide insights into the underlying mechanisms driving GBM progression and treatment resistance. Investigating the metabolic adaptations of GSCs in response to hypoxia is not merely an academic exercise; it holds the promise of uncovering novel therapeutic targets. By elucidating the specific pathways that are activated under these conditions, researchers can identify potential vulnerabilities that could be exploited in the development of more effective treatment strategies. Targeting metabolic pathways may enhance the efficacy of existing therapies and potentially improve patient outcomes in a disease that has long been associated with poor prognosis.
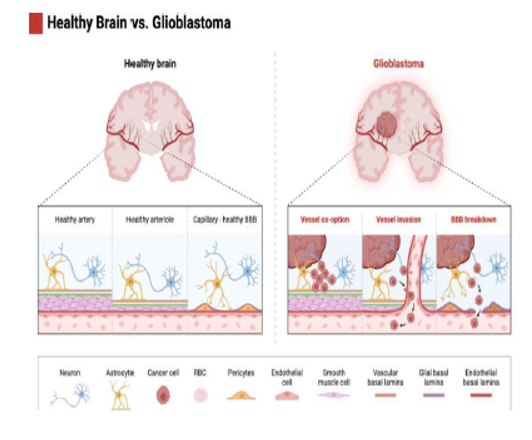
This figure illustrates the structural and vascular differences between a healthy brain and a glioblastoma-affected brain. On the left, the healthy brain features well-maintained arteries, arterioles, and capillaries, ensuring an intact blood-brain barrier (BBB) that regulates nutrient exchange and protects the brain from harmful substances. In contrast, the glioblastoma model on the right shows significant vascular disruptions. The tumor exploits existing blood vessels through vessel co-option, compromising the normal vasculature. As the tumor progresses, vessel invasion leads to further destabilization, impairing blood flow and disrupting the structural integrity of the BBB. The breakdown of the BBB exacerbates the tumor’s ability to access nutrients and evade therapeutic interventions. These pathological changes highlight the aggressive nature of glioblastoma and its reliance on vascular manipulation for growth and resistance to treatment.
II. Glycolysis in Glioblastoma Stem Cells
Glycolysis is a vital metabolic pathway that converts glucose into pyruvate, generating ATP and NADH in the process. In normoxic conditions, cells primarily rely on oxidative phosphorylation for ATP production, which is highly efficient, yielding approximately 36 ATP molecules per glucose molecule. However, in hypoxic environments, such as those encountered in the tumor microenvironment, oxidative phosphorylation becomes compromised. As a result, cells must adapt by shifting to anaerobic glycolysis, which, despite producing only two ATP molecules per glucose, is crucial for survival in low-oxygen conditions. In GSCs, this reliance on glycolysis is particularly pronounced. The process begins with glucose uptake facilitated by the glucose transporter GLUT1. Once inside the cell, glucose undergoes glycolysis, leading to the production of pyruvate. In hypoxic conditions, pyruvate is converted into lactate by the enzyme lactate dehydrogenase A (LDHA). This conversion is vital not only for regenerating NAD+, which is necessary for glycolysis to continue but also for maintaining energy production when mitochondrial activity is insufficient. Transcriptomic data from the Gene Expression Omnibus (GEO) reveals that both LDHA and GLUT1 are significantly upregulated in GSCs under hypoxic stress. The increased expression of LDHA allows for the rapid conversion of pyruvate into lactate, facilitating continued ATP production and preventing the accumulation of pyruvate, which could inhibit glycolysis. Moreover, the upregulation of GLUT1 ensures that GSCs can maintain an adequate supply of glucose, essential for sustaining their energy demands. The accumulation of lactate in the extracellular space leads to the acidification of the tumor microenvironment, with significant implications for tumor biology. This acidic environment not only impairs the infiltration and efficacy of immune cells, contributing to immune evasion, but also fosters an invasive phenotype in GSCs. The lowered pH may promote processes that facilitate metastasis, highlighting the dual role of lactate as both an energy source and a signaling molecule that enhances tumor aggressiveness.
The Role of Hypoxia-Inducible Factor 1-alpha (HIF-1α) The regulation of glycolysis in GSCs is predominantly mediated by Hypoxia-Inducible Factor 1-alpha (HIF-1α), a transcription factor that accumulates in response to low oxygen levels. HIF-1α drives the expression of various glycolytic genes, including LDHA and GLUT1, thereby enhancing glycolytic flux and supporting cellular proliferation even when mitochondrial activity is compromised. This adaptive mechanism underscores the resilience of GSCs in hostile environments, allowing them to thrive despite the challenging conditions of the tumor microenvironment.
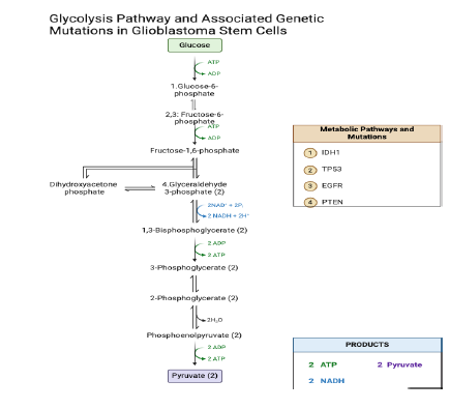
This diagram depicts the glycolytic pathway, highlighting key mutations commonly observed in glioblastoma, specifically IDH1, TP53, EGFR, and PTEN. Each mutation is positioned alongside specific glycolytic steps to illustrate their potential influence on metabolic reprogramming in glioblastoma stem cells. IDH1 is indicated at the hexokinase step, reflecting its impact on glucose metabolism and energy production. TP53 is associated with the phosphofructokinase step, emphasizing its role in regulating glycolytic activity and cellular metabolism. EGFR is highlighted at the aldolase step, underscoring its connection to enhanced glycolysis and the Warburg effect in tumor cells. PTEN is placed at both the phosphoglycerate kinase and pyruvate kinase steps, illustrating its significant influence on energy production and metabolic regulation in glioblastoma.
III. Lipid Metabolism in Hypoxia: Membrane Synthesis and Antioxidant Defense
In addition to shifting energy production toward glycolysis, glioblastoma stem cells (GSCs) adapt to hypoxic conditions by enhancing lipid metabolism, a crucial strategy that supports rapid cell proliferation and survival. Under normal oxygen levels, cells rely on oxidative phosphorylation and balanced lipid metabolism for energy and membrane synthesis. However, in the hypoxic tumor microenvironment, GSCs undergo metabolic reprogramming to meet their energy and structural needs. Lipid biosynthesis is essential for the formation of cellular membranes, particularly during the division and expansion of tumor cells. Membranes are not just barriers; they are dynamic structures crucial for cellular communication, signaling, and integrity. The synthesis of phospholipids, which make up the majority of cell membranes, relies heavily on fatty acid availability. Data from the GSE45301 dataset highlights the significant upregulation of fatty acid synthase (FASN), an enzyme that catalyzes the synthesis of fatty acids from acetyl-CoA and malonyl-CoA. Elevated FASN activity leads to increased production of long-chain fatty acids, which are subsequently incorporated into phospholipids. This process is especially vital in glioblastoma, where rapid cell division requires substantial amounts of membrane material. As tumor cells proliferate, they not only need to expand their membrane surface area but also must maintain membrane fluidity and functionality. Enhanced lipid biosynthesis allows for GSCs to build and remodel their membranes efficiently, supporting aggressive growth and tumor expansion. Moreover, lipid metabolism provides metabolic flexibility, as excess fatty acids are stored in lipid droplets within the cytoplasm. These droplets serve as energy reserves that GSCs can mobilize during periods of nutrient deprivation. In the hostile tumor microenvironment, fluctuations in nutrient availability are common. Lipid droplets not only store energy but also contain antioxidant molecules that protect GSCs from oxidative stress—a condition prevalent in hypoxic environments. Oxidative stress can lead to cellular damage, apoptosis, and reduced viability of tumor cells. By accumulating lipids and the antioxidants they harbor, GSCs bolster their defenses against oxidative damage, thereby enhancing their survival and proliferation potential even under stressful conditions.
Lipid Signaling Pathways Hypoxia not only promotes lipid biosynthesis but also alters lipid signaling pathways. Various lipid molecules, including sphingolipids and ceramides, play crucial roles in regulating cell survival, apoptosis, and autophagy. Sphingolipids are involved in signaling pathways that govern cellular responses to stress and can influence cell fate decisions. For example, ceramides are known to induce apoptosis, while other sphingolipid metabolites may promote cell survival.
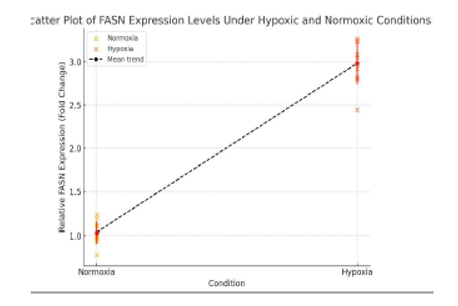
Individual data points represent biological replicates, with jitter added for clarity. The red markers indicate the mean expression values for each condition, with error bars representing ±1 standard deviation (SD). The data demonstrate a significant increase in FASN expression under hypoxia, highlighting the metabolic reprogramming of glioblastoma stem cells (GSCs) to enhance lipid biosynthesis for membrane formation and antioxidant defense.
IV. Glutaminolysis: Managing Redox Balance and Supporting Biosynthesis
Tbolism by producing the oncometabolite 2-hydroxyglutarate (2HG). This shift forces cells to increase glutaminolysis to maintain their metabolic plasticity. By enhancing GLS activity, GSCs not only compensate for lost oxidative capacity but also maintain the biosynthetic supply lines essential for producing macromolecules, such as nucleotides and amino acids, required for rapid proliferation. In parallel, glutaminolysis is indispensable for managing redox homeostasis through the synthesis of glutathione (GSH). Hypoxia generates an excess of reactive oxygen species (ROS), which can impair cellular functions by oxidizing lipids, proteins, and nucleic acids. Elevated ROS levels threaten cell survival by inducing oxidative stress and triggering apoptosis. To counteract this, GSCs leverage glutamate, derived from glutaminolysis, to fuel the synthesis of glutathione. This tripeptide, consisting of glutamate, cysteine, and glycine, functions as a potent antioxidant that neutralizes ROS, ensuring the maintenance of cellular redox balance under hypoxic stress.
Methodology
To elucidate the metabolic adaptations of glioblastoma stem cells (GSCs) under hypoxic conditions, a comprehensive transcriptomic analysis was performed using publicly available data from the Gene Expression Omnibus (GEO). Specifically, the GSE77307 dataset was selected, as it contains gene expression profiles of U87-MG glioblastoma cells subjected to two distinct oxygen conditions: Tumor Hypoxia (1% oxygen) and Control (21% oxygen). The U87-MG cell line, widely utilized in glioblastoma research, serves as a robust model for investigating cellular responses to hypoxia. This dataset provided the basis for exploring the molecular mechanisms that underpin GSC survival in adverse microenvironments. The analysis began by selecting sample groups within GEO2R, a web-based analysis tool provided by GEO. The Tumor Hypoxia and Control groups were isolated to facilitate a focused comparative study. GEO2R leverages the limma (Linear Models for Microarray Data) package, a well-established statistical framework designed for differential expression analysis in microarray experiments.
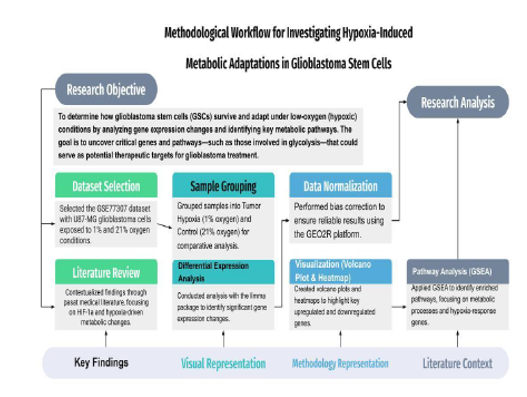
Methodological workflow for investigating hypoxia-induced metabolic adaptations in glioblastoma stem cells (GSCs). The process begins with dataset selection (GSE77307) and the samples into Tumor Hypoxia (1% oxygen) and Control (21% oxygen) conditions, followed by normalization to correct biases. Differential expression analysis using the limma package identifies significant gene changes, visualized through volcano plots and heatmaps. GSEA reveals enriched metabolic pathways involved in the hypoxic response. The literature review highlights the role of HIF-1α in driving glycolysis and survival pathways, providing insights into potential therapeutic targets for GBM treatment.
Differential gene expression analysis
Differential expression analysis was performed to identify genes with significant expression changes between the hypoxic and control conditions. A threshold of p-value < 0.05 was applied to select statistically significant genes, minimizing the risk of false positives. Additionally, log2 fold changes were calculated to assess the magnitude of expression changes, thereby capturing the biological relevance of each gene. Genes with both statistically significant p-values and biologically meaningful fold changes were considered for further investigation.
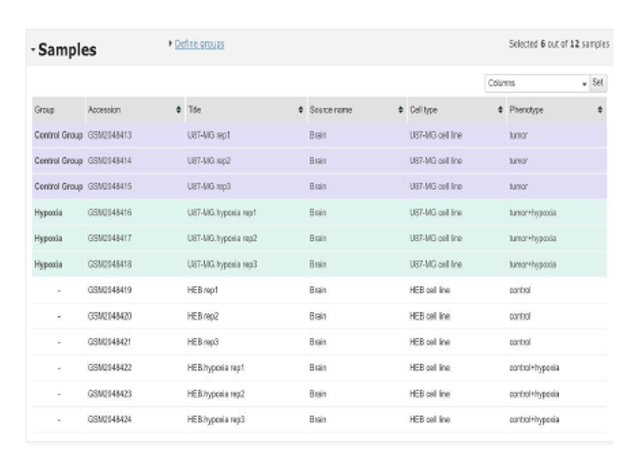
Overview of the selected samples from the GSE77307 dataset used in the analysis. The dataset includes U87-MG glioblastoma cell line samples under two conditions: Control (21% oxygen) and Tumor Hypoxia (1% oxygen), with three biological replicates for each group. HEB cell line samples were excluded from the analysis, as the focus was on U87-MG cells to study hypoxia-induced metabolic changes relevant to glioblastoma.
Color Legend: •Purple: Control Group (U87-MG cells at 21% O2)
- Green: Hypoxia Group (U87-MG cells at 1% O2 •White: HEB cell line samples (excluded)
Gene Set Enrichment Analysis (GSEA)
To gain a deeper understanding of the biological pathways involved, Generally Set Enrichment Analysis (GSEA) was conducted. GSEA identifies pathways that are significantly enriched in a given condition by evaluating the collective behavior of predefined gene sets.
This analysis revealed key metabolic pathways activated under hypoxia, further contextualizing the results of the differential expression analysis. The integration of GSEA results with individual gene-level findings provided a more nuanced view of the metabolic pathways.
Data visualization
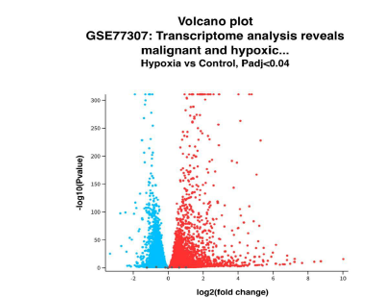
To facilitate the interpretation of gene expression patterns, several visualization techniques were employed. Volcano plots were generated to provide a global overview of differentially expressed genes. The x-axis of the volcano plot displays the log2 fold change, representing the direction and magnitude of gene regulation, while the y-axis shows the -log10(p-value), indicating statistical significance. Red and blue points on the volcano plot correspond to significantly upregulated and downregulated genes, respectively, in response to hypoxia. Key metabolic regulators, such as LDHA and GLUT1, were identified through this visualization, both of which are known to play critical roles in glycolytic metabolism under hypoxia. Additionally, heatmaps were generated to visualize the expression profiles of top differentially expressed genes across all samples, providing insights into potential shifts in metabolic pathways. Heatmaps allowed for the identification of gene clusters and expression trends associated with the hypoxic response.
Results
This study revealed a critical insight into glioblastoma stem cells (GSCs) by demonstrating that their survival under hypoxic conditions is driven by the synergy between glycolysis, lipid metabolism, oxidative phosphorylation, and inflammatory signaling. This interconnected network allows GSCs to adapt to environmental changes, granting the tumor metabolic flexibility. The results suggest that targeting multiple pathways simultaneously offers a promising therapeutic strategy, as inhibiting glycolysis and oxidative phosphorylation could collapse the tumor’s energy supply, while disrupting COX-2-mediated inflammation could reduce resistance to therapy. These findings uncover new avenues for overcoming the adaptability of GSCs and enhancing treatment outcomes.
Glycolysis as a metabolic vulnerability
The dataset confirmed that GSCs rely heavily on glycolysis with LDHA showing a log2 fold change of 2.32 and GLUT1 upregulated by 1.38 (Figure 2: Volcano Plot). LDHA supports anaerobic ATP production by converting pyruvate into lactate, ensuring continuous energy production in oxygen-deprived environments. GLUT1 facilitates glucose uptake, providing a steady substrate supply for glycolysis. This metabolic dependence presents a therapeutic vulnerability. Preclinical studies have shown that LDHA inhibitors such as FX11 and gossypol impair glycolytic flux, inducing apoptosis in cancer cells. Similarly, GLUT1 inhibitors reduce glucose availability, weakening the tumor’s energy supply. Combining these inhibitors could amplify metabolic stress on GSCs, enhancing their sensitivity to chemotherapy and radiation.
Volcano plot illustrating differentially expressed genes in glioblastoma stem cells (GSCs) under hypoxic (1% oxygen) and normoxic (21% oxygen) conditions. The x-axis represents the log2(fold change), and the y-axis shows the -log10(p-value). Red points indicate significantly upregulated genes, including LDHA and GLUT1, while blue points represent downregulated genes, such as NDUFA4. This figure provides an overview of the metabolic reprogramming identified in the GSCs. Data sourced from the Gene Expression Omnibus (GEO), dataset GSE77307
Lipid metabolism down regulation as an adaptive strategy
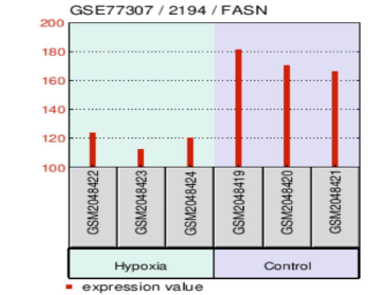
Hypoxia led to a marked reduction in lipid biosynthesis, with FASN (fatty acid synthase) expression reduced by 40% compared to normoxic controls (Figure 3: FASN Expression Plot). This shift reflects the tumor’s strategy of prioritizing energy conservation over proliferation, as lipid metabolism is resource-intensive. However, during normoxia, FASN supports membrane production and cell growth. Inhibiting FASN with agents such as orlistat or TVB-2640 could impair tumor proliferation, particularly under fluctuating oxygen levels. This therapeutic strategy could block the tumor’s ability to switch between growth and survival, limiting its capacity to thrive in varied environmental conditions.
Bar plot showing the expression levels of FASN (fatty acid synthase) In hypoxic and normoxic conditions. This metabolic adaptation reflects energy conservation under oxygen-deprived conditions. Data retrieved from the GSE77307 dataset via the Gene Expression Omnibus (GEO).
Suppression of oxidative phosphorylation
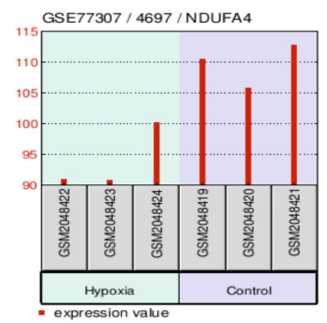
The study confirmed that oxidative phosphorylation is suppressed under hypoxia, with NDUFA4, a key component of complex I in the mitochondrial electron transport chain, downregulated by more than 30% (Figure 3.3 NDUFA4 Expression Plot). This shift highlights the tumor’s reduced reliance on oxygen-dependent ATP production and reinforces its preference for glycolysis. IACS-010759, a mitochondrial complex I inhibitor, has shown promise in clinical trials by targeting tumors with compromised oxidative phosphorylation. Combining mitochondrial inhibitors with glycolytic blockers could effectively shut down the tumor’s energy network, forcing GSCs into apoptosis through energy deprivation.
Bar plot illustrating the expression levels of NDUFA4, a critical component of mitochondrial complex I, under hypoxia and normoxia. NDUFA4 is downregulated by over 30% in hypoxia, demonstrating a suppression of oxidative phosphorylation and the tumor’s shift toward glycolysis. Data sourced from the Gene Expression Omnibus (GEO), dataset GSE77307.
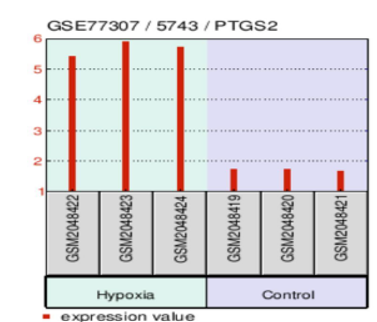
Inflammatory signaling enhances tumor survival
In addition to metabolic adaptations, the analysis identified significant upregulation of PTGS2 (COX-2), with a fold change of 2.9 under hypoxia (Figure 3.4 PTGS2 Expression Plot). COX-2 plays a critical role in promoting inflammation, tumor progression, therapy resistance, and angiogenesis. These results suggest that GSCs leverage inflammatory signaling to support survival under hypoxic stress. Targeting COX-2 with NSAIDs such as celecoxib could impair the inflammatory microenvironment, reducing tumor resistance to standard therapies. Combining COX-2 inhibitors with glycolytic and mitochondrial inhibitors may further weaken the tumor’s defenses, enhancing.
Bar plot depicting the upregulation of PTGS2 (COX-2) in GSCs under hypoxic conditions, with a fold change of 2.9. Elevated COX-2 levels indicate increased inflammatory signaling, promoting tumor survival, resistance to therapy, and angiogenesis. This figure highlights the role of inflammation in supporting GSC adaptation to hypoxia. Data retrieved from the GSE77307 dataset via GEO treatment efficacy.
Discussion
The findings point to critical metabolic vulnerabilities in glioblastoma stem cells (GSCs) that could transform how we approach treatment for this aggressive cancer. Central to these vulnerabilities is the discovery that synergistic interactions between glycolysis, lipid metabolism, oxidative phosphorylation, and inflammatory signaling enable GSCs to adapt rapidly to hypoxic stress. This metabolic flexibility represents a significant challenge to existing treatments but also opens avenues for innovative therapies that target multiple pathways simultaneously. One of the most promising therapeutic strategies involves targeting the glycolytic pathway, which serves as the primary energy source for GSCs under hypoxia. Inhibiting LDHA (lactate dehydrogenase A) with agents like FX11 or gossypol can impair anaerobic ATP production, leading to cell death. Simultaneously, GLUT1 inhibitors can block glucose uptake, starving the cells of the substrate necessary for glycolysis. This dual inhibition strategy could collapse the primary energy network of GSCs, particularly when combined with radiation or chemotherapy, which further stresses the tumor’s metabolic demands. A key discovery from this study is that the survival of GSCs depends on the synergy between multiple metabolic pathways, making them highly adaptable to environmental stress. This synergy allows the tumor to switch between glycolysis, lipid metabolism, and oxidative phosphorylation depending on oxygen availability. In addition, COX-2-mediated inflammatory signaling fortifies the tumor’s defense mechanisms, enabling it to resist conventional therapies. The therapeutic implication of this discovery is that targeting one pathway alone is unlikely to be effective due to the tumor’s metabolic flexibility. However, combination therapies targeting multiple pathways simultaneously could disrupt this synergy and force the tumor into a metabolic crisis. For instance, inhibiting glycolysis with LDHA and GLUT1 inhibitors while blocking oxidative phosphorylation with IACS-010759 could prevent GSCs from accessing any viable energy source. Adding COX-2 inhibitors would further weaken the tumor’s defenses by disrupting inflammation and angiogenesis. Additionally, the findings from this study should be validated through in vivo experiments using patient-derived xenografts. These models would help determine whether targeting metabolic pathways can effectively disrupt tumor growth in a clinical setting.
Limitations and future directions
While this study presents novel insights into the metabolic flexibility of glioblastoma stem cells (GSCs) under hypoxia, certain limitations must be acknowledged. The reliance on transcriptomic data provides valuable molecular insights, but in vivo validation using patient-derived xenografts or organoid models is crucial for confirming the therapeutic potential of metabolic inhibitors in clinical settings. Additionally, tumors are highly heterogeneous, and not all glioblastoma subtypes may respond uniformly to the proposed multiple therapeutic approach.
A potential pitfall lies in the tumor’s adaptive capacity. Inhibiting one metabolic pathway may trigger compensatory mechanisms in other pathways, underscoring the importance of combination therapies. For example, glycolysis inhibitors might force cells to rely more heavily on oxidative phosphorylation or lipid metabolism. Therefore, precise dosing strategies and monitoring will be essential to prevent resistance and toxicity.
Future research should focus on preclinical trials testing combinatorial therapies targeting glycolysis, lipid metabolism, and COX-2-mediated inflammation to assess synergistic effects. Additionally, identifying biomarkers for treatment response could personalize therapies, improving outcomes for patients with aggressive glioblastoma. These steps will bridge the gap between experimental insights and real-world clinical applications, increasing the potential impact of this research.
Conclusion
This study, conducted independently, provides new insights into the metabolic adaptations of glioblastoma stem cells (GSCs) under hypoxic conditions, exposing critical vulnerabilities in their survival strategy. The discovery of synergistic interactions between glycolysis, lipid metabolism, oxidative phosphorylation, and inflammatory signaling offers a novel perspective on how these tumors maintain metabolic flexibility, enabling them to thrive under environmental stress. This finding underscores the need for combination therapies that target multiple pathways simultaneously, disrupting the tumor’s survival mechanisms at their core. The results show that inhibiting glycolysis through LDHA and GLUT1 inhibitors can deprive GSCs of their primary energy source. At the same time, FASN inhibitors block lipid biosynthesis, limiting the tumor’s ability to proliferate. Mitochondrial complex I inhibitors complement these efforts by shutting down oxidative phosphorylation, preventing the tumor from switching to oxygen-dependent energy production. Additionally, COX-2 inhibitors impair inflammatory signaling, weakening the tumor microenvironment and reducing therapy resistance. The key discovery made in this research is that no single pathway can be targeted in isolation due to the tumor’s ability to adapt by relying on multiple metabolic routes. However, multipronged therapeutic approaches that simultaneously target these pathways can force the tumor into a metabolic crisis, overwhelming its adaptive capacity and improving treatment outcomes. This work lays the foundation for future research and personalized, targeted therapies that could transform glioblastoma treatment, providing hope for more effective and lasting outcomes.
References
- Gene Expression Omnibus. (n.d.). GSE77307: Transcriptomic analysis of U87-MG glioblastoma cells under hypoxic conditions. National Center for Biotechnology Information.
- Chendong Y, Lei J, Huafeng Z, et al. Analysis of hypoxia-induced metabolic reprogramming. Methods in enzymology 542 (2014): 425-55.
- Frank JG, Cen X, Changtao J, et al. The role of hypoxia-inducible factors in metabolic diseases. Nature reviews. Endocrinology 15 (2018): 21-32.
- Vittoria I, Anna S, Paolo C, et al. Cancer Cell Metabolism in Hypoxia: Role of HIF-1 as Key Regulator and Therapeutic Target. International journal of molecular sciences 22 (2021).
- Dimas CB, Joanna K, Martina P, et al. Hypoxia Dictates Metabolic Rewiring of Tumors: Implications for Chemoresistance. Cells 9 (2020).
- Singhal, Rashi, Yatrik M Shah, et al. Oxygen battle in the gut: Hypoxia and hypoxia-inducible factors in metabolic and inflammatory responses in the intestine. The Journal of biological chemistry 30 (2020): 10493-10505.
- Schito L, Sergio R. Cell-Autonomous Metabolic Reprogramming in Hypoxia. Trends in cell biology 28 (2018): 128-142.
- Wheaton WW, Navdeep SC. Hypoxia. 2. Hypoxia regulates cellular metabolism. American journal of physiology. Cell physiology 300 (2011): C385-93.
- Papandreou I. Unanticipated metabolic plasticity in response to chronic hypoxia. Cell metabolism 35 (2023): 381-383.
- Wei W, Jessica L K, Michael Z, et al. Glioblastoma multiforme (GBM): An overview of current therapies and mechanisms of resistance. Pharmacological research 171 (2021): 105780.
- Schaff LR, Ingo KM. Glioblastoma and Other Primary Brain Malignancies in Adults: A Review. JAMA 329 (2023): 574-587.
- Alejandro RC, José GFV, Júlia MM, et al. Glioblastoma Treatment: State-of-the-Art and Future Perspectives. International journal of molecular sciences 23 (2022): 7207.
- Jiawei L, Lili F, Yingmei L. Glioblastoma multiforme: Diagnosis, treatment, and invasion. Journal of biomedical research 37 (2022): 47-58.


 Impact Factor: * 4.2
Impact Factor: * 4.2 Acceptance Rate: 72.62%
Acceptance Rate: 72.62%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 