The Differences among Full Sternotomy, Partial Sternotomy, and Right Infra-axillary Thoracotomy with Stonehenge Technique for Aortic Valve Surgery
Yasushi Tashima*, Ryo Fujita, Taichi Sano, Noriyuki Nakamura, Koichi Adachi, Naoyuki Kimura, Atsushi Yamaguchi
1Department of Cardiovascular Surgery, Yokosuka General Hospital Uwamachi, Kanagawa, Japan
²Department of Cardiovascular Surgery, Saitama Medical Center, Jichi Medical University, Saitama, Japan
3Department of General Surgery, Yokosuka Kyosai Hospital, Kanagawa, Japan
*Corresponding Author: Yasushi Tashima, Department of Cardiovascular Surgery, Yokosuka General Hospital Uwamachi, Kanagawa, Japan
Received: 07 April 2023; Accepted: 17 April 2023; Published: 25 April 2023
Article Information
Citation: Yasushi Tashima, Ryo Fujita, Taichi Sano, Noriyuki Nakamura, Koichi Adachi, Naoyuki Kimura, Atsushi Yamaguchi. The Differences among Full Sternotomy, Partial Sternotomy, and Right Infra-axillary Thoracotomy with Stonehenge Technique for Aortic Valve Surgery. Journal of Surgery and Research. 6 (2023): 156-166.
View / Download Pdf Share at FacebookAbstract
Background: The surgical outcomes of trans-right axillary aortic valve replacement (AVR) with Stonehenge technique (SHAVR), which involves in pulling the heart closer to the right chest wall with retraction sutures of the pericardium to improve the surgical view, remains unknown although the partial sternotomy (PAVR) is widely recognized as a minimally invasive approach for AVR. We evaluated the surgical outcomes of the respective approaches compared to the conventional approach (CAVR).
Methods: A retrospective analysis of 137 consecutive patients who underwent isolated and initial AVR was performed at our institution between January 2009 and December 2020. After matching propensity scores with preoperative characteristics, surgical outcomes were compared between the two groups (PAVR vs. CAVR: n = 22 each, SHAVR vs. CAVR: n = 28 each).
Results: The SHAVR group did not show any significant differences in operative time, aortic cross-clamp time, CPB time, postoperative complications, and hospital death compared with the CAVR group. The length of hospital stay was likely to be shorter in the SHAVR group and the PAVR group than in the CAVR group (P = 0.043, P = 0.047). However, in the PAVR group, operative time, aortic cross-clamp time, and CPB time were significantly longer than in the CAVR group (P = 0.029, P = 0.015, P = 0.003), although there were no significant differences in postoperative complications and hospital death. Based on Multivariate risk analysis, PAVR (in comparison to SHAVR) was an independent risk factor for more than 2 hours of prolonged CPB time (P = 0.034).
Conclusion: These findings suggest that SHAVR can be a safe technique and has cosmetic benefits and a faster CPB time than PAVR.
Keywords
<p>Minimally invasive surgery, AVR, Right infra-axillary thoracotomy</p>
Article Details
Introduction
Various techniques for minimally invasive cardiac surgery are being developed to avoid the full median sternotomy traditionally required for aortic valve replacement (AVR) [1-3]. AVR through partial sternotomy approach (PAVR) is the most widely used minimally invasive AVR approach (MIAVR) and has several advantages over conventional AVR (CAVR) with full sternotomy [4-7]. However, PAVR does not necessarily have cosmetic advantages over the standard median sternotomy because anterior chest wounds can be easily recognized [8-12]. Furthermore, potential complication risks, such as sternal infection and internal thoracic artery injury, still possible with the partial sternotomy [13-16]. On the other hand, AVR through a small right infra-axillary thoracotomy has more cosmetic advantages than other MIAVR methods, such as partial sternotomy and right anterior mini-thoracotomy approaches [17]. Moreover, it enables effective early social reintegration because it does not require any sternotomy or postoperative exercise limits. However, AVR through the right infra-axillary thoracotomy may be considered a more complicated surgical procedure than other MIAVR since the distance between the skin wound and the ascending aortic root in the infra-axillary thoracotomy is much deeper than in other procedures. It may lead to prolonged operative time and time on cardiopulmonary bypass (CPB) [5,18,19]. However, trans-right infra-axillary AVR with the Stonehenge technique (SHAVR) reportedly improves the surgical field by pulling the heart closer to the right chest wall with retraction sutures of the pericardium [20] (Figure 1). It can reduce operative time and CPB time [18]. There are limited reports on the differences in the surgical outcomes among CAVR, PAVR, and SHAVR. We compared CAVR with PAVR, and SHAVR by using propensity score matching to reveal the objective benefits of PAVR, and SHAVR at a single center. Furthermore, we conducted a multivariate analysis in MIAVR (PAVR and SHAVR) to determine the factors that may influence the length of time that a CPB is used.
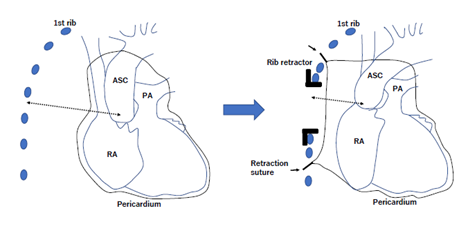
Figure 1: Schema of aortic valve replacement with Stonehenge technique
In the example illustrated, the thoracotomy incision is made through the fourth intercostal space. The pericardium is pulled up using several braided polyester sutures to put the ascending aorta and aortic root close to the chest wall. In the schema, the retraction sutures and the distance from the ascending aorta to the fourth intercostal incision are shown by arrow and broken arrow, respectively. ASC, ascending aorta; RA, right atrium; PA, pulmonary artery
Materials and Methods
Patients
A retrospective analysis was performed on 137 consecutive patients who underwent isolated and initial AVR at Yokosuka General Hospital Uwamachi from January 2009 to December 2020. Full sternotomy (CAVR), partial sternotomy (PAVR), and trans-right axillary AVR with Stonehenge technique (SHAVR) were performed in 67 (49%), 31 (23%), and 39 patients (28%), respectively. The choice of the approach was left to the surgeon’s preference. The following conditions were excluded from SHAVR: previous pneumectomy, ascending aorta enlargement (diameter > 50, shaggy aorta, difficulty of differential lung ventilation, deformity of the thoracic cage, and significant anatomical shift of aortic root to the left side. Patients who needed an emergency AVR because of endocarditis were excluded if they had a periannular abscess requiring annulus implantation and reconstruction with pericardium. With propensity score matching, the patients were adjusted with age, gender, comorbidity, New York Heart Association (NYHA) class, preoperative hemoglobin, and preoperative EF. Well-matched 22 (PAVR vs. CAVR) and 28 (SHAVR vs. CAVR) pairs were evaluated for surgical outcomes. Our selection process and the number of selected patients are shown in figure 2. The Institutional Review Board approved this retrospective observational study. The approval included a waiver of informed consent.
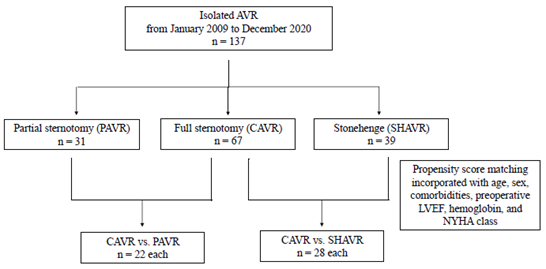
Figure 2: Patient selection flowchart
AVR, aortic valve replacement; CAVR, conventional AVR; LVEF, left ventricular ejection fraction; PAVR, AVR through partial sternotomy approach; SHAVR, trans-right infra-axillary AVR with Stonehenge technique
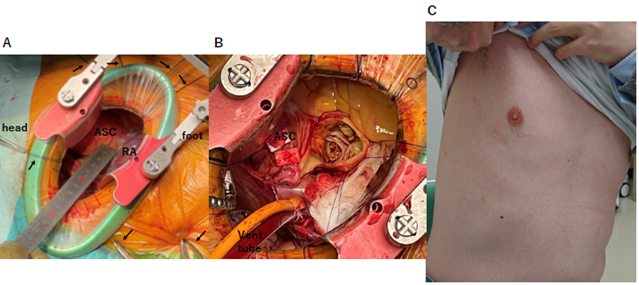
Figure 3: Representative pictures of trans-right axillary AVR with Stonehenge technique (SHAVR)
(A), (B), and (C) depict representative intraoperative and postoperative pictures from the patients in the SHAVR group. (A) After pulling the heart closer to the right chest wall with retraction sutures of pericardium, the distance between the fourth intercostal incision and ascending aorta was more shorten than before. The retraction sutures are shown by arrows in the picture. (B) The good surgical view of aortic valve was shown in the intraoperative picture of the Stonehenge technique, after established cardiopulmonary bypass and incised ascending aorta. (C) The right infra-axillary thoracotomy approach had better cosmetic results than traditional approaches through the anterior chest wall.
ASC, ascending aorta; RA, right atrium; SHAVR, trans-right infra-axillary AVR with Stonehenge technique
Data collection
Medical records and preoperative examinations were used to collect the variables. Preoperative characteristics, such as sex, body mass index (BMI), NYHA classification, European System for Cardiac Operative Risk Evaluation (EuroSCORE II) scores, and comorbidities, including hyperlipidemia, hypertension, diabetes mellitus, chronic kidney disease (CKD), peripheral arterial disease, ischemic heart disease, old stroke, chronic obstructive pulmonary disease, and atrial fibrillation, were compared between the groups [21-23]. Anemia was defined as preoperative hemoglobin (Hb) <13 g/dL and <12 g/dL in males and females. CKD was defined as estimated glomerular filtration rate (eGFR) < 60 ml/min/1.73m2 in preoperative blood test. Surgical variables, including operation time, CPB time, surgical procedure, intensive care unit (ICU) and hospital stay, and hospital death, and postoperative complications, such as stroke, pneumonia, mediastinitis, bleeding, postoperative aortic regurgitation (≥moderate), tracheotomy, and postoperative hemodialysis, were also compared between the groups.
Surgical procedure
Conventional surgical aortic valve replacement was performed in all procedures without using a suture less aortic valve prosthesis.
Stonehenge technique
SHAVR was performed as described in previous reports [18,20]. An 8 cm skin incision was made in the right anterior axillary line along the edge of the pectoralis major muscle, followed by the fourth or third intercostal thoracotomy. CPB was established via the subclavian artery or femoral artery for arterial cannulation and the femoral vein for venous cannulation. A longitudinal incision was made on the pericardium 2-3cm away from the phrenic nerve. The pericardium was pulled up using several braided polyester sutures to put the ascending aorta and aortic root close to the chest wall according to the references [20], which improved the surgical view shortening the distance from the aortic root to the chest wall. Aortic cross-clamping was performed using a Cygnet Flexible Clamp (Vitalitec Inc., Plymouth, MA, USA) from within the wound. To protect myocardium, cold blood cardioplegia was administered in an antegrade fashion into the aortic root and the coronary ostia approximately every 20 min (Figure 3).
Partial sternotomy
Partial upper or lower sternotomy was performed standardized [4,24,25]. The 8 cm midline skin incision was made. The upper or lower sternotomy was chosen based on each patient’s anatomical position of the aortic valve and surgeons’ preference (upper sternotomy; n =24 [77.4%], lower sternotomy; n = 7 [22.6%]). The sternum was incised from the sternal notch down to the left or right fourth intercostal space during an upper sternotomy. However, in lower sternotomy, the sternum was incised from the left or right second intercostal space to the xiphoid. For CPB, the ascending aorta, subclavian artery, or femoral artery was used for arterial cannulation. The right atria or femoral vein was used for venous cannulation. The left ventricle was vented through a small cannula placed in the right upper pulmonary vein. Through the incision, the aortic cross-clamp was applied. Cold blood cardioplegia was administered for myocardial protection in an antegrade fashion into the aortic root and the coronary ostia approximately every 20 min.
Conventional approach
The conventional full sternotomy was performed. CPB was established with the ascending aorta, subclavian artery, or femoral artery for arterial cannulation and the right atria or femoral vein for venous cannulation. For myocardial protection, cold blood cardioplegia was administered into the aortic root in an antegrade fashion and the coronary ostia approximately every 20 min.
Statistical analysis
Continuous data were expressed as the median (25–75 interquartile range). The Mann–Whitney U test was used to compare continuous data between groups in this study. Categorical data are expressed as frequencies (%) and evaluated by using the Chi-square test or Fisher’s exact test. Propensity score methodology was adopted to reduce the confounding in the statistical comparison of surgical outcomes in two groups by accounting for differences in baseline patient characteristics, using a 1:1 nearest-neighbor-matching algorithm with a ±0.2 caliper and no replacement. It produced 22 (PAVR vs. CAVR) and 28 (STAVR vs. CAVR) pairs of propensity score-matched observations. The propensity score was calculated using a multivariate logistic regression model with an indication for the selection of patients for the two groups, using the preoperative variables. The preoperative patient variables considered clinically relevant were included as explanatory covariates, namely age, comorbidities (hypertension, hyperlipidemia, diabetes mellitus, CKD, peripheral arterial disease, ischemic heart disease, atrial fibrillation, chronic obstructive pulmonary disease, and old stroke), preoperative hemoglobin, preoperative LVEF, and NYHA class. The risk factors for prolonged CPB time > 2 h and extended hospital stay of more than 2 weeks in MIAVR (SHAVR and PAVR) groups were included in the univariate analysis, and any variable with a P-value ≤ 0.1 was incorporated in the multivariate logistic regression model. All statistical analyses were performed using EZR (Saitama Medical Center, Jichi Medical University, Saitama, Japan) or Prism version 8.0 (GraphPad Software, San Diego, CA, USA). A P-value < 0.05 was considered statistically significant.
Result
PAVR vs. CAVR
Preoperative patient characteristics in the unmatched cohort of PAVR (n = 31) vs. CAVR (n = 67) are shown in table 1. The hyperlipidemia and NYHA III class frequencies in the CAVR group were more than the PAVR group (P = 0.051, P = 0.066. respectively). NYHA I class in the PAVR group was significantly more frequent than the CAVR group (P = 0.047). Propensity score matching produced 22 (PAVR vs. CAVR) matched pairs. Table 1 lists comparisons of preoperative patient backgrounds after propensity score matching. There were no significant differences in all covariates and the models fitted well. Surgical outcomes in matched comparison (PAVR vs. CAVR) are shown in table 2. Intriguingly, in the PAVR group, operative time, aortic cross-clamp time, and CPB time were longer than CAVR group (223 [200-268] min vs. 261 [241-288] min, P = 0.029; 90 [75-101] min vs. 109 [89-131] min, P = 0.015; 112 [95-116] min vs. 136 [119-156] min, P = 0.003; respectively), although there were no significant differences in transfusion, prosthetic valve size, incubation time > 48 h, postoperative complications, and hospital death within 30 days between CAVR and PAVR groups. The duration of hospital stay was significantly shorter in the PAVR group than in the CAVR group (P = 0.047).
SHAVR vs. CAVR
Preoperative patient characteristics in the unmatched cohort of SHAVR (n = 39) vs. CAVR (n = 67) are shown in table 3. CKD, NYHA III, NYHA IV class, and Euro Score II were more frequent in the CAVR group than in the SHAVR group (P = 0.103, P = 0.022, P = 0.046, P < 0.001, respectively), and NYHA I and II classes in the SHAVR group were more frequent than in the CAVR group (P = 0.035, P = 0.07, respectively). Propensity score matching produced 28 (SHAVR vs. CAVR) matched pairs. A comparison of patient backgrounds based on propensity scores can be found in table 3. There were no significant differences in all covariates, and the models fitted well. Surgical outcomes in matched comparison (SHAVR vs. CAVR) are shown in table 4. Compared to the CAVR group, there were no significant differences in operative time, aortic cross-clamp time, CPB time, prosthetic valve size, incubation time >48 h, postoperative complications, and hospital death within 30 days in the SHAVR group. However, the duration of the hospital stay was likely to be shorter in the SHAVR groups than in the CAVR group (P = 0.043).
Risk factors for prolonged CPB time of more than 2 hours and an extended hospital stay of more than 2 weeks in MIAVR (SHAVR and PAVR groups) groups
Univariate and multivariate analyses were performed to assess which preoperative factors impact prolonged CPB time of more than 2 hours in MIAVR groups. The prolonged CPB time estimated by univariate analysis was negatively affected by NYHA III or IV status, CKD, severe aortic stenosis, and PAVR (vs. SHAVR) (Table 5). Multivariate risk analysis revealed that severe aortic stenosis and PAVR (vs. SHAVR) were independent predictors of the prolonged CPB time in the MIAVR groups [severe aortic stenosis: P = 0.034, odds ratio 4.69, 95% CI: 1.13–19.5; PAVR (vs. SHAVR): P = 0.009, odds ratio 5.04, 95% CI: 1.5–17].
In the same way, to assess which preoperative factors impact extended hospital stay of more than 2 weeks in the MIAVR groups, univariate and multivariate analyses were performed. The extended hospital stay estimated by the univariate analysis was negatively impacted by anemia, age > 75 years old, BMI < 18.5 kg/m2, NYHA III or IV class, preoperative albumin < 3.5 g/dl, severe aortic stenosis, and postoperative complications (Supplemental Table). Multivariate risk analysis revealed that preoperative albumin < 3.5 g/dl and postoperative complications were independent predictors of the extended hospital stay in the MIAVR groups (preoperative albumin < 3.5g/dl: P = 0.03, odds ratio 10.1, 95% CI: 1.22–83.6; postoperative complications: P = 0.008, odds ratio 30.4, 95% CI: 2.46–376). PAVR (vs. SHAVR) was not the predictor of the extended hospital stay in the MIAVR groups.
|
Characteristics |
Unmatched comparison |
Matched comparison |
||||
|
CAVR |
PAVR |
P value |
CAVR |
PAVR |
P value |
|
|
(n=67) |
(n=31) |
(n=22) |
(n=22) |
|||
|
Age |
79 (74.5-82) |
81 (75-85) |
0.194 |
80.5 (75-85.5) |
82 (74-85) |
0.879 |
|
BMI |
22.4 (20.5-24.7) |
21.5 (19.3-22.7) |
0.134 |
21.5 (20.7-23) |
22.1 (20-23.3) |
0.935 |
|
Female |
44 (65.7%) |
22 (71%) |
0.65 |
13 (59.1%) |
15 (68.2%) |
0.755 |
|
Comorbidity |
||||||
|
Hyperlipidemia |
34 (50.7%) |
9 (29%) |
0.051 |
8 (36.4%) |
9 (40.9%) |
> 0.99 |
|
Hypertension |
48 (71.6%) |
23 (74.2%) |
> 0.99 |
16 (72.7%) |
16 (72.7%) |
> 0.99 |
|
Diabetes mellitus |
14 (20.9%) |
6 (19.4%) |
> 0.99 |
3 (13.6%) |
4 (18.2%) |
> 0.99 |
|
Chronic kidney disease |
43 (64.2%) |
20 (64.5%) |
> 0.99 |
11 (50%) |
14 (63.6%) |
0.543 |
|
Peripheral arterial disease |
3 (4.5%) |
4 (12.9%) |
0.203 |
0 (0%) |
1 (4.5%) |
> 0.99 |
|
Ischemic heart disease |
14 (20.9%) |
7 (22.6%) |
> 0.99 |
6 (27.3%) |
5 (22.7%) |
> 0.99 |
|
Old stroke |
7 (10.4%) |
2 (6.5%) |
0.725 |
2 (9.1%) |
2 (9.1%) |
> 0.99 |
|
Chronic obstructive pulmonary disease |
1 (1.5%) |
1 (3.2%) |
0.535 |
1 (4.5%) |
0 (0%) |
> 0.99 |
|
Atrial fibrillation |
10 (14.9%) |
3 (9.7%) |
0.75 |
3 (13.6%) |
3 (13.6%) |
> 0.99 |
|
Emergency |
3 (4.5%) |
2 (6.5%) |
> 0.99 |
0 (0%) |
1 (4.5%) |
> 0.99 |
|
Infective endocarditis |
3 (4.5%) |
0 (0%) |
0.549 |
0 (0%) |
0 (0%) |
> 0.99 |
|
Severe aortic stenosis |
50 (74.6%) |
22 (71%) |
0.806 |
17 (77.3%) |
16 (72.7%) |
> 0.99 |
|
Preoperative LVEF (%) |
63 (49.5-71.5) |
64 (54.5-71.5) |
0.734 |
64 (49.8-71.3) |
62.5 (54.8-68) |
0.91 |
|
NYHA class |
||||||
|
1 |
5 (7.5%) |
7 (22.6%) |
0.047 |
3 (13.6%) |
2 (9.1%) |
> 0.99 |
|
2 |
30 (44.8%) |
13 (41.9%) |
0.83 |
9 (40.9%) |
11 (50%) |
0.763 |
|
3 |
18 (26.9%) |
3 (9.7%) |
0.066 |
6 (27.3%) |
3 (13.6%) |
0.457 |
|
4 |
14 (20.9%) |
8 (25.8%) |
0.61 |
4 (18.2%) |
6 (27.3%) |
0.721 |
|
EuroSCORE II |
2.9 (1.7-4) |
2.7 (2-4.9) |
0.598 |
2.8 (1.6-8.1) |
2.8 (2.1-5.1) |
0.824 |
AVR, aortic valve replacement; BMI, body mass index; CAVR, conventional AVR; LVEF, left ventricular ejection fraction; PAVR, AVR through partial sternotomy approach
Table 1: Preoperative clinical characteristics (CAVR vs. PAVR)
|
Surgical Outcomes |
Matched comparison |
||
|
CAVR |
PAVR |
P value |
|
|
(n=22) |
(n=22) |
||
|
Prosthetic valve size (mm) |
20 (19-21) |
19 (19-23) |
0.875 |
|
CPB Time (minutes) |
112 (95-116) |
136 (119-156) |
0.003 |
|
Aortic Cross-clamp Time (minutes) |
90 (75-101) |
109 (89-131) |
0.015 |
|
Operation Time (minutes) |
223 (200-268) |
261 (241-288) |
0.029 |
|
Blood transfusion (unit) |
4 (5-7.5) |
6 (4-8) |
0.137 |
|
Complications |
|||
|
Stroke |
0 (0%) |
0 (0%) |
> 0.99 |
|
Pneumonia |
2 (9.1%) |
1 (4.5%) |
> 0.99 |
|
Mediastinitis |
1 (4.5%) |
2 (9.1%) |
0.607 |
|
Postoperative hemodialysis |
1 (4.5%) |
0 (0%) |
> 0.99 |
|
Bleeding |
0 (0%) |
0 (0%) |
> 0.99 |
|
Tracheotomy |
1 (4.5%) |
0 (0%) |
> 0.99 |
|
Postoperative AR (?moderate) |
0 (0%) |
1 (4.5%) |
> 0.99 |
|
Incubation time (> 48 hours) |
2 (9.1%) |
1 (4.5%) |
> 0.99 |
|
ICU Stay (days) |
5 (3.3-5.8) |
4 (3-5) |
0.245 |
|
Hospital Stay (days) |
16 (13-22.8) |
11 (8-18.8) |
0.047 |
|
Hospital Death (within 30 days) |
0 (0%) |
0 (0%) |
> 0.99 |
|
Postoperative LVEF (%) |
61.5 (57.3-69.3) |
65 (55-68) |
0.681 |
AR, aortic regurgitation; AVR, aortic valve replacement; CAVR, conventional AVR; CPB, cardiopulmonary bypass; ICU, intensive care unit; LVEF, left ventricular ejection fraction; PAVR, AVR through partial sternotomy approach
Table 2. Surgical outcomes in the matched cohort (CAVR vs. PAVR)
|
Characteristics |
Unmatched comparison |
Matched comparison |
||||
|
CAVR |
SHAVR |
P value |
CAVR |
SHAVR |
P value |
|
|
(n=67) |
(n=39) |
(n=28) |
(n=28) |
|||
|
Age |
79 (74.5-82) |
77 (71.5-84) |
0.687 |
75.5 (70-82.3) |
76 (73-82.5) |
0.472 |
|
BMI |
22.4 (20.5-24.7) |
22.1 (20.9-25.6) |
0.694 |
22.4 (20.6-24.7) |
22.6 (21.1-26) |
0.572 |
|
Female |
44 (65.7%) |
25 (64.1%) |
>0.99 |
19 (67.9%) |
17 (60.7%) |
0.781 |
|
Comorbidity |
||||||
|
Hyperlipidemia |
34 (50.7%) |
16 (41%) |
0.42 |
13 (46.4%) |
12 (42.9%) |
>0.99 |
|
Hypertension |
48 (71.6%) |
26 (66.7%) |
0.663 |
20 (71.4%) |
19 (67.9%) |
0.789 |
|
Diabetes mellitus |
14 (20.9%) |
8 (20.5%) |
>0.99 |
8 (28.6%) |
7 (25%) |
>0.99 |
|
Chronic kidney disease |
43 (64.2%) |
18 (46.2%) |
0.103 |
11 (39.3%) |
14 (50%) |
0.591 |
|
Peripheral arterial disease |
3 (4.5%) |
0 (0%) |
0.296 |
0 (0%) |
0 (0%) |
>0.99 |
|
Ischemic heart disease |
14 (20.9%) |
4 (10.3%) |
0.189 |
3 (10.7%) |
4 (14.3%) |
>0.99 |
|
Old stroke |
7 (10.4%) |
2 (5.1%) |
0.49 |
2 (7.1%) |
1 (3.6%) |
>0.99 |
|
Chronic obstructive pulmonary disease |
1 (1.5%) |
0 (0%) |
>0.99 |
0 (0%) |
0 (0%) |
>0.99 |
|
Atrial fibrillation |
10 (14.9%) |
3 (7.7%) |
0.365 |
5 (17.9%) |
3 (10.7%) |
0.705 |
|
Emergency |
3 (4.5%) |
0 (0%) |
0.296 |
0 (0%) |
0 (0%) |
>0.99 |
|
Infective endocarditis |
3 (4.5%) |
1 (2.6%) |
>0.99 |
0 (0%) |
0 (0%) |
>0.99 |
|
Severe aortic stenosis |
50 (74.6%) |
29 (74.4%) |
>0.99 |
24 (85.7%) |
22 (78.6%) |
0.729 |
|
Preoperative LVEF (%) |
63 (49.5-71.5) |
68 (57-72) |
0.241 |
65.5 (57.8-74.3) |
67.5 (57.5-70.3) |
0.676 |
|
NYHA class |
||||||
|
1 |
5 (7.5%) |
9 (23.1%) |
0.035 |
4 (14.3%) |
7 (25%) |
0.503 |
|
2 |
30 (44.8%) |
25 (64.1%) |
0.07 |
20 (71.4%) |
16 (57.1%) |
0.403 |
|
3 |
18 (26.9%) |
3 (7.7%) |
0.022 |
1 (3.6%) |
3 (10.7%) |
0.611 |
|
4 |
14 (20.9%) |
2 (5.1%) |
0.046 |
3 (10.7%) |
2 (7.1%) |
>0.99 |
|
EuroSCORE II |
2.9 (1.7-4) |
1.7 (1.1-2.6) |
<0.001 |
1.9 (1.4-2.7) |
1.9 (1.1-2.7) |
0.793 |
AVR, aortic valve replacement; BMI, body mass index; CAVR, conventional AVR; LVEF, left ventricular ejection fraction; SHAVR, trans-right infra-axillary AVR with Stonehenge technique
Table 3: Preoperative clinical characteristics (CAVR vs. SHAVR)
|
Surgical Outcomes |
Matched comparison |
||
|
CAVR |
SHAVR |
P value |
|
|
(n=28) |
(n=28) |
||
|
Prosthetic valve size (mm) |
19 (19-21) |
21 (20-21) |
0.161 |
|
CPB Time (minutes) |
115 (103-129) |
113 (103-126) |
0.743 |
|
Aortic Cross-clamp Time (minutes) |
94 (81-104) |
94 (85-102) |
0.799 |
|
Operation Time (minutes) |
240 (212-260) |
223 (210-260) |
0.528 |
|
Blood transfusion (unit) |
4 (0-6) |
2 (0-6) |
0.799 |
|
Complications |
|||
|
Stroke |
0 (0%) |
0 (0%) |
>0.99 |
|
Pneumonia |
1 (3.6%) |
2 (7.1%) |
>0.99 |
|
Mediastinitis |
2 (7.1%) |
0 (0%) |
0.491 |
|
Postoperative hemodialysis |
0 (0%) |
1 (3.7%) |
>0.99 |
|
Bleeding |
0 (0%) |
0 (0%) |
>0.99 |
|
Tracheotomy |
2 (6.1%) |
2 (6.1%) |
>0.99 |
|
Postoperative AR (? moderate) |
0 (0%) |
1 (3.7%) |
0.491 |
|
Incubation time (> 48 hours) |
2 (7.1%) |
2 (7.1%) |
>0.99 |
|
ICU Stay (days) |
4 (3-5) |
4 (3-8) |
0.97 |
|
Hospital Stay (days) |
15 (12-19.3) |
12 (10.8-14.3) |
0.043 |
|
Hospital Death (within 30 days) |
0 (0%) |
0 (0%) |
>0.99 |
|
Postoperative LVEF (%) |
64.5 (61-69.3) |
66 (61-70) |
0.899 |
AR, aortic regurgitation; AVR, aortic valve replacement; CAVR, conventional AVR; CPB, cardiopulmonary bypass; ICU, intensive care unit; LVEF, left ventricular ejection fraction; SHAVR, trans-right infra-axillary AVR with Stonehenge technique
Table 4: Surgical outcomes in the matched cohort (CAVR vs. SHAVR)
|
time >2 hours in MIAVR |
||||
|
Variables |
Univariable |
Multivariable |
||
|
OR (95% CI) |
P value |
OR (95% CI) |
P value |
|
|
Prolonged CPB time >2 hours |
||||
|
Age >75 |
1.36 (0.51-3.62) |
0.534 |
- |
- |
|
Female |
0.96 (0.35-2.6) |
0.936 |
- |
- |
|
BMI <18.5 kg/m2 |
1.83 (0.47-7.17) |
0.384 |
- |
- |
|
BMI >28 kg/m2 |
3.6 (0.36-36.4) |
0.278 |
- |
- |
|
NYHA 3,4 |
4.71 (1.34-16.6) |
0.016 |
2.6 (0.55-12.3) |
0.229 |
|
Preoperative albumin <3.5 g/dl |
0.71 (0.18-2.78) |
0.626 |
- |
- |
|
Anemia |
1.22 (0.47-3.16) |
0.678 |
- |
- |
|
Chronic kidney disease |
2.62 (0.99-6.95) |
0.052 |
2.81 (0.88-8.98) |
0.08 |
|
Low LVEF <50% |
0.59 (0.16-2.24) |
0.439 |
- |
- |
|
Severe aortic stenosis |
3.82 (1.2-12.1) |
0.023 |
4.69 (1.13-19.5) |
0.034 |
|
Blood transfusion (+) during surgery |
0.55 (0.19-1.6) |
0.274 |
- |
- |
|
PAVR (vs. SHAVR) |
4.72 (1.71-13) |
0.003 |
5.04 (1.5-17) |
0.009 |
AVR, aortic valve replacement; BMI, body mass index; CPB, cardiopulmonary bypass; LVEF, left ventricular ejection fraction; MIAVR, minimally invasive AVR; PAVR, AVR through partial sternotomy approach; SHAVR, trans-right infra-axillary AVR with Stonehenge technique
Table 5: Univariate and multivariate analyses of the factors related with prolonged CPB time >2 hours in MIAVR
Discussion
This study illustrated the following: 1) The propensity score when matched with preoperative characteristics, showed that the PAVR group was associated with longer aortic cross-clamp time, CPB time, and operation time as compared to the CAVR group. However, interestingly, there were no significantly difference between the SHAVR group and the CAVR group; 2) In matched cohort, hospital stay was significantly shorter in the PAVR and the SHAVR groups as compared to the CAVR group, although the frequencies of postoperative complications and hospital mortality were nearly same when compared to the CAVR group; 3) Multivariate risk analysis revealed that PAVR (vs. SHAVR) was an independent risk factor of prolonged CPB time of more than 2 hours in the MIAVR groups; 4) Preoperative albumin < 3.5 g/dl and postoperative complications were marked down as independent risk factors for a prolonged hospital stay of more than 2 weeks in the MIAVR groups. The treatment of AVR by means of partial sternotomy, parasternal approach, and anterior mini-thoracotomy has been identified as an effective option to reduce blood transfusion and improve postoperative course [7,26-28]. However, these traditional minimally invasive approaches for AVR using anterior chest skin incisions do not always have cosmetic superiority over conventional median sternotomy. Because anterior chest wounds usually lead to hypertrophic scarring and can easily be recognized. Reportedly, AVR is done through a vertical infra-axillary thoracotomy and limited small skin incision with endoscopic assistance could be a cosmetically superior option for the selected patients undergoing AVR [17,20]. However, the distance between the incision and the ascending aorta in the infra-axillary thoracotomy approach is much deeper than any other MIAVR, which might restrict the surgical field and lead to prolonged CPB time and operative time. Interestingly, in the present study, we found no such inferiority in the CPB time and operative time in the SHAVR group over the CAVR group, although those in the PAVR group were found to have prolonged CPB time and operative time as compared to the CAVR group. It was revealed that the PAVR (vs. SHAVR) was an independent risk factor for prolonged CPB time in the MIAVR groups. These results suggested that the surgical field in the SHAVR approach may be better than the PAVR approach. Ghoreishi et al. [29] examined the surgical outcomes among CAVR, PAVR, and AVR with right anterior mini-thoracotomy in patients undergoing isolated primary AVR from the Society of Thoracic Surgeons database [29]. It was found that the aortic cross-clamp time, CPB time, and operative time were the longest in the patients with AVR treated via the right anterior mini-thoracotomy and shortest in those who underwent CAVR. Verifying this report, our findings suggested that SHAVR might be superior in terms of CPB time and operative time as compared to the anterior right mini-thoracotomy approach. The infra-axillary thoracotomy, which is incised more from the outside of the sternum than the right anterior mini-thoracotomy, validates the intercostal through the incision that is more widely open with the rib retractor. Furthermore, in SHAVR, pulling the heart closer to the right chest wall with retraction sutures improves the depth of the surgical view between the incision and the ascending aorta. Therefore, SHAVR can improve the depth and width of the surgical field and may shorten the CPB time and the operative time when compared with other MIAVR. The most common limitations of this study were its retrospective nature, a few number of participants, and single-center design, which are considered as the potential sources of bias. Furthermore, the difficulty in differential lung ventilation, previous pneumectomy, shaggy aorta, ascending aorta enlargement (diameter > 50 mm), deformity of the thoracic cage, and significant shift of aortic root to the left side were excluded for SHAVR group, which might cause selection bias. Further studies must focus on a larger number of patients and a greater emphasis on hemodynamic and biomechanical parameters. Our findings illustrated new significant insights into the differences in surgical outcomes among SHAVR, PAVR, and CAVR groups, and could potentially help the selection of appropriate approaches in the MIAVR groups.
Conclusion
After matching propensity scores with the preoperative characteristics, there was no significantly difference in CPB time between the SHAVR group and the CAVR group, although the PAVR group was associated with longer CPB time as compared to the CAVR group. Furthermore, hospital stay was likely to be shorter in the PAVR and the SHAVR groups as compared to the CAVR group, although the frequencies of postoperative complications and hospital mortality were nearly same. Multivariate risk analysis revealed that PAVR (vs. SHAVR) was an independent risk factor of prolonged CPB time of more than 2 hours in the MIAVR groups. Our findings in the present study suggested that SHAVR can be safely performed with not only a superior cosmetic advantage but also a shorter CPB time compared to PAVR.
Acknowledgements
The authors would like to thank Eigoexperts (www.eigoexperts.com) for the English language review.
Conflict of interests
The authors declare that there are no conflicts of interests.
Author contributions
Conception and design: YT, RF, NN, TS, KA, NK, and AY
Analysis and interpretation: YT, RF, NN, TS, KA, NK, and AY
Data collection: YT, RF, NN, and TS
Writing the article: YT
Critical revision of the article: YT, RF, NN, TS, KA, NK, and AY
Final approval of the article: YT, RF, NN, TS, KA, NK, and AY
Statistical analysis: YT, RF, NN, and TS
Obtained funding: Not applicable
Overall responsibility: YT
Date and number of IRB approval
Date: 2020/10/9
IRB number: 2020018
Data availability statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Funding
None
References
Supplemetary table
|
Variables |
Univariable |
|
Multivariable |
||
|
OR (95% CI) |
P value |
|
OR (95% CI) |
P value |
|
|
Extended hospital stay >2 weeks |
|||||
|
Age >75 |
4.39 (1.47-13) |
0.008 |
3.68 (0.81-16.7) |
0.09 |
|
|
Female |
2.39 (0.83-6.86) |
0.107 |
- |
- |
|
|
BMI <18.5 kg/m2 |
6.43 (1.26-33) |
0.026 |
1.29 (0.14-12.4) |
0.823 |
|
|
BMI >28 kg/m2 |
1.28 (0.17-9.61) |
0.813 |
- |
- |
|
|
NYHA 3,4 |
3.74 (1.13-12.3) |
0.03 |
1.25 (0.24-6.42) |
0.79 |
|
|
Preoperative albumin <3.5 g/dl |
6.43 (1.26-33) |
0.026 |
10.1 (1.22-83.6) |
0.03 |
|
|
Anemia |
2.43 (0.92-6.42) |
0.073 |
0.77 (0.19-3.05) |
0.708 |
|
|
Chronic kidney disease |
1.04 (0.4-2.68) |
0.934 |
- |
- |
|
|
Low LVEF <50% |
1.63 (0.45-5.95) |
0.458 |
- |
- |
|
|
Severe aortic stenosis |
4.7 (1.37-16) |
0.014 |
3.26 (0.59-18) |
0.175 |
|
|
Blood transfusion (+) during surgery |
1.52 (0.51-4.5) |
0.446 |
- |
- |
|
|
Postoperative complications (+) |
27.4 (3.33-226) |
0.002 |
30.4 (2.46-376) |
0.008 |
|
|
PAVR (vs. SHAVR) |
2.17 (0.83-5.68) |
0.115 |
- |
- |
|
AVR, aortic valve replacement; BMI, body mass index; CPB, cardiopulmonary bypass; LVEF, left
ventricular ejection fraction; MIAVR, minimally invasive AVR; PAVR, AVR through partial sternotomy
approach; SHAVR, trans-right infra-axillary AVR with Stonehenge technique
Supplemental Table: Univariate and multivariate analyses of the factors related with extended hospital stay >2 weeks in MIAVR
- Almeida AS, Ceron RO, Anschau F, et al. Conventional Versus Minimally Invasive Aortic Valve Replacement Surgery: A Systematic Review, Meta-Analysis, and Meta-Regression. Innovations (Phila). 17 (2022): 3-13.
- Condello I, Santarpino G, Speziale G. Minimally invasive aortic valve surgery: What approach shall I use? J Card Surg 37 (2022): 464.
- Di Bacco L, Miceli A, Glauber M. Minimally invasive aortic valve surgery. J Thorac Dis. 13 (2021): 1945-1959.
- Raja SG, Benedetto U, Amrani M. Aortic valve replacement through J-shaped partial upper sternotomy. J Thorac Dis 5 (2013): S662-S668.
- Shibata K, Matsushiro T, Murai Y. Comparison of Two Minimally Invasive Aortic Valve Replacement Approaches;Right Infra-axillary Thoracotomy versus Partial Sternotomy. Kyobu Geka 72 (2019): 327-331.
- Szwerc MF, Benckart DH, Wiechmann RJ, et al. Partial versus full sternotomy for aortic valve replacement. Ann Thorac Surg 68 (1998): 2209-2213.
- Welp HA, Herlemann I, Martens S, et al. Outcomes of aortic valve replacement via partial upper sternotomy versus conventional aortic valve replacement in obese patients. Interact Cardiovasc Thorac Surg 27 (2018): 481-486.
- Furukawa N, Kuss O, Emmel E, et al. Minimally invasive versus transapical versus transfemoral aortic valve implantation: A one-to-one-to-one propensity score-matched analysis. J Thorac Cardiovasc Surg 156 (2018): 1825-1834.
- Miceli A, Murzi M, Gilmanov D, et al. Minimally invasive aortic valve replacement using right minithoracotomy is associated with better outcomes than ministernotomy. J Thorac Cardiovasc Surg 148 (2014): 133-137.
- Murzi M, Cerillo AG, Bevilacqua S, et al. Traversing the learning curve in minimally invasive heart valve surgery: a cumulative analysis of an individual surgeon's experience with a right minithoracotomy approach for aortic valve replacement. Eur J Cardiothorac Surg 41 (2012): 1242-1246.
- Totsugawa T, Suzuki K, Hiraoka A, et al. Concomitant septal myectomy during minimally invasive aortic valve replacement through a right mini-thoracotomy for the treatment of aortic stenosis with systolic anterior motion of the mitral valve. Gen Thorac Cardiovasc Surg 65 (2017): 657-660.
- Young CP, Sinha S, Vohra HA. Outcomes of minimally invasive aortic valve replacement surgery. Eur J Cardiothorac Surg 53 (2018): ii19-ii23.
- Beckmann A, Funkat AK, Lewandowski J, et al. German Heart Surgery Report 2015: The Annual Updated Registry of the German Society for Thoracic and Cardiovascular Surgery. Thorac Cardiovasc Surg 64 (2016): 462-474.
- Ito T. Minimally invasive mitral valve surgery through right mini-thoracotomy: recommendations for good exposure, stable cardiopulmonary bypass, and secure myocardial protection. Gen Thorac Cardiovasc Surg 63 (2015): 371-378.
- Nishi H, Miyata H, Motomura N, et al. Propensity-matched analysis of minimally invasive mitral valve repair using a nationwide surgical database. Surg Today 45 (2015): 1144-1152.
- Sakaguchi T. Minimally invasive mitral valve surgery through a right mini-thoracotomy. Gen Thorac Cardiovasc Surg 64 (2016): 699-706.
- Ito T, Maekawa A, Hoshino S, et al. Right infraaxillary thoracotomy for minimally invasive aortic valve replacement. Ann Thorac Surg 96 (2013): 715-717.
- Yamazaki M, Yoshitake A, Takahashi T, et al. Stonehenge technique is associated with faster aortic clamp time in group of minimally invasive aortic valve replacement via right infra-axillary thoracotomy. Gen Thorac Cardiovasc Surg 66 (2018): 700-706.
- Tokoro M, Ito T, Maekawa A, et al. Trans-right axillary aortic valve replacement: propensity-matched comparison with standard sternotomy approach. Interact Cardiovasc Thorac Surg 25 (2017): 521-525.
- Yamazaki M, Kin H, Kitamoto S, et al. Efficacy of the Stonehenge Technique for Minimally Invasive Aortic Valve Replacement via Right Infraaxillary Thoracotomy. Ann Thorac Cardiovasc Surg 23 (2017): 45-48.
- Caraballo C, Desai NR, Mulder H, et al. Clinical Implications of the New York Heart Association Classification. J Am Heart Assoc 8 (2019): e014240.
- Yancy CW, Jessup M, Bozkurt B, et al. American College of Cardiology F and American Heart Association Task Force on Practice G. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation 128 (2013): 1810-1852.
- Nashef SA, Roques F, Sharples LD, et al. Euroscore II. Eur J Cardiothorac Surg 41 (2012): 734-744.
- Cosgrove DM, Sabik JF. Minimally invasive approach for aortic valve operations. Ann Thorac Surg 62 (1996): 596-597.
- Svensson LG. Minimal-access "J" or "j" sternotomy for valvular, aortic, and coronary operations or reoperations. Ann Thorac Surg 64 (1997): 1501-1503.
- Haunschild J, Van Kampen A, Von Aspern K, et al. Supracommissural replacement of the ascending aorta and the aortic valve via partial versus full sternotomy-a propensity-matched comparison in a high-volume centre. Eur J Cardiothorac Surg 61 (2022): 479-487.
- Balmforth D, Harky A, Lall K, et al. Is ministernotomy superior to right anterior minithoracotomy in minimally invasive aortic valve replacement? Interact Cardiovasc Thorac Surg 25 (2017): 818-821.
- Mikus E, Calvi S, Campo G, et al. Full Sternotomy, Hemisternotomy, and Minithoracotomy for Aortic Valve Surgery: Is There a Difference? Ann Thorac Surg 106 (2018): 1782-1788.
- Ghoreishi M, Thourani VH, Badhwar V, et al. Less-Invasive Aortic Valve Replacement: Trends and Outcomes From The Society of Thoracic Surgeons Database. Ann Thorac Surg 111 (2021): 1216-1223.


 Impact Factor: * 4.2
Impact Factor: * 4.2 Acceptance Rate: 72.62%
Acceptance Rate: 72.62%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 