Low Dose Dabigatran-Induced Severe Pulmonary Hemorrhage and Cardiogenic Shock, Salvaged by Reversal Agent and Extracorporeal Membrane Oxygenation
Article Information
Chih-Chun Fu1, Ying-Cheng Chen2, Bing-Jung Yang1, Ching-Pei Chen1,3*
1Department of Cardiovascular Medicine, Changhua Christian Hospital, Changhua, Taiwan
2Department of Cardiovascular Surgery, Changhua Christian Hospital, Changhua, Taiwan
3Department of Beauty Science and Graduate Institute of Beauty Science, Chienkuo Technology University, Changhua, Taiwan
*orresponding Author: Dr. Ching-Pei Chen, Department of Cardiovascular Medicine, Changhua Christian Hospital, No. 135, Nanxiao St., Changhua 500, Taiwan (R.O.C.)
Received: 26 May 2020; Accepted: 15 June 2020; Published: 10 July 2020
Citation: Chih-Chun Fu, Ying-Cheng Chen, Bing-Jung Yang, Ching-Pei Chen. Low Dose Dabigatran-Induced Severe Pulmonary Hemorrhage and Cardiogenic Shock, Salvaged by Reversal Agent and Extracorporeal Membrane Oxygenation. Archives of Clinical and Medical Case Reports 4 (2020): 658-663.
View / Download Pdf Share at FacebookAbstract
A 67-year-old man with newly diagnosed posterior mitral leaflet flail and atrial fibrillation was given dabigatran for stroke prevention upon admission. Within 12 hours of taking two doses of 110 mg dabigatran, the patient suffered from severe pulmonary hemorrhage, acute respiratory failure, and cardiogenic shock. He was rescued by a single dose of 5 g idarucizumab in addition to extracorporeal membrane oxygenation (ECMO) support. The patient recovered to stable hemodynamic status then successfully underwent surgical intervention for mitral valve repair. He was discharged 2 weeks after surgery.
Keywords
Atrial fibrillation; Dabigatran; Pulmonary hemorrhage; Cardiogenic shock; Idarucizumab
Atrial fibrillation articles, Dabigatran articles, Pulmonary hemorrhage articles, Cardiogenic shock articles, Idarucizumab articles
Atrial fibrillation articles Atrial fibrillation Research articles Atrial fibrillation review articles Atrial fibrillation PubMed articles Atrial fibrillation PubMed Central articles Atrial fibrillation 2023 articles Atrial fibrillation 2024 articles Atrial fibrillation Scopus articles Atrial fibrillation impact factor journals Atrial fibrillation Scopus journals Atrial fibrillation PubMed journals Atrial fibrillation medical journals Atrial fibrillation free journals Atrial fibrillation best journals Atrial fibrillation top journals Atrial fibrillation free medical journals Atrial fibrillation famous journals Atrial fibrillation Google Scholar indexed journals Dabigatran articles Dabigatran Research articles Dabigatran review articles Dabigatran PubMed articles Dabigatran PubMed Central articles Dabigatran 2023 articles Dabigatran 2024 articles Dabigatran Scopus articles Dabigatran impact factor journals Dabigatran Scopus journals Dabigatran PubMed journals Dabigatran medical journals Dabigatran free journals Dabigatran best journals Dabigatran top journals Dabigatran free medical journals Dabigatran famous journals Dabigatran Google Scholar indexed journals Pulmonary hemorrhage articles Pulmonary hemorrhage Research articles Pulmonary hemorrhage review articles Pulmonary hemorrhage PubMed articles Pulmonary hemorrhage PubMed Central articles Pulmonary hemorrhage 2023 articles Pulmonary hemorrhage 2024 articles Pulmonary hemorrhage Scopus articles Pulmonary hemorrhage impact factor journals Pulmonary hemorrhage Scopus journals Pulmonary hemorrhage PubMed journals Pulmonary hemorrhage medical journals Pulmonary hemorrhage free journals Pulmonary hemorrhage best journals Pulmonary hemorrhage top journals Pulmonary hemorrhage free medical journals Pulmonary hemorrhage famous journals Pulmonary hemorrhage Google Scholar indexed journals Cardiogenic shock articles Cardiogenic shock Research articles Cardiogenic shock review articles Cardiogenic shock PubMed articles Cardiogenic shock PubMed Central articles Cardiogenic shock 2023 articles Cardiogenic shock 2024 articles Cardiogenic shock Scopus articles Cardiogenic shock impact factor journals Cardiogenic shock Scopus journals Cardiogenic shock PubMed journals Cardiogenic shock medical journals Cardiogenic shock free journals Cardiogenic shock best journals Cardiogenic shock top journals Cardiogenic shock free medical journals Cardiogenic shock famous journals Cardiogenic shock Google Scholar indexed journals Cardiogenic articles Cardiogenic Research articles Cardiogenic review articles Cardiogenic PubMed articles Cardiogenic PubMed Central articles Cardiogenic 2023 articles Cardiogenic 2024 articles Cardiogenic Scopus articles Cardiogenic impact factor journals Cardiogenic Scopus journals Cardiogenic PubMed journals Cardiogenic medical journals Cardiogenic free journals Cardiogenic best journals Cardiogenic top journals Cardiogenic free medical journals Cardiogenic famous journals Cardiogenic Google Scholar indexed journals treatment articles treatment Research articles treatment review articles treatment PubMed articles treatment PubMed Central articles treatment 2023 articles treatment 2024 articles treatment Scopus articles treatment impact factor journals treatment Scopus journals treatment PubMed journals treatment medical journals treatment free journals treatment best journals treatment top journals treatment free medical journals treatment famous journals treatment Google Scholar indexed journals CT articles CT Research articles CT review articles CT PubMed articles CT PubMed Central articles CT 2023 articles CT 2024 articles CT Scopus articles CT impact factor journals CT Scopus journals CT PubMed journals CT medical journals CT free journals CT best journals CT top journals CT free medical journals CT famous journals CT Google Scholar indexed journals surgery articles surgery Research articles surgery review articles surgery PubMed articles surgery PubMed Central articles surgery 2023 articles surgery 2024 articles surgery Scopus articles surgery impact factor journals surgery Scopus journals surgery PubMed journals surgery medical journals surgery free journals surgery best journals surgery top journals surgery free medical journals surgery famous journals surgery Google Scholar indexed journals Oxygenation articles Oxygenation Research articles Oxygenation review articles Oxygenation PubMed articles Oxygenation PubMed Central articles Oxygenation 2023 articles Oxygenation 2024 articles Oxygenation Scopus articles Oxygenation impact factor journals Oxygenation Scopus journals Oxygenation PubMed journals Oxygenation medical journals Oxygenation free journals Oxygenation best journals Oxygenation top journals Oxygenation free medical journals Oxygenation famous journals Oxygenation Google Scholar indexed journals Idarucizumab articles Idarucizumab Research articles Idarucizumab review articles Idarucizumab PubMed articles Idarucizumab PubMed Central articles Idarucizumab 2023 articles Idarucizumab 2024 articles Idarucizumab Scopus articles Idarucizumab impact factor journals Idarucizumab Scopus journals Idarucizumab PubMed journals Idarucizumab medical journals Idarucizumab free journals Idarucizumab best journals Idarucizumab top journals Idarucizumab free medical journals Idarucizumab famous journals Idarucizumab Google Scholar indexed journals
Article Details
1. Introduction
Dabigatran is an effective non-Vitamin K antagonist oral anticoagulant (NOAC) that is used for prevention of stroke and systemic embolism in patients with non-valvular atrial fibrillation (NVAF). However, in trials and serial case reports, dabigatran has been associated with major bleeding events, including intracerebral hemorrhage, retroperitoneal bleeding and massive gastrointestinal bleeding. Currently, such major and life-threatening bleeding events are treated with idarucizumab, a high affinity and high specificity agent that to binds dabigatran, and rapidly reverses its anticoagulant activity.
2. Case Report
A 67-year-old man with a history of hypertension was admitted after months of intermittent chest tightness and palpitations. The patient denied exertional dyspnea and any bleeding tendency. His blood pressure was 107/70 mmHg, and body temperature was 36.1°C. On physical examination, no abnormalities were found except a grade III/VI systolic murmur at apex; the patient’s body weight was 62.6 kg (body mass index: 26 kg/m2). Electrocardiogram (ECG) showed atrial fibrillation with a heart rate of 108 beats per minute. Laboratory data revealed moderately impaired renal function (creatinine clearance [CrCl] 49.3 mL/min), hemoglobin: 16.8 g/dL, platelet count: 200,000/µL, prothrombin time (PT): 12 sec and activated partial thromboplastin time (aPTT): 36.7 sec. Echocardiography was arranged, which demonstrated flail of posterior mitral leaflet with severe mitral regurgitation (Figure 1) and severe tricuspid regurgitation.
The patient’s CHA?DS?-VASc score was 2 points (age 67, hypertension). He received 100 mg aspirin and 0.125 mg digoxin on the day of admission. One the same day, 110 mg dabigatran (Pradaxa) was given at 18:00. In the morning of following day, aspirin was discontinued, and second dose of 110 mg dabigatran was given at 09:00. He suffered from cough with blood-tinged sputum (Figure 2) after 30 minutes later after receiving second dose dabigatran.
Due to a suspected bleeding tendency, dabigatran was discontinued. However, the patient experienced progressive dyspnea with desaturation two hours later. Emergent intubation was performed due to impending hypoxemic respiratory failure. Follow-up laboratory data showed PT and aPTT in the normal ranges, without any evidence of anemia or thrombocytopenia. Chest X-ray revealed diffuse interstitial fluffy opacity shadows and consolidations of bilateral parahilar areas with mild pleural effusions (Figure 3).
Following intubation, the patient was transferred to a coronary care unit, where 5 g idarucizumab (Praxbind) was given intravenously. His condition quickly progressed to cardiogenic shock despite administration of a vasopressor and fluid resuscitation. Veno-Arterial (VA-) mode ECMO was applied. By that time, pulmonary hemorrhage was ceased, and the chest X-ray had also improved. The patient received robotic-assisted mitral valve repair with neo-chordae creation and annuloplasty for flail of posterior mitral leaflet one week later. After surgical intervention, his cardiac and pulmonary functions both improved. The patient was successfully weaned off ECMO and he was extubated one week after the operation with stable hemodynamic status (Figure 4).
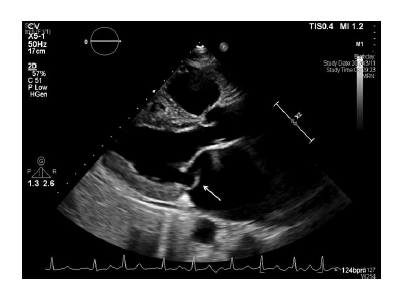
Figure 1: Flail of posterior mitral leaflet (white arrow).
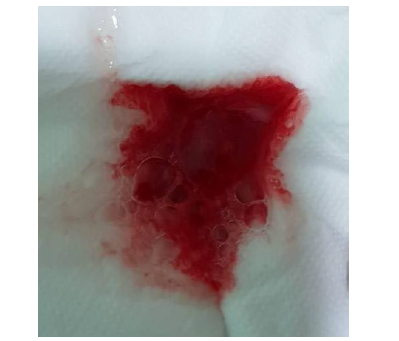
Figure 2: Blood-tinged sputum after receiving second low dose of dabigatran.
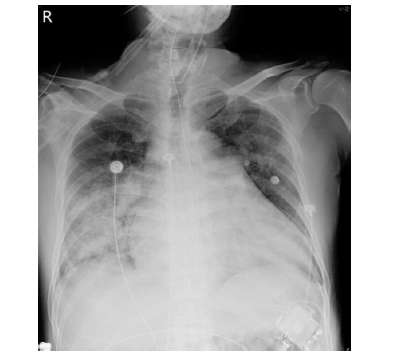
Figure 3: Diffuse interstitial fluffy opacity consistent with clinical pulmonary hemorrhage.
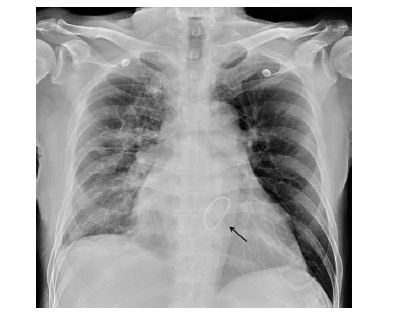
Figure 4: Status following mitral valve repair (black arrow) and extubation.
3. Discussion
NOACs are currently prescribed worldwide to prevent stroke in patients with NVAF at the expense of increased risk of bleeding. The CHA?DS?-VASc and HAS-BLED scores are used to respectively evaluate the risks of stroke and NOAC-related bleeding; a higher HAS-BLED score indicates risk of NOAC-related bleeding. Previous randomize studies revealed the rate of dabigatran-associated major bleeding was 2.71% per year in patients receiving 110 mg and 3.11% per year in those receiving 150 mg [1, 2]. According to its pharmacokinetic profile, dabigatran exhibits a peak plasma concentration (Cmax) 1.5 to2 hr after oral administration, which means bleeding is most likely to occur approximately two hours after taking the medication [3]. Current data supports several risk factors for bleeding after NOAC administration, including renal insufficiency, concomitant use of P-glycoprotein inhibitors (e.g., verapamil or diltiazem) and extreme body weight [4, 5]. Although a reduced dose of dabigatran (75 mg orally twice daily) is only recommend for patients with CrCl of 15 to 30 mL/min, dabigatran-receiving patients with CrCl <50 mL/min have a more than two-fold increased risk of major bleeding compared to those with CrCl ≥80 mL/min [6]. Since our patient has a HAS-BLED score of only 1 point (> 65 years of age), the specific factor likely to have predisposed him to bleeding was moderate renal impairment. Also, it is possible that receiving one dose of aspirin might have contributed to the development of hemorrhage [7].
To our knowledge, there has been no randomized trial or case report discussing the time interval between starting NOAC treatment and incidence of life-threatening major bleeding or severe pulmonary hemorrhage. Our patient developed massive pulmonary hemorrhage and cardiogenic shock only 15 hours after taking the first of two low doses of dabigatran. His condition deteriorated rapidly and required ECMO as a live-saving measure. In such emergent situations, the reversal agent is a crucial component of the treatment. Idarucizumab is able to quickly alleviate bleeding if the patient develops life-threatening bleeding required urgent intervention [8]. In the RE-VERSE AD study, a single 5 g dose of idarucizumab was effective to abolish dabigatran activity in 98% of patients, and this effect was maintained for 24 hours in most patients. Among patients with overt bleeding that could be evaluated in the first 24 hours, the median time to the cessation of bleeding was 2.5 hours [9]. Thus, the benefit of early intervention with idarucizumab when a patient only shows mild clinical manifestations remains unknown. As a result of this major bleeding event, NOACs were discontinued in our patient. Instead, he underwent heart surgery and may benefit from left atrial appendage (LAA) occluders or primary LAA suture [10].
4. Conclusion
Our patient with low bleeding risk was placed on NOAC therapy for stroke prevention. However, a potentially lethal major bleeding event occurred rapidly after treatment with only two low doses of dabigatran. Use of ECMO was required for life salvage, and in this emergent condition, it was essential to reverse drug activity prior to performing an invasive procedure to correct the underlying condition.
Conflicts of Interest
The authors have no conflicts of interest to declare.
References
- Gulseth MP, Wittkowsky AK, Fanikos J, et al. Dabigatran etexilate in clinical practice: confronting challenges to improve safety and effectiveness. Pharmacotherapy 31 (2011): 1232.
- Stuart J. Connolly, Michael D. Ezekowitz MB, Ch B, et al. Dabigatran versus Warfarin in Patients with Atrial Fibrillation. N Engl J Med 361 (2009): 1139-1151.
- Blech S, Ebner T, Ludwig-Schwellinger E, et al. The metabolism and disposition of the oral direct thrombin inhibitor, dabigatran, in humans. Drug Metab Dispos 36 (2008): 386-399.
- Giugliano RP, Ruff CT, Braunwald E, et al. ENGAGE AF-TIMI 48. Investigators. Edoxaban versus warfarin in patients with atrial fibrillation. N Engl J Med 369 (2013): 2093-2104.
- Martin K, Beyer-Westendorf J, Davidson BL, et al. Use of the direct oral anticoagulants in obese patients: guidance from the SSC of the ISTH. J Thromb Haemost 14 (2016: 1308-1313.
- Eikelboom JW, Wallentin L, Connolly SJ, et al. Risk of bleeding with 2 doses of dabigatran compared with warfarin in older and younger patients with atrial fibrillation: an analysis of the randomized evaluation of long-term anticoagulant therapy (RE-LY) trial. Circulation 123 (2011): 2363-2372.
- Antonio L. Dans, Stuart J. Connolly, Lars Wallentin, et al. Concomitant Use of Antiplatelet Therapy with Dabigatran or Warfarin in the Randomized Evaluation of Long-Term Anticoagulation Therapy (RE-LY) Trial. Circulation 127 (2013): 634.
- Schiele F, van Ryn J, Canada K, et al. A specific antidote for dabigatran: functional and structural characterization. Blood 121 (2013): 3554.
- Pollack CV Jr, Reilly PA, van Ryn J, et al. Idarucizumab for Dabigatran Reversal —Full Cohort Analysis. N Engl J Med 377 (2017): 431-441.
- Sievert H, Lesh MD, Trepels T, et al. Percutaneous left atrial appendage transcatheter occlusion to prevent stroke in high-risk patients with atrial fibrillation: early clinical experience. Circulation 105 (2002): 1887-1889.


 Impact Factor: * 3.1
Impact Factor: * 3.1 CiteScore: 2.9
CiteScore: 2.9  Acceptance Rate: 11.01%
Acceptance Rate: 11.01%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 
