A Case Series of 13 Patients with COVID-19 Associated Respiratory Symptoms Treated with Nebulized OTR4120 (Cacipliq20®)
El Husseini Ali1 and Barritault Denis2*
1Mar Elias Medical Center Chehadeh Bulding Mar Elias Street Beirut Lebanon
2Laboratory of Cell Growth and Tissue Repair (CRRET), UPEC 4397/ ERL CNRS 9215, Université Paris Est Créteil, Université Paris Est, F-94000 Créteil, France
*Corresponding Author: Barritault Denis, Laboratory of Cell Growth and Tissue Repair (CRRET), UPEC 4397/ ERL CNRS 9215, Université Paris Est Créteil, Université Paris Est, F-94000 Créteil, France
Received: 14 July 2022; Accepted: 25 July 2022; Published: 26 August 2022
Article Information
Citation: El Husseini Ali and Barritault Denis. A Case Series of 13 Patients with COVID-19 Associated Respiratory Symptoms Treated with Nebulized OTR4120 (Cacipliq20®). Archives of Clinical and Medical Case Reports 6 (2022): 604-610.
View / Download Pdf Share at FacebookAbstract
We report a series of 13 patients with COVID-19 treated with Cacipliq20®, an heparan sulfate mimetic approved for the treatment of hard to heal cutaneous ulcers. Heparan sulfates play important roles in tissue repair and possess antiviral activity. Cacipliq20® was administered through nebulization at a dose of 45 μg twice a day for 5.5 consecutive days. All patients presented respiratory symptoms with some dyspnea and in most cases pulmonary abnormalities on chest CT-Scan. Eight patients presented with a moderate form of the disease, three patients with a severe form, one with a mild form, and one with a critical form. In all patients the treatment was added to the standard of care. Ten patients were treated during the acute stage of the disease (<4 weeks from symptoms onset) while 3 patients were in the postacute stage (>4 weeks from symptoms onset). A second treatment was administered for another 5.5 days in 6 patients. All patients showed clinically improvement after treatment. The time to first improvement ranged from 2 to 4 days after first treatment onset with a median of 3 days. Time to full clinical recovery ranged between 6 to 27 days from treatment onset with a median of 6 days. Lung CT scans followed clinical impression and showed a clear improvement of the lesions in most cases. The treatment was well tolerated in all patients. These preliminary observations should justify further evaluation through a well-designed placebo-controlled therapeutic trial.
Keywords
<p>COVID-19; Cacipliq20®; OTR4120</p>
Article Details
1. Introduction
Coronavirus Disease 2019 (COVID-19) caused by the new SARS-CoV-2 coronavirus, has affected the entire world population. There is still a need for treatments that could accelerate the recovery after COVID-19 infection and prevent long-term sequelae [1]. OTR4120 is a linear chain of about 250 α-1-6 glucose carboxymethyl sulfate units engineered as a structural and functional biodegradable bio-mimetic of heparan sulfates. Heparan sulfates act as mechanical nano element in the scaffolding of the extracellular matrix, bridging large matrix proteins (such as collagens, elastin, laminin) and protecting the cellular communication peptides from degradation [2,3]. OTR4120 mimics in all their functions heparan sulfates [4,5]. However, unlike natural heparan sulfates that possess β 1-4 links between the sugar units, the α-1-6 links of OTR4120 are resistant to heparanases and glycanases present at the site of injury. Consequently, OTR4120, when introduced at the site of injury replaces degraded heparan sulfates, restore the extracellular matrix architecture and protect the newly synthetized communication peptides from further degradation, hence creating a cellular micro-environment permissive to tissue regeneration. OTR4120 is commercially available in many countries, including Lebanon [6,7], under the tradename Cacipliq20® for the treatment of hard to heal cutaneous ulcers as a topical solution [8]. Here we report a case series of 13 patients with COVID-19 and respiratory symptoms who were treated off-label with repeated nebulizations of Cacipliq20®. The treatment appeared to be safe and showed some promising efficacy.
2. Methods
Dr A.E.’s decided to administer Cacipliq20® in nebulization first to himself. After noticing spectacular improvement of his own respiratory symptoms (see case #1 below), Dr A.E. decided to treat other patients referred to him with COVID-19 respiratory symptoms. In all 13 cases, infection by SARS-COV2 was confirmed by PCR or serology depending on whether the patient was in the acute phase or in the post-COVID phase. COVID-19 diagnosis was confirmed by the presence of ground-glass opacities on chest CT scans. Dr A.E. followed the patients clinically and with chest CT scans. Patients were followed in the context of routine clinical practice with no formal protocol. Dr A.E. used a “global impression approach” as well as Chalder fatigue scale, and MMRC dyspnea scale to assess the “time to first clinical improvement”. Time to recovery was defined as the time when the patient returned to normal life. CT Scans were sent to an independent contract research organization, “Visible patients, Strasbourg, France”, which performed 3D image reconstruction and provided a semi-automated quantification of pulmonary lesions (https://www.visiblepatient.com). Information was provided to, and consent letters were signed by each patient treated as individual case and at clinician’s own responsibility for off label use of Cacipliq20® with no further need for approval by ethical comity. Each patient received the same treatment. The nebulizing device was an OMRON C28P air jet nebulizer, available at local pharmacies or on the internet, which delivers droplets in the 50-100µm size range. Each nebulization lasted 10 minutes and delivered 4.5 mL of a solution of commercially available Cacipliq20® at an initial concentration of 100µg/mL diluted 10 folds in water, for a final concentration of 10µg/mL. Consequently, each nebulization delivered 45µg of Cacipliq20®. Nebulizations were repeated twice a day (morning and evening) for 5.5 consecutive days with a total of 11 nebulizations resulting in a total dose per patient of 495 µg. In 6 cases, a second series of nebulizations of Cacipliq20® was administered based on the patients’ demand after experiencing self-improvement from the first series, and on the intuition that it did not provide enough benefit or with the hope that the second series would consolidate the effects of the first one.
3. Overall Results
Thirteen patients diagnosed with Covid were treated with nebulized Cacipliq20® (table 1). Patients were between 27- and 75-year-old. All patients were ambulatory at the time of treatment onset and were treated in an out-clinic setting except case2 who was hospitalized. Ten patients were treated during the acute stage of the disease (<4 weeks from symptoms onset) while 3 patients (cases 1, 4, 5) were in the post-acute stage (>4 weeks from symptoms onset). Nine patients presented with a moderate form of the disease according to the NIH classification whereas 3 patients (cases 2, 4 and 6) presented with a severe form and another patient (case 5) with a critical form which required mechanical ventilation. The nebulized Cacipliq20® onset date ranged between 1 and 70 days after symptoms onset, with a median of 5 days. In all patients the treatment was added to the standard of care. In 6 patients (cases 1, 2, 4, 5, 6, 13), a second treatment was administered for another 5 days starting from 1 to 17 days after the end of the first treatment. All patients exhibited an improvement of their symptoms after treatment. The time to first improvement ranged from 2 to 4 days after first treatment onset with a median of 3 days. Time to full clinical recovery ranged between 6 to 27 days from treatment onset with a median delay of 6 days. The percentage of lung lesions ranged between 0 and 41% before treatment (median = 2%) to 0 to 44.6% after treatment (median = 0.8%). In 6 cases (cases 1, 3, 5, 6, 7, 9) the CT scan evolution showed a clear improvement of the lesions, and these improvements were observed respectively 29, 21, 50, 22, 12 and 5 days after the first scan. In 4 cases (cases 4, 8, 11, 12) no significant pneumopathy was observed at baseline and no worsening occurred after treatment. In 2 patients (cases 10 and 13) no improvement or even a slight worsening was observed on follow-up CT scans performed 11 and 6 days after the first CT-scan. In both latter cases, patients eventually clinically recovered 11 and 6 days after the start of Cacipliq20®.
Table 1: Summary of the 13 patients treated with Cacipliq20®. Mod: moderate, Sev: severe, Crit: critical according to the NIH classification (https://www.covid19treatmentguidelines.nih.gov). Of note, the second scan quantification of case 9 seems to be an artifact, since the percentage of affected lung increases from 8.62 to 15.59% while the images clearly show improvement, see Figure 7).
4. Brief Summary of Cases
4.1 Case 1
Dr AE, a 69-year-old radiologist was contaminated by a patient in January 2021. Clinical symptoms appeared on Day 2 and intensified the following days, including cough, fatigue and chest pain. Drug regiment started on Day 13, with aspirin, Azithromycin 500, followed by favipiravir. During the period from Day 17 till Day 25, SpO2 was between 99 and 96%, and temperature between 38 and 39°C. The patient experienced intense fatigue with sensation of fainting, low systolic blood pressure and anorexia resulting in a weight loss of 12 kg. Constipation was also noticed. Blood samples were analyzed every 2 or 3 days, and showed high inflammatory markers including ferritin, D-Dimers and CRP. Most of symptoms resolved spontaneously by Day 26 but extreme fatigue and exertional dyspnea persisted, blocking any attempt of walking more than few meters. A second chest CT scan performed at day 34 showed a diffuse interstitial pneumopathy (figure 1). Treatment with nebulized Cacipliq20® was started the evening of day 34 for 5.5 consecutive days. Concomitant treatment included Symbicort®320. The day after the first administration, the patient noticed an improvement of the dyspnea. He could walk again for 10, 20 minutes then 30 minutes on Days 37, 38, 39, respectively. By day 40, exertional dyspnea, pain and fatigue had completely disappeared. A control CT scan performed on Day 51 (17 days after the start of the first Cacipliq20® treatment) indicated an improvement of the interstitial pneumonia (Figure 1). It was decided to resume treatment for another 5 Days. Another CT scan performed 29 days after the start of the first Cacipliq20® treatment showed further reduction of the interstitial pneumonia (Figure 1).
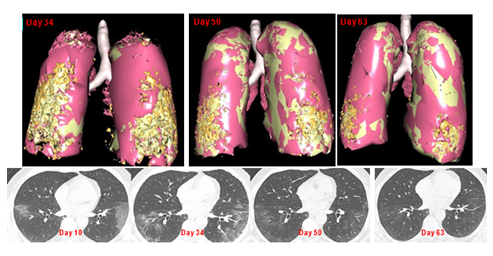
Figure 1: Chest CT-scan evolution of case 1. Upper panel: 3D reconstruction. Lesions appear in orange. Lower panel: native axial images. Cacipliq20® was started on Day 34. Day 0 = symptoms onset.
4.2 Case 2
Case 2 is a 63-year-old male, medical doctor, admitted in the emergency room in February 2021 (Day 1) with cough, a 39°C fever and dyspnea grade 3. SpO2 was 90%. A chest CT scan showed multiple pulmonary nodular infiltration with bilateral basal ground-glass opacities (Figure 18). Cacipliq20® was started the evening of day 1. On day 3, some cough improvement was noticed. On Day 6, the patient was allowed to go back home. Another Cacipliq20® treatment was administered at home for 5 days with no interruption between the two Cacipliq20® treatments. Ten days after the first Cacipliq20® treatment, the patient had completely recovered. A CT scan performed at 51 days after symptoms onset showed a disappearance of the lesions (Figure 2).
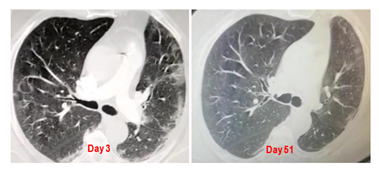
Figure 2: Chest CT-scan evolution of case 2. Native axial images. Cacipliq20® was started on Day 3. Day 0 = symptoms onset.
4.3 Case 3
This 45-year-old female was diagnosed with Covid: First symptoms started in February 2021, with sinusitis, frequent sneezing, cephalalgia, fever, deep fatigue, general muscle pain, diarrhea, anorexia and exertional dyspnea grade 1. A CT scan performed 14 days after symptoms onset showed a right lobe pneumopathy (Figure 3). Cacipliq20® treatment was started the same day. Concomitant treatments included antibiotics, paracetamol and aspirin. As early as the next day, dyspnea started to improve. The patient recovered within one week and went back to a normal life. Three weeks after the start of Cacipliq20®, a new chest CT indicated a complete disappearance of the pneumopathy.
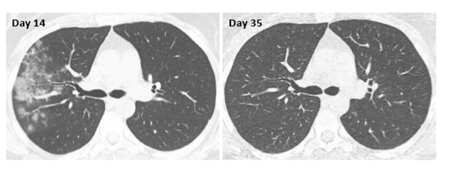
Figure 3: Chest CT-scan evolution of case 3. Native axial images. Cacipliq20® was started on Day 14. Day 0 = symptoms onset.
4.4 Case 4
This 58-year-old man was hospitalized for severe Covid in January 2021. His symptoms encompassed muscle pain, fatigue, dyspnea with low SpO2 and low arterial blood pressure. He was hospitalized in an intensive care unit for 2 weeks until being discharged. He was still complaining of a slight dyspnea at rest and severe exertional dyspnea grade 3 after short walking distances. He was kept under nasal oxygen at 2 liters/min with prophylactic anticoagulation. A CT-Scan performed Day 52 after symptoms onset indicated a slight Day 3 Day 51 pneumopathy with ground-glass opacities. Dyspnea, overwhelming fatigue, and dry cough remained. Cacipliq20® was started on Day 59, leading to rapid clinical improvement. On Day 62, the patient was able to walk 400 m and he had fully recovered by Day 65. A Day 66 CT scan was found normal.
4.5 Case 5
This 63-year-old woman contracted Covid a week before Christmas 2020. She presented to the emergency department with cough, fever, dyspnea, fatigue, cephalalgia, low Sp02 necessitating tracheal intubation and mechanical ventilation. She was discharged from hospital one month later. Since then, she suffered from extreme fatigue and dyspnea with pO2 value around 94 mmHg. A chest CT scan performed 70 days after symptoms onset (Day 70) revealed a mild interstitial pneumopathy (Figure 4). Cacipliq20® treatment was started the same day. A significant improvement was noted on the 3rd day, however, at the end of the first series of nebulizations, since dyspnea persisted, it was decided to continue the treatment for another 5 days. By Day 85, the patient had fully recovered from dyspnea and fatigue. A CT scan performed on Day 94 indicated a regression of the bilateral interstitial pneumopathy (Figure 4), that was confirmed on Day 120 (Figure 4).
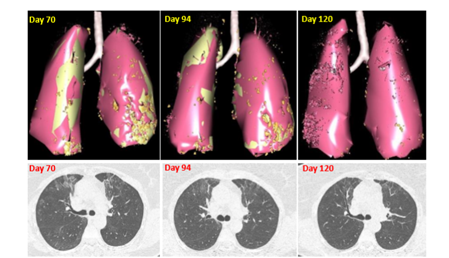
Figure 4: Chest CT-scan evolution of case 5. Upper panel: 3D reconstruction. Lesions appear in orange. Lower panel: native axial images. Cacipliq20® was started at Day 70. Day 0= symptoms onset.
4.6 Case 6
This 75-year-old-female suffering from a chronic lymphoid leukemia was diagnosed with Sars-CoV2 infection. She suffered from dyspnea, fatigue, and diffuse abdominal pain. A CT scan performed 5 days after symptoms onset, showed a diffuse interstitial pneumopathy. Treatment with Cacipliq20® was started the same day. Symptoms improved after one week. A second treatment was administered starting 22 days after the first treatment but resulted in no further improvement. A CT scan performed 24 days after Cacipliq20® started showed a clear reduction of the pneumopathy (Figure 5).
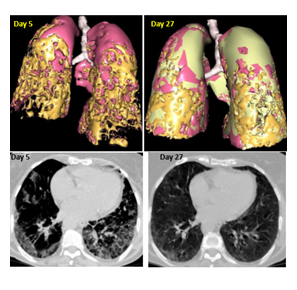
Figure 5: Chest CT-scan evolution of case 6. Upper panel: 3D reconstruction. Lesions appear in orange. Lower panel: native axial images. Cacipliq20® was started on Day 5. Day 0 = symptoms onset.
4.7 Case 7
This 47-year-old-man was diagnosed with Covid. Clinical symptoms started in March 2021 with abdominal pain, dry cough, dyspnea, fatigue. Cacipliq20® was proposed 4 days after symptoms onset, after a first CT scan revealed a bilateral interstitial pneumonia (Figure 6). On the second day of Cacipliq20® treatment, fatigue and dyspnea improved significantly and disappeared on the 3rd day. A second CT scan performed 12 days after the first one showed an almost disappearance of the lesions.
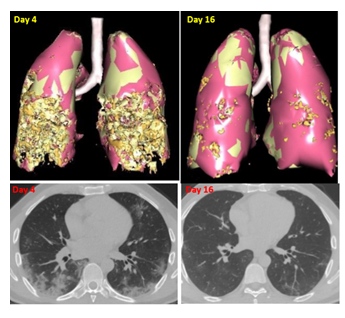
Figure 6: Chest CT-scan evolution of case 7. Upper panel: 3D reconstruction. Lesions appear in orange. Lower panel: native axial images. Cacipliq20® was started on Day 4. Day 0 = symptoms onset.
4.8 Case 8
This 27-year-old-female patient was diagnosed with Covid. She had experienced muscle pain, sore throat, slight general fatigue, moderate dyspnea, dry cough and fever. A first CTscan was performed 24 days after symptoms onset, indicating slight (below 1-2% interstitial pneumopathy). Treatment with Cacipliq20® was started the same day. Cough and dyspnea disappeared on the third day. A second CT scan performed 15 days after the first one was considered normal.
4.9 Case 9
This 63-year-old female positive for Covid, presented with a dyspnea grade 3. A CT scan performed 11 days after symptoms onset revealed a bilateral interstitial pneumopathy (Figure 7). Cacipliq20® was started the same day. Symptoms started to improve 3 days later and recovered 6 days after Cacipliq20® start. A second CT scan done 5 days after the first one showed an improvement of the lesions (Figure 7).
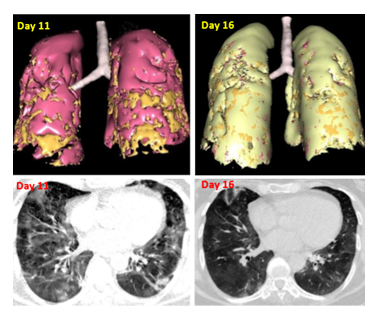
Figure 7: Chest CT-scan evolution of case 9. Upper panel: 3D reconstruction. Lesions appear in orange. Lower panel: native axial images. Cacipliq20® was started on Day 11. Day 0= symptoms onset.
4.10 Case 10
A 47-year-old woman presented with abdominal pain, fever, fatigue and dyspnea. A CT scan showed an interstitial sub-pleural pneumopathy, compatible with Covid 19 infection of the pulmonary bases. A treatment with Cacipliq20® was started 2 days after symptoms onset. Abdominal pain and dyspnea disappeared on the 3rd day of treatment and complete recovery was noticed on the 6th day. A control chest CT scan performed 11 days after the first one did not show any modification of the images (not shown).
4.11 Case 11
A 39-year-old woman presented with muscle pain, cough, dyspnea and fatigue. A chest CT-scan performed 4 days after symptoms onset showed a sub-pleural, bilateral mild COVID-19 lung disease with barely detectable ground-glass opacities. A Cacipliq20® treatment was started 4 days after symptoms onset. Three days later, muscle pain, dyspnea and fatigue improved, and the patient recovered on the 6th day. A follow-up CT scan 16 days later was found unchanged (not shown).
4.12 Case 12
A 54-year-old Woman was diagnosed with COVID-19. The patient had experienced cough, dyspnea, a 38°C fever, fatigue, and chest pain. A thoracic CT scan showed barely detectable sub-pleural interstitial opacities. A Cacipliq20® treatment was introduced 2 days after symptoms onset. Dyspnea, fatigue, chest pain and fever disappeared on the 3rd day. A followup CT scan performed one month later was considered normal (not shown).
4.13 Case 13
A 41-year-old woman was diagnosed with COVID-19. Her symptoms encompassed dyspnea, fatigue, cough, a 37.8° C fever. A thoracic CT scan showed a sub-pleural pneumopathy of the right middle lobe (Figure 8). A treatment with Cacipliq20® was started. Regression of dyspnea, fatigue and cough was noticed on the 2nd day. However, fever at 39°C, fatigue, and dyspnea reappeared on the fifth day, after the last nebulization. A CT scan (performed 6 days after the first one) showed a regression of the right anterior original lesion with appearance of bilateral new ground-glass opacities (Figure 8). A 2nd series of Cacipliq20® nebulizations was administered the same day leading to re-improvement of the symptoms.
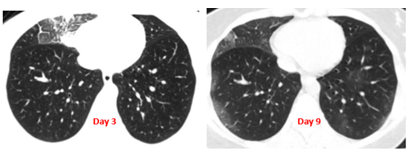
Figure 8: Chest CT-scan evolution of case 13. Cacipliq20® was started at Day 3. See the regression of the anterior lesion and the appearance of new ground-glass opacities at Day 9. Day 0 = symptoms onset.
5. Discussion
Here we report the clinical and radiological evolution of 13 consecutive patients with COVID-19 infection treated with Nebulized Cacipliq20®, an heparan sulfate mimetic approved for the treatment of hard to heal chronic ulcers. Patients received Cacipliq20® at the dose of 45µg per nebulization, twice a day for 11 days. Six patients received two full treatment regimens. Twelve patients were ambulatory at the time of treatment. Some patients were treated during the acute stage of the disease while 3 patients were in the post-acute stage. Nine patients exhibited signs of pneumopathy on their initial CT scan. In all cases, the evolution was favorable with no adverse effects related to the nebulization of Cacipliq20®. All patients eventually recovered clinically. In most cases, the radiological improvement followed clinical improvement. In 2 patients, no radiological improvement could be detected, however, in both cases, the follow-up CT scan was probably performed too soon (<12 days after the initial CT-Scan) to see any improvement. OTR4120 is a biomimetic of heparan sulfate, resistant to heparinase and glycanase digestion. OTR4120 serves both as a scaffold element and as a site for communication peptides storage and protection. It was designed to replace degraded heparan sulfates in damaged tissues, accelerating the speed and enhancing the quality of tissue repair. Its unique property has been subject to intensive pre-clinical and clinical studies in various type of lesions [4,5,8-11]. Beside this repair effect of OTR4120 which is supposed to be independent of the cause of the tissue damage and which might have played a role in the recovery of patients treated with nebulized Cacipliq20®, OTR4120 is also expected to have anti-viral properties. Indeed, it is well known that several viruses, including herpes and coronaviruses, bind to heparan sulfate during infection [12-17]. It was shown that binding of SARS-CoV to heparan sulfates (HS) is required for viral attachment and infection of target cells and that HS can serve as an anchor point to facilitate endocytosis of SARS-CoV-2 related coronaviruses. Recently, it was also shown that SARS-CoV-2 spike protein interacts with HS through its receptor-binding domain (RBD). Consequently, OTR4120 could wrap the virus envelope. And would act as a decoy, preventing the virus from entering the host cell. To our knowledge this is the first report that RGTA administered by nebulization appears to be safe and might potentially facilitate lung tissue repair. It is acknowledged that this study is a collection of cases from which it is difficult to draw any robust conclusion regarding the link between Cacipliq20® treatment and improvement which may have followed the natural evolution of the disease. This cases report has only ambition to provide some safety evidence and to illustrate the potential efficacy of a rapid treatment for lung injury in COVID-19. These clinical observations should justify further evaluation through a well-designed placebo-controlled study.
Funding
3D image reconstruction and semi-automated quantification of pulmonary lesions (www.visiblepatient.com) were funded by the company OTR3.
Conflicts of Interest
Dr Ali El Husseini declares no conflict of interest. Denis Barritault is co-inventor and co-owner in the patents describing Cacipliq20® technology and applications. He is founder, president and shareholder of the company OTR3 which manufactures and commercializes Cacipliq20®.
References
- Nalbandian A, Sehgal K, Gupta A, et al. Post-acute COVID-19 syndrome. Nat Med 27 (2021): 601-615.
- Olczyk P, L. Mencner L, Komosinska-Vassev K. Diverse Roles of Heparan Sulfate and Heparin in Wound Repair. Biomed Res Int 2015 (2015): 549417.
- Van Neck J, Tuk B, Barritault D, et al. Heparan Sulfate Proteoglycan Mimetics Promote Tissue Regeneration: An Overview in P. J. Davies (Ed.), Tissue Regeneration - From Basic Biology to Clinical Application (Vol. 4, pp. 69-92): InTech. Ward, A. F. Carlin and J. D. Esko (2020). SARS-CoV-2 Infection Depends on Cellular Heparan Sulfate and ACE2. Cell (2012).
- Barritault D, Gilbert-Sirieix M, Rice KL, et al. RGTA(R) or ReGeneraTing Agents mimic heparan sulfate in regenerative medicine: from concept to curing patients. Glycoconj J 34 (2017): 325-338.
- Rouet V, Meddahi-Pelle A, Miao HQ, et al. (2006). Heparin-like synthetic polymers, named RGTAs, mimic biological effects of heparin in vitro. J Biomed Mater Res 78 (2006): 792-797.
- Hayek S, Dibo S, Baroud J, et al. Refractory sickle cell leg ulcer: is heparan sulphate a new hope?. Int Wound J 13 (2016): 35-38
- Hayek S, Atiyeh B, Zgheib E. Steward Bluefarb Syndrome: Review of the literature and case report of chronic ulcer treatment with heparan sulfate mimetic (CACIPLIQ 20®) International Wound Journal 12 (2013): 169-172.
- Desgranges P, Louissaint T, Godeau B, et al. Matrix therapy is a cost-effective solution to reduce amputation risk and improve quality of life: pilot and case studies. Regen Med Res 7(2019): 2.
- Aifa A, Gueudry J, Portmann A, et al. Topical treatment with a new matrix therapy agent (RGTA) for the treatment of corneal neurotrophic ulcers. Invest Ophthalmol Vis Sci 53 (2012): 8181-8185.
- Deback C, Rousseau A, Breckler M, et al. Antiviral effects of Cacicol ((R)), a heparan sulfate biomimetic for corneal regeneration therapy, for herpes simplex virus type-1 and varicella zoster virus infection. Antivir Ther 23 (2018): 665-675.
- Escartin Q, Lallam-Laroye C, Baroukh B, et al. A new approach to treat tissue destruction in periodontitis with chemically modified dextran polymers. FASEB J 17(2003): 644-651.
- Cagno V, Tseligka ED, Jones ST, et al. Heparan Sulfate Proteoglycans and Viral Attachment: True Receptors or Adaptation Bias?. Viruses 11 (2019): 596.
- Clausen TM, Sandoval DR, Spliid CB, et al. SARS-CoV-2 Infection Depends on Cellular Heparan Sulfate and ACE2. Cell 183(2020): 1043-1057.
- De Pasquale V, Quiccione MS, Tafuri S, et al. Heparan Sulfate Proteoglycans in Viral Infection and Treatment: A Special Focus on SARS-CoV-2. Int J Mol Sci 22 (2021): 6574.
- Lang J, Yang N, Deng J, et al. Inhibition of SARS pseudovirus cell entry by lactoferrin binding to heparan sulfate proteoglycans. PLoS One 6 (2011): e23710.
- Milewska A, Zarebski M, Nowak P, et al. PyrcHuman coronavirus NL63 utilizes heparan sulfate proteoglycans for attachment to target cells. J Virol 88 (2014): 13221-13230.
- National Institutes of Health. COVID-19 Treatment Guidelines Panel. Coronavirus Disease 2019 (COVID-19) Treatment Guidelines (2020).


 Impact Factor: * 5.3
Impact Factor: * 5.3 Acceptance Rate: 75.63%
Acceptance Rate: 75.63%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 
