Lipoma-like Pleomorphic Adenoma of the Trachea
Jung Seop Eom1, Chang Hun Lee2*, Ah Rong Kim2, So Jung Lee2, Se Jin Jung2
1Department of Internal Medicine, Pusan National University School of Medicine, Busan, South Korea
2Department of Pathology, Pusan National University School of Medicine, Busan, South Korea
*Corresponding Author: Dr. Chang Hun Lee, Department of Pathology, Pusan National University School of Medicine, 179 Gudeok-ro, Seo-gu, Busan 602-739, South Korea
Received: 04 March 2020; Accepted: 19 March 2020; Published: 04 May 2020
Article Information
Citation:
Jung Seop Eom, Chang Hun Lee, Ah Rong Kim, So Jung Lee, Se Jin Jung. Lipoma-like Pleomorphic Adenoma of the Trachea. Archives of Clinical and Medical Case Reports 4 (2020): 329-335.
View / Download Pdf Share at FacebookAbstract
Endotracheal pleomorphic adenoma is an extremely rare disease. A 67-year-old Korean woman, who had complained of progressive dyspnea and wheezing presented with an endotracheal mass in the upper trachea on a chest CT scan. The tumor was removed by rigid bronchoscopy with diode laser ablation at the base. From histologic examination, the mass consisted of extensive mature fat tissue (about 80% of tumor volume) and strips of usual pleomorphic adenoma with diverse histology. Nuclear atypia or mitotic activity of epithelial cells was not identified. Therefore, it was diagnosed as lipoma-like pleomorphic adenoma. Lipoma-like pleomorphic adenoma arising in the trachea is an extremely rare condition. To the best of our knowledge, this is the first such trachea case reported in the English literature.
Keywords
<p>Trachea; Pleomorphic adenoma; Lipoma-like; Rigid bronchoscopy</p>
Article Details
1. Introduction
Pleomorphic adenoma is ‘‘a circumscribed epithelial tumor which consists of clearly recognizable epithelial tissue that is intermingled with mesenchymal tissues of mucoid, myxoid, and chondroid.” [1]. The term “pleomorphic” refers to both the histogenesis and histology of the tumor. As the term “pleomorphic” shows, the tumor is characteristic of the diversity of the histology. Lipomatous component focally can be included in pleomorphic adenoma. Extensive lipomatous change of the tumor is, however, a very rare finding in pleomorphic adenoma, and only a few such cases have been reported in the literature [2-5]. These all arose in parotid glands, and lipomatous changes are often observed in the normal parotid glands of adults as they age. However, there is little fatty tissue in the trachea, so lipoma-like pleomorphic adenomas arising in the trachea are extremely rare. To the best of our knowledge, this is the first such trachea case reported in the English literature.
2. Case Report
A 67-year-old Korean woman presented to her primary care physician with progressive dyspnea, productive cough, and wheezing during about 10 years. She had been treated for asthma. Her chest roentgenogram was unremarkable, except for evidence of inactive old tuberculosis on the right upper lobe of the lung and mild focal thickening of the right pleura. The patient denied personal tobacco use, but consumed alcohol and had longtime exposure to secondhand smoke. In her past significant medical history, she underwent low anterior resection of the left colon at another local university hospital 3 years ago due to sigmoid colon cancer. But there was no radiologic evidence of recurrence from a computed tomography (CT) scan of the abdomen. Otherwise, she was in no acute distress and had no labored breathing. No abnormal findings were evident from laboratory tests. On pulmonary function test, moderate obstructive pattern, normal lung volume, and mild reduction in diffusion lung capacity for carbon monoxide were noted. A CT scan of the thorax showed a tumor confined to the upper trachea, in its right lateral wall (Figure 1A). Flexible bronchoscopy performed under conscious sedation revealed a polypoid, hypervascular sessile tumor arising from the right lateral wall of the upper trachea occluding approximately 90% of its lumen (Figure 1B). To resolve the patient’s respiratory difficulty and her symptoms, we scheduled an immediate rigid bronchoscopy under general anesthesia to remove the endobronchial tumor. The tumor was resected with a mechanical debulking procedure using the sharp bevel of a rigid tube (Karl Storz, Tuttlingen, Germany). Thereafter, the base of the endobronchial tumor was ablated with a diode laser (Ceralas D50, Jena, Germany) to prevent recurrence. The removed endobronchial tumor was a 15 mm × 20 mm-sized oval-shaped mass (Figure 1C).
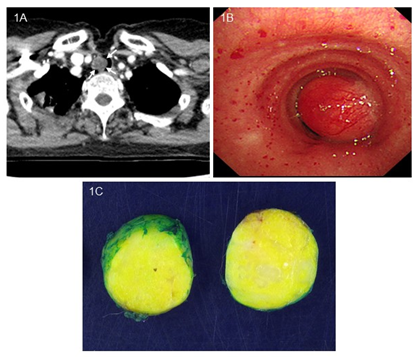
Figure 1: (A) Computed tomography scan of the chest revealing endotracheal soft tissue mass (white arrow), protruding into the lumen of the upper trachea. (B) Flexible bronchoscopy revealing a polypoid sessile tumor with a wide-based pedicle arising from the right lateral wall of the upper trachea. (C) Gross finding of the tumor (cut surface) showing about 2 cm-sized well-circumscribed tumor with a lipoma-like yellowish cut surface.
On histologic examination at low power view, the tumor consisted of a bland, basaloid epithelial proliferation against extensive fatty stroma and focal hyalinized fibrous tissue (Figure 2A). Proliferating epithelial cells were growing in orderly cords with multifocal areas of squamous differentiation, tubules, and frequent cribriform structures (Figure 2B), and they gradually merged into the surrounding mesenchymal tissues. At a glance, the cribriform structures were reminiscent of the growth pattern of adenoid cystic carcinoma. On high power view, cribriform nests were composed of two cell types; that is, cuboidal cells with eosinophilic cytoplasm and vesicular nuclei lining true ductal structures and small cells with scant cytoplasm and basophilic nuclei with finely stippled chromatin (Figure 2C). There was also no evidence of vascular or perineural invasion. Peripherally, the tumor showed focal involvement of the resection margin. Nuclear atypia or mitotic activity of epithelial cells was not identified. Adipocytes were univacuolar in shape, and neither lipoblasts nor malignant findings were identified. In some areas, the cells showed a transition with adjacent stromal myoepithelial cells (Figure 2D).
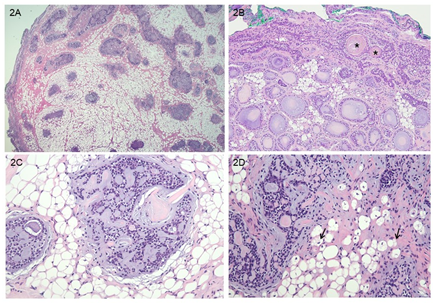
Figure 2: Microscopic findings of the tumor (H&E stain). A: On low power view, a well-demarcated lesion with bland, basaloid epithelial proliferation against extensive fatty stroma and focal hyalinized fibrous tissue. B: Proliferating epithelial cells growing in orderly cords with multifocal areas of squamous differentiation (asterisks), tubules, and cribriform structures. C: Cribriform nests composed of two cell types; that is, cuboidal cells with eosinophilic cytoplasm and vesicular nuclei lining true ductal structures and small cells with scant cytoplasm. D: In some areas, stromal myoepithelial cells showed transition into adipocytes (black arrows) (original magnifications: A, x20; B, x100; C, x400; D, x200).
Around and within the lumina of tubules and cribriform structures, mucoid substances that are reactive to Alcian blue stain at pH 2.5 (Figure 3A) and periodic acid-Schiff stain (data not shown) were present. Immunohistochemically, the main tumor cells around tubular lumina and cribriform structures were strongly positive for myoepithelial cell markers p63 and smooth muscle actin (Figure 3B). c-kit marker highlighted scattered stromal mast cells, but was negative on most of tumor cells of tubules and cribriform structures. Tubular epithelial cells showed positivity to epithelial membrane antigen (Figure 3C). The Ki67 proliferation index of tumor cells was less than 5% (Figure 3D). Altogether, lipoma-like pleomorphic adenoma was given as the pathological diagnosis.
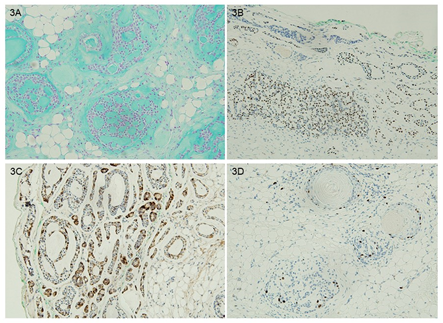
Figure 3: (A) Alcian blue (at pH 2.5) stain showing mucoid substances reactive to the special stain Around and within lumina of tubules and cribriform structures. B to D describing immunohistochemical findings, (B) tumor cells around tubular lumina and of cribriform structures strongly positive for myoepithelial cell marker p63. (C) Tubular-lining epithelial cells showing positivity to epithelial membrane antigen. D: Tumor cells showing a low Ki67 proliferation index (about less than 5%).
The postoperative course was uneventful, and the patient was discharged the second day after the procedure. Follow-up bronchoscopy with a biopsy 3 months later revealed a small scar-like area of tissue at the removal site. Its pathologic report showed it to be a residual pleomorphic adenoma that reflected a marginal involvement at the initial tumor resection. About 11 months later, the patient was well without evidence of recurrence.
3. Discussion
Primary tumors of the trachea account for 0.2% of all airway neoplasms, affecting 0.1/100,000 individuals per year. Ninety percent of these lesions in adults are malignant, with squamous cell carcinoma and adenoid cystic carcinoma being the commonest [6]. So, benign tumors in the trachea, which include xanthogranuloma, pleomorphic adenoma, etc. are very uncommon, with an incidence of 0.26 per 100,000 population [7].
Pleomorphic adenoma, which is the most common benign tumor of the parotid gland, rarely develops in the trachea, with less than 50 patients being reported in the literature up to 2016. The average age of these patients was 48 years old (range, 26–71 years old) and the sex distribution was almost equal [8]. Nearly half of these tumors were located in the upper third of the trachea as in the present case, and 12% were located in the lower third. Like other tracheal tumors, pleomorphic adenoma often leads to obstructive symptoms such as dyspnea and wheezing. These symptoms mimic those of asthma and therefore may result in a delay of primary diagnosis, as in the present case [9]. Although extremely rare, tracheobronchial pleomorphic adenoma may present with great clinical significance due to its interruption of breathing [8]. Chest roentgenograms are not helpful because the tumors are rarely visualized [10]. CT and magnetic resonance images of the thorax have a diagnostic yield of up to 90% and also allow for the evaluation of near and distant structures, so play an essential radiological role in its diagnosis [9, 10]. Flexible bronchoscopy remains the gold standard for diagnosing tracheal tumors. A precise evaluation of tumor characteristics, localization, and extension can be made by a bronchoscopic examination, along with the possibility of biopsy sampling [11]. Thus, both CT and flexible bronchoscopy play a central role in diagnosis and treatment planning. Histologically, the tumor is characterized by neoplastic proliferation of parenchymal glandular cells along with myoepithelial components. It generally grows slowly and lacks a complete capsule [12]. The most interesting point in the present case was that a large part of the tumor parenchyma was replaced by lipoma-like fat tissue. Similarly, in 1999, Seifert et al. [3] coined the term lipomatous pleomorphic adenoma for an otherwise typical pleomorphic adenoma with adipose tissue components occupying more than 90% of the tumor. Fat contents of the present case were abundant, but the amount approximated less than 90% of the tumor. So, we named this case lipoma-like pleomorphic adenoma. Although foci of adipose tissue are sometimes encountered within the stroma of pleomorphic adenoma, extensive replacement by adipose tissue as in this case is regarded as a very rare finding. Differential diagnosis concerning lipoma (pure lipomatous tumor) in particular is essential. The existence of an epithelial or myoepithelial component is a diagnostic clue [13]. From a histopathological point of view, a correct diagnosis is not difficult, with presences of the islets of typical ductal and myxochondroid structures. However, diagnostic problems may arise when a bronchoscopic fine needle aspiration specimen contains only adipose cells, and the tumor thus may be misdiagnosed as a lipoma.
In addition to abundant adipose tissue, the present case histologically showed frequent occurrence of tubular and cribriform structures, which is characteristic of adenoid cystic carcinoma. Adenoid cystic carcinoma is an aggressive malignant tumor with foci of peritumoral and perineural invasion [14]. The differential diagnosis between pleomorphic adenoma and adenoid cystic carcinoma may be most difficult. Regarding cribriform nests, tumor cell nests of adenoid cystic carcinoma are sharply separated from the stroma, as compared with pleomorphic adenoma [15], and are composed of a single type of small basophilic cells with angular hyperchromatic nuclei.
The histogenesis of lipomatous or lipoma-like pleomorphic adenoma is not still clear, but metaplastic transformation of myoepithelial cells to adipocytes and entrapment of fat tissue are known to be two possible mechanisms [5]. In the present case, some stromal myoepithelial cells within the tumor showed foci of probable lipocytic transformation, so we favor the metaplastic theory by myoepithelial cells as suggested by some researchers [2, 4].
Because pleomorphic adenoma of the trachea, including the present case, rarely occurs, standard management has not been established. In general, endoscopic resection or surgical segmental resection is selected as a treatment on the basis of tumor status and physician preference. Endoscopic removal may be used when rapid respiratory symptom improvement or hemorrhage control is required. Segmental resection is the only curative treatment for this tumor, and can decrease the possibility of local recurrence [3, 16].
4. Conclusion
Lipoma-like pleomorphic adenoma of the trachea is considered an extremely rare tumor. In this benign tumor, we suggest that abundant fatty contents would be derived from lipocytic metaplasia of stromal myoepithelial cells. As with other tracheal tumors, obstructive respiratory symptoms could mimic asthma and may cause a delay in the diagnosis with ordinary anti-asthmatic treatment. At this point, CT and flexible bronchoscopy help in confirming the diagnosis and treatment planning. Surgical resection is considered a traditional treatment of choice; however, less-invasive rigid bronchoscopic resection with careful observation could also be a treatment option.
Conflicts of Interest
No potential conflict of interest relevant to this article was reported. The authors do not declare any conflict of interest.
Contribution of the Authors
All authors have read and approved the final version of the manuscript.
Acknowledgments
This research was supported by a 2-year research grant from Pusan National University. The funder of the study had no role in the study design, data collection, data analysis, data interpretation, or writing of the manuscript.
Ethics Approval and Consent to Participate
Not applicable.
References
- Langdon JD. Tumors of the salivary glands: clinical analysis of 68 cases. J Oral Maxillofac Surg 43 (1985): 688-692.
- Ng WK, Ma L. Pleomorphic adenoma with extensive lipometaplasia. Histopathology 27 (1995): 285-288.
- Seifert G, Donath K, Schäfer R. Lipomatous pleomorphic adenoma of the parotid gland. Classification of lipomatous tissue in salivary glands. Pathol Res Pract 195 (1999): 247-252.
- Skálová A, Stárek I, Simpson RH, et al. Spindle cell myoepithelial tumours of the parotid gland with extensive lipomatous metaplasia. A report of four cases with immunohistochemical and ultrastructural findings. Virchows Arch 439 (2001): 762-767.
- Korkmaz H, Adsay NV, Jacobs JR, et al. Lipomatous pleomorphic adenoma of parotid gland. Histopathology 40 (2002): 487-488.
- Gelder CM, Hetzel MR. Primary tracheal tumours: a national survey. Thorax 48 (1993): 688-692.
- Schneider P, Schirren J, Muley T, et al. Primary tracheal tumors: experience with 14 resected patients. Eur J Cardiothorac Surg 20 (2001): 12-18.
- Liu L, Yan CH, Tao SD. Radiofrequency Ablation Is Low Invasive and Effective in Treat Pleomorphic Adenoma in Trachea Without Recurrence for At Least Five Years. J Craniofac Surg 27 (2016): 978-980.
- Aribas OK, Kanat F, Avunduk MC. Pleomorphic adenoma of the trachea mimicking bronchial asthma: report of a case. Surg Today 37 (2007): 493-495.
- Honings J, Gaissert HA, van der Heijden HF, et al. Clinical aspects and treatment of primary tracheal malignancies. Acta Otolaryngol 130 (2010): 763-772.
- Casillas-Enríquez JD, Álvarez-Maldonado P, Salguero-Cruz L, et al. Pleomorphic adenoma of the trachea: A case report. J Bronchology Interv Pulmonol 21 (2014): 51-53.
- Foresta E, Torroni A, Di Nardo F, et al. Pleomorphic adenoma and benign parotid tumors: extracapsular dissection vs superficial parotidectomy--review of literature and meta-analysis. Oral Surg Oral Med Oral Pathol Oral Radiol 117 (2014): 663-676.
- Kondo T. A case of lipomatous pleomorphic adenoma in the parotid gland: a case report. Diagn Pathol 4 (2009): 16.
- Nicolini EM, Montessi J, Vieira JP, et al. Adenoid Cystic Carcinoma of the Trachea: A Case Report. Am J Case Rep 20 (2019): 1373-1377.
- Jaso J, Malhotra R: Adenoid cystic carcinoma. Arch Pathol Lab Med 135 (2011): 511-515.
- Park KS, Sung WJ. Pleomorphic Adenoma of the Trachea: A Case Report. Korean J Pathol 47 (2013): 399-401.


 Impact Factor: * 5.3
Impact Factor: * 5.3 Acceptance Rate: 75.63%
Acceptance Rate: 75.63%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 
