Chemotherapy-Induced Fulminant, Severe Hyperactive Delirium in A Patient with A New Diagnosed Multiple Myeloma
Olesya Pavlova1, Wolfram Weinrebe2*
1Department of Acute Geriatrics and Rehabilitation, Fribourg Cantonal Hospital (HFR), Fribourg, Switzerland
2Department of Internal Medicine and Geriatrics, Campus of Hirslanden, Salem Hospital, Bern, Switzerland
*Corresponding Author: Dr. med. Wolfram Weinrebe, Department of Internal Medicine and Geriatrics, Campus of Hirslanden, Salem Hospital, Bern, Switzerland
Received: 19 February 2020; Accepted: 30 March 2020; Published: 04 May 2020
Article Information
Citation:
Olesya Pavlova, Wolfram Weinrebe. Chemotherapy-Induced Fulminant, Severe Hyperactive Delirium in A Patient with A New Diagnosed Multiple Myeloma. Archives of Clinical and Medical Case Reports 4 (2020): 312-328.
View / Download Pdf Share at FacebookAbstract
Multiple myeloma is an incurable haematological malignancy with complex pathogenesis, affecting mostly the elderly and characterized by the presence of monoclonal plasma cells within the bone marrow secreting an abnormal immunoglobulin, which results in gammapathy with organ or tissue injury. The chemotherapy used for the treatment of multiple myeloma causes various adverse effects, including serious psychiatric and cognitive problems, which pose a significant challenge to the clinician, present an impact on the quality of patient’s life and augment hospital stays and health care costs. We reported a clinical case of 73-year-old women with new diagnosed multiple myeloma developing severe, rapidly progressing and persisting hyperactive delirium with psychotic symptoms and mania-episodes during Velcade-Revlimid-Dexamethasone-based treatment. We discuss possible causes, clinical presentation, treatment and deficits maintained after recovery. Our report demonstrates the rare possibility of an association between chemotherapy and the development of combined psychiatric disorders.
Keywords
<p>Multiple myeloma; Chemotherapy; Chemo-brain; hyperactive delirium; Psychosis; Mania; Geriatric patients</p>
Article Details
Abbreviations:
Abbreviations: ADL - activities of daily living; ASCT - autologous stem cell transplantation; BM - bone marrow; CAM-S LF - confusion assessment method severity long form; 3D-CAM - 3-minute diagnostic interview for CAM-defined delirum; CCI - Charlson comorbidity index; CNS - central nervous system; FNA - fine-needle aspiration; IADL - instrumental ADL; ISS - international staging system; MGUS - monoclonal gammopathy of undetermined significances; MM - multiple myeloma; MMSE - mini mental state examination; MoCA - Montreal cognitive assessment; PCL - plasma cell leukemia; R-ISS - revising international staging system; ROS - reactive oxygen species; SAMM - smoldering asymptomatic multiple myeloma; SMM - symptomatic multiple myeloma; TDP-43 - transactive response DNA-binding protein 43; VD - velcade-dexamethasone; VRD - velcade-revlimid-dexamethasone; VTD - velcade-thalidomide-dexamethasone
1. Introduction
Multiple myeloma (MM) is a rare, aggressive and incurable B-cell neoplasia with complex pathogenesis, that accounts for approximately 10% of hematological malignancies and is characterized by uncontrolled proliferation and dissemination of monoclonal plasma cells within the bone marrow (BM) producing a monoclonal protein, which is detected in the blood and/or urine and leads to organ or tissue impairment [1-3]. During progression, MM evolves from a pre-malignant non-IgM- /IgM- /light chain- monoclonal gammopathy of undetermined significances (MGUS), and progresses to smoldering asymptomatic multiple myeloma (SAMM) or directly to manifest symptomatic multiple myeloma (SMM), resulting in infiltration of the bone marrow and osteolytic lesions [1-6]. Occasionally, MM progresses to bone-marrow independent plasma cell leukemia (PCL) [3]. Another rare clinical form of MM is non-secretory myeloma with similar monoclonal proliferation within the bone marrow, clinical and radiological features but without secretion of atypical immunoglobulin by plasma cells [4]. Older age, male sex, and African racial background, exposure to the ionizing radiation, pesticides, benzol, pre-existing immunosuppression, chronic infection, obesity, and severe comorbidities increase the probability of MM development [1, 3-5, 7]. Heterogeneous chromosomal aberrations and multiple gene mutations testify the poor prognosis and play an important role in MM pathogenesis, leading to uncontrolled proliferation, resistance to apoptosis and abnormal immune response [1, 2, 4]. More detailed information can be found in the elegant review of Rajkumar et al. [5, 8].
During MM-progression the malignant cells produce monoclonal immunoglobulins or light chains, disordering the normal balance between osteoblastic and osteoclastic activity and leading to lytic bone lesions and hypercalcemia [9]. Accumulation of free light chains in tissues provokes amyloidosis [7]. Atypical tumour clone invasion of bone marrow causes anemia and pancytopenia [5]. The abnormal function of lymphocytes producing dysfunctional immunoglobulins results in leukopenia, immunosuppression, recurrent infections, and hyperviscosity, responsible for congestive heart failure, neurologic symptoms, coma, and confusion [10]. Renal failure is multifactorial and principally induced by nephropathy, amyloidosis, plasma cell infiltration, interstitial nephritis and light/heavy chain deposition disease, hypercalcemia, hyperuricemia, volume depletion, hyperviscosity, nephrotoxic drugs, bisphosphonates and secondary infections [1, 3, 7, 10].
The diagnosis of multiple myeloma requires typical clinical, laboratory and radiological findings, including amyloidosis, the end-organ damage with hyper-Calcemia, Renal insufficiency, Anemia and Bone lytic lesions, pathologic fractures or severe osteopenia (CRAB), increased numbers of immature, abnormal plasma cells in the bone marrow, a monoclonal protein (M-protein) and/or light chains (kappa or lambda) in the serum or urine (except in patient with true no secretory MM) and characteristic gene alterations [1-3, 5, 7, 11].
The genetic complexity makes the treatment of MM tricky. First-line chemotherapy of younger fit seniors with few comorbidities comprises two- or three-drug combination, followed by autologous stem cell transplantation (ASCT) [1, 2, 4-6, 8, 12]. Older patients with multiple comorbidities, ineligible for ASCT, are treated with precautions according to one of the proposed myeloablative chemotherapy-schemas [5, 6, 11]. The gold standard in the treatment for multiple myeloma remains dexamethasone-based chemotherapy [1, 2, 5, 10]. Multiple clinical trials have demonstrated that the addition of novel agents, like the proteasome inhibitor Velcade ([bortezomib], the VD protocol), immunomodulatory drugs thalidomide (the VTD protocol) and Revlimid ([lenalidomide], the VRD protocol), increases response rates, prolongs overall survival and progression-free survival [1, 2, 5, 6, 9, 10-14]. Supportive treatment is used to manage myeloma-associated organ damage and therapy-induced adverse effects and includes pain therapy, bisphosphonates, prophylaxis against infection, thromboprophylaxis, immunoglobulins, and hematopoietic growth factors, irradiation of skeletal/extramedullary lesions [1, 2, 5, 11].
The chemotherapy provokes multiple side effects related to their administration way [15, 16] and drug interactions with previous medication [9, 11, 14], correlated with dose, duration of treatment, and used substances (Table 1), and offen aggravated by age-related injuriousness. Many chemotherapeutic agents traverse the blood-brain barrier and alter cognitive function both acutely and chronically [16]. We report a clinical case of severe combined psychiatric disorder, including rapidly progressing and persisting hyperactive delirium with psychotic symptoms and mania-episodes, appearing after the first cycle of the VRD-based treatment in a 73-year-old patient, who was diagnosed with multiple myeloma at the HFR Tafers Hospital (the part of HFR Fribourg cantonal hospital, Switzerland).
2. Methods
Available patient’s data were extracted from the DPI electronic database of HFR Fribourg cantonal hospital. Collected data included demographic data at the time of diagnosis, details of treatment, laboratory and radiological investigations. The patient gave its informed consent before any data were entered into the database. The consent mentions that data from the electronic database of HFR Fribourg may be used for collaborative projects and publications. We used the multidisciplinary complex geriatric assessment, including estimation of clinical status, severity und number of comorbidities with the Charlson Comorbidity Index (CCI), multipharmacology, socioeconomic status, nutrition status, risk of falls. Moreover, we investigated risk factors for incident delirium in older patients according to previous publications [17]. To evaluate the severity of cognitive impairment of our patient we used standard cognitive screening tests, like Mini Mental State Examination (MMSE), Montreal Cognitive Assessment (MoCA), Confusion Assessment Method Severity Long Form (CAM-S LF), and 3-Minute Diagnostic Interview for CAM-defined Delirum (3D-CAM). To estimate self-care activities, independence status and charges of household management the Katz and Akpom’s basic activities of daily living (ADL) and the Lawton and Brody’s instrumental ADL (IADL) scales were used.
3. Case Presentation
A 73-year-old woman with no past psychiatric history was initially admitted for unspecific increased fatigue and weakness, cough with yellowish expectoration for three to four weeks, and known irregularity of the chair with intermittent diarrhoea without concomitant abdominal pain. She denied the urinary retention, urinary and faecal incontinence, fever, night sweats, weight loss or any other symptoms.
Her medical history was significant for chronic heart failure with hypertensive, dysrhythmic and valvular cardiopathy NYHA III; chronic renal insufficiency stage 2-3a; colonic diverticulosis with diverticulitis; subclinical hypothyroidism; severe manifest osteoporosis with spontan vertebral fructures, status after hormone therapy, and multiple orthopaedic surgeries; chronic back pain. The important medication taken at the time were oxycontin (5 mg/d), torasemide (20 mg/d), metoprolol (50 mg/d), irbesartan/ hydrochlorothiazide (150/12.5 mg/d), calcimagon with vitamin D3, imodium, pantoprazol (40 mg/d), daflon (1g/d).
On physical examination, the patient was alert, active, oriented to person, place and time, and in acute pain. Vital signs were as following: blood pressure of 111/89 mm Hg, heart rate of 82 beats/min (regular), and temperature of 36.1°C. The cardiac examination revealed a systolic heart murmur with a maximum over the aortic valve and bilateral leg oedema. No lymphadenopathy or hepatosplenomegaly was noted. The osteo-articular examination found pain in the palpation of the lower lumbar area. There was no paraspinal muscle tenderness, no superficial lesions noted, no costovertebral angle tenderness, and a negative straight leg raise test. There were normal bilateral lower extremity reflexes. The strength and sensation were intact. The rest of the clinical examination was without any particularities.
The patient has profited from a biological assessment, which did not indicate an inflammatory syndrome with normal erythrocyte sedimentation rate and CRP-test. Laboratory findings at that time demonstrated normocytic, normochromic anaemia (101 g/L), white blood cells and platelets were within normal limits. There was a mild increase in serum creatinine concentration (101 µmol/L), serum uric acid levels (374 µmol/L), lactatdehydrogenase and lipase levels (528 and 78 U/l, respectively), decreased total protein and albumin (39 and 23.7 g/l, respectively). Serum calcium was within normal limits, and the patient had subnormal magnesium levels (0.43 mmol/l) (Table 2). Urinalysis detected proteinuria (0.46 g/l) with albuminuria (10 mg/l), and increased albumin/creatinine ratio (3.2 mg/mmol). Moreover, additional investigations were performed for further evaluation. Serum protein electrophoresis with immunofixation revealed significant hypogammaglobulinemia with an excess of free kappa-(κ)-light chain of 715 mg/l (Table 2, Figure 1). Bone marrow biopsy and aspirate discovered high-grade infiltration by a mature plasma cell myeloma (degree of infiltration 90%, with aberrant co-expression of cyclin D1, CD56 negative, restriction for kappa light chains) with a subtotal displacement of resident haematopoiesis. The Congo red stain for amyloid on the trephine biopsy was negative. The fine-needle aspiration (FNA) of the abdominal fat showed AL-amyloid-deposits of the kappa-(κ)-light chain. The cytogenetic analysis revealed t(11;14)(q13.3;q32.3) rearrangement involving the CCND1 gene associated with multiple myeloma, as well as additional abnormalities (Table 3). Radiography of the dorsolumbar spine showed diffuse osteoporosis. MRI findings displayed diffuse degenerative changes, spondylolysis of L5 without spondylolisthesis, hypertrophic spondylarthrosis of the lower lumbar spine sections and Th11/12-kyphosis, without evidence of newly occurring fractures. Ultrasonography findings were within normal limits.
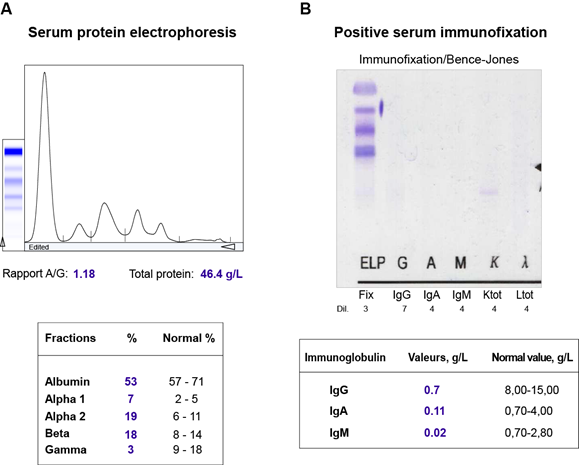
Figure 1: Some of the laboratory findings seen in our patient with multiple myeloma. (A) Serum protein electrophoresis showing hypogammaglobulinemia. (B) Positive serum immunofixation with an excess of free kappa-(κ)-light chain.
|
Substance |
Side effects * |
|||
|
Very common |
Frequent |
Occasional |
Rare |
|
|
Lenalidomide (Revlimid)
|
1. Psychiatric side effects: Insomnia, Depression. 2. Nervous system disorders: Peripheral neuropathy, Disturbance of sense of taste, Dizziness, Paraesthesia, Headache. |
1. Psychiatric side effects: Sedation, Asthenia, Confusion, Hallucinations, Mood swings, Anxiety, Irritability, Drowsiness. 2. Nervous system disorders: Circulatory disorders of the brain, Syncope, Numbness, Tremors, Memory problems, Neuralgia, Dysesthesia. |
1. Psychiatric side effects: Psychosis, Hypomania, Fixed thoughts, Low libido, Personality changes, Nervousness, Aggression, Nightmares.
2. Nervous system disorders: Stroke, Leuko-encephalopathy, Speech disorders, Attention disorders, Balance disorders, Motor disorders, Oral paraesthesia, Psychomotor hyperactivity, Anosmia, Ataxia, Dyskinesia, Motor dysfunction, Myasthenic syndrome. |
NO** |
|
Bortezomib (Velcade) |
1. Nervous system disorders: Peripheral neuropathy, Peripheral sensory neuropathy, Dysaesthesia, Neuralgia. |
1. Psychiatric side effects: Fluctuations in mood, Anxiety, Sleep disorders. 2. Nervous system disorders: Peripheral motor neuropathy, Loss of consciousness (including syncope), Headache, Dizziness, Taste disturbances, Lethargy. |
1. Psychiatric side effects: Psychosis, Hallucinations, Confusion, Impatience, Posterior reversible encephalopathy syndrome.
2. Nervous system disorders: Tremor, Peripheral sensorimotor neuropathy, Dyskinesia, Impaired memory, Encephalopathy, Reversible posterior encephalopathy syndrome (RPES), Coordination and balance disorder of cerebellar origin (including ataxia), Neurotoxicity, Convulsion, Post-herpetic neuralgia, Slurred speech, Restless legs syndrome, Migraine, Sciatica, Attention disorders, Abnormal reflexes, Parosmia, Autonomic neuropathy. |
1. Psychiatric side effects: Suicidality, Abnormal dreams, Maladaptation, Delirium, Psychosis, Low libido. 2. Nervous system disorders: Intracranial haemorrhage, Cerebral oedema, Cerebral haemorrhage, Transient ischemic attack, Coma, Cerebral palsy, Paralysis, Brain stem syndrome, Cerebrovascular disorder, Motor neuropathy, Nerve root injury, Spinal cord compression, Cognitive impairment, Paresis, Pre-syncope, Psychomotor hyperactivity, Autonomic nervous system imbalance, Motor dysfunction, Radiculitis, Nervous system disorders, Salivation, Hypotonia. |
|
Steroids (Dexamethasone) |
1. Psychiatric side effects: Psychosis, Mania, Hypomania, Depression, Hallucinations, Emotional lability, Mood Swings, Increased Energy, Insomnia, Pressured speech, Memory deficits, Decreased concentration, Agitation, Anxiety, Irritability, Increased initiative, Euphoria, Inner agitation, Suicidal behaviour. 2. Nervous system disorders: Elevation of intracranial pressure with papillary stasis (cerebral pseudotumor), Onset or aggravation of epilepsy, Convulsions, Dizziness, Headache. |
|||
* The frequency of chemotherapy-induced adverse effects is defined as follows: very common (≥1/10), frequent (≥1/100 to <1/10), occasional (≥1/1'000 to <1/100), rare (≥1/10'000 to <1/1000), very rare (<1/10'000), unknown (frequency cannot be estimated from the available data).
** NO, not observed.
Table 1: Common neuro-psychiatric adverse effects of several substances used for the treatment of MM in our patient (1) (12) (46).
|
Parameter |
Case |
Reference range |
Parameter |
Case |
Reference range |
|
Urea |
7.5 |
2.8-7.0 mmol/l |
Cell count: |
||
|
Creatinine in plasma |
101 |
50-95 μmol/l |
Leucocytes |
9,3 |
4.0-10.0 g/l |
|
Total protein |
39 |
65.0-80.0 g/l |
Erythrocytes |
3,27 |
4,00-5,00 T/l |
|
Albumin |
23.7 |
37.0-51.0 g/l |
Haemoglobin |
99 |
120-160 g/L |
|
Uric acid |
374 |
119-339 μmol/l |
Haematocrit |
0,29 |
0.37-0.47 l/l |
|
C-reactive protein |
<5 |
<5 mg/l |
MCV |
90 |
80-98 fl |
|
Troponin T hs |
39 |
<14 ng/l |
MCH |
30 |
29-35 pg |
|
NT-proBNP |
1’550 |
<300 ng/l |
MCHC |
338 |
320-370 g/l |
|
RDW (% anisocytosis) |
16 |
<15% |
|||
|
Platelets |
270 |
150-300 G/l |
|||
|
Sodium |
138 |
136-146 mmol/l |
Differentiation %: |
||
|
Potassium |
4.1 |
3.7-5.0 mmol/l |
Neutrophils |
57.6 |
45-75% |
|
Calcium |
2.21 |
2.20-2.55 mmol/l |
Barred Neutro. |
2 |
< 8.0% |
|
Ca correct. Alb 40g/l |
2.49 |
2.20-2.55 mmol/l |
Segmented nuclear neutro. |
67.5 |
45.0-70.0% |
|
Phosphate |
1.6 |
0.80-1.60 mmol/l |
Eosinophils |
1.5 |
< 4.0% |
|
Magnesium |
0.43 |
0.80-1.15 mmol/l |
Basophils |
0 |
< 2.0% |
|
AST |
21 |
< 40 U/l |
Monocytes |
3 |
< 8.0% |
|
ALT |
20 |
< 40 U/l |
Lymphocytes |
25.5 |
25.0-40.0% |
|
LDH |
528 |
< 450 U/l |
Plasma cells |
0.5 |
% |
|
Alkaline phosphatase |
103 |
35-105 U/l |
Blasts |
0 |
% |
|
Gamma-GT |
37 |
< 40 U/l |
Promyelocytes |
0 |
% |
|
Total bilirubin |
3.9 |
3.1-18.8 μmol/l |
Myelocytes |
0 |
% |
|
Direct bilirubin |
1.2 |
< 3.4 μmol/l |
Metamyelocytes |
0 |
% |
|
Lipase |
78 |
0-60 U/l |
Other cells |
0 |
% |
|
|
Erythroblasts |
0 |
% |
||
|
|
Erythro. morphology |
** |
|||
|
|
Leuko. morphology |
*** |
|||
|
Thrombo. Morphology |
**** |
||||
|
Serum protein electrophoresis: |
Absolute differentiation: |
|
|||
|
53 |
57.0-71.0% |
Neutrophils # |
4.95 |
2.0-7.5 G/l |
|
|
Albumin |
0.23 |
0.16-0.40 g/l |
Stab nuclear Neutro # |
0.19 |
0.3-0.8 G/l |
|
Prealbumin |
Segmented Neutro # |
6.28 |
1.5-7.0 G/l |
||
|
7 |
1.9-4.5% |
Eosinophils # |
0.14 |
0-0.7 G/l |
|
|
Alpha-1 globulin |
19 |
5.5-10.5% |
Basophils # |
0 |
0-0.2 G/l |
|
Alpha-2 globulin |
18 |
8.0-13.5% |
Monocytes # |
0.28 |
0.1-0.8 G/l |
|
Beta-1 + 2 globulins |
3 |
9,0-18,0% |
Lymphocytes # |
2.37 |
1.0-4.0 G/l |
|
Gamma-globulins |
Pos. * |
Neg. |
|||
|
Immunofixation |
0.11 |
0.70-4.00 g/l |
Reticulocytes: |
||
|
Immunoglobulin IgA |
0.7 |
8,00-15,00 g/l |
Reti (automatic) |
18 |
6-22 p. mille |
|
Immunoglobulin IgG |
< 0.02 |
0.70-2.80 g/l |
Reti absolute value |
60 |
25-105 G/l |
|
Immunoglobulin IgM |
|||||
|
Report free Kappa/Lambda |
715 |
3.3-19.4 mg/l |
|||
|
Free light chains Kappa- serum |
1 |
5.7-26.3 mg/l |
|||
|
Free light chains Lambda-serum |
715 |
0.26-1.65 |
|||
|
Kappa/Lambda free light chains ratio |
* Presence of a free kappa monoclonal band. ** Ovalocytes +/++, poikilocytosis +/++, anisochromasia, anisocytosis +++, microcytes +/++, money-roll formation of erythrocytes +/++. *** Döhle body: coarse granulation +, maturation disorder, pycnotic nuclei, isolated cell nuclei, atypical lymphocytes, stimulated lymphocytes +. **** Anisocytosis of thrombocytes + /+ +, giant thrombocytes + /+ +.
Table 2: Laboratory results of the patient.
|
Type of anomaly |
Involved genes/chromosomes |
Results |
Frequency |
Methods |
|
Anomalies with prognostic value |
||||
|
Hyperdiploidy |
- |
ND* |
- |
puce ADN |
|
Perte 1p |
FAF1, CDKN2C/FAM46C |
ND |
- |
puce ADN |
|
Gain 1q |
CKSIB |
ND |
- |
puce ADN |
|
Perte 17p13.1 |
TP53 |
ND |
- |
puce ADN |
|
Rearrangement |
IGH |
detected |
95,5% |
FISH |
|
t(11;14)(q13.3;q32.3) |
IGH-CCNDl |
detected |
97% |
FISH |
|
t(4;14)(p16.3;q32.3) |
IGH-FGFR3 |
NS** |
- |
FISH |
|
t(14;16)(q32.3;q23.2) |
IGH-MAF |
NS |
- |
FISH |
|
Mutation |
TP53 |
NS |
- |
FISH |
|
Anomalies with no clear prognostic value |
||||
|
gain 6p21.33p21.33 |
- |
detected |
90% |
puce ADN |
|
gain 11q13.2q13.2 |
- |
detected |
90% |
puce ADN |
|
perte 11q13.3q13.3 |
- |
detected |
90% |
puce ADN |
|
gain 11q13.3q13.3 |
CCNDl |
detected |
90% |
puce ADN |
|
gain 11q13.4q13.4 |
- |
detected |
90% |
puce ADN |
|
perte 13q31.1q34 |
- |
detected |
90% |
puce ADN |
|
gain 17q11.2q12 |
- |
detected |
90% |
puce ADN |
|
gain 19p13.3p13.11 |
- |
detected |
90% |
puce AND |
|
monosomie X |
- |
detected |
20% |
puce AND |
* ND, not detected. ** NS, not sought. This analysis revealed an unbalanced t (11;14) (q13;q32) complex involving the CCND1 gene and additional abnormalities.
Table 3: Found genomic abnormalities.
In addition, esophagogastroduodenoscopy and colonoscopy revealed amyloid deposits in biopsies from the stomach, duodenum, terminal ileum, descending colon, sigmoid, and rectum. According to cardio-oncologic consilium and based on the clinic features of our cardiac decompensated, markedly hypotensive patient with known diastolic cardiac insufficiency and hypertensive and valvular cardiopathy with moderate aortic stenosis, persistent atrial fibrillation, we suspect also the cardiac involvement by multiple organ AL-amyloidosis.
Given the combination of patient’s chronic back pain, multiple vertebral fractures and severe osteoporosis, multiorgan AL-amyloidosis, unexplained anemia and acute kidney injury on laboratory analysis, hypogammaglobulinemia with excess of free kappa-(κ)-light chain, more than 90% plasma cells on bone marrow biopsy, the presence of t(11;14)(q13.3;q32.3) rearrangement and immunophenotype CD56(-), the diagnosis of light chain-multiple myeloma with secondary AL-amyloidosis was established. The patient was in clinical stage II according to the both International Staging System (ISS) and revising ISS (R-ISS) for plasma cell myeloma and stage IIA according to the Durie-Salmon staging system [3, 18]..
The patient was referred to the oncology center for further management, where she was treated with a chemotherapy regimen included initially the proteasome inhibitor bortezomib (Velcade, 2.15 mg) administrated subcutaneous on days 1, 4, 8, 11; immunomodulatory agent lenalidomide (Revlimid, 25 mg) administrated per os on days 1 through 14; and dexamethasone (Fortecortin, 8 mg) administrated per os on days 1, 2, 4, 5, 8, 9, 11, 12. During this treatment, the patient withdrew from the agranulocytosis and developed pancytopenia, which was interpreted in the context of the high degree of myeloid infiltration with plasma cells with suppression of haematopoiesis, aggravated by chemotherapy. The first cycle of chemotherapy was terminated prematurely and the second chemotherapy cycle with Velcade 2.18 mg subcutaneous weekly was taken in monotherapy for better compatibility.
Since the begin of the chemotherapy, the patient’s family noticed some changes in her mood, sleep disturbances and significantly worsened cognitive impairment with a marked impact on ADL and IADL. One week after the hasty cessation of the first chemotherapy cycle, the symptoms became more prominent and the patient was admitted again to our hospital for cognitive assessment. On physical examination, the patient was alert, markedly elated, agitated, hyperactive, and very talkative, total disoriented to place and time, but oriented to person and in acute pain. Our patient had episodes of elevated and excited mood, increased energy, restlessness, decreased sleep (less than 3 hours each night), maniac episodes, racing thoughts, logorrhoea and pressured speech. Moreover, she also had periods of high distraction and poor concentration, forgetfulness, loss of interest for the therapy and cognitive impairment with verbal memory deficits. Persecutory delusion, grandiose delusion, and conversations with voices were suspected. She complained that the nurses took her for someone else with the same last and first name. She called more than 10 times per day her family physician in order to say that we made a mistake about the patient and she should not be here. She was not depressed. According to her daughter, she understood about her cancer diagnosis and there was no report of other psychological stressors at the time.
Previously, we performed imaging of the head, which revealed a leucocytopathy of stage 2 and osseous involvement of the calvaria, without evidence of circulatory failure, encephalitis, or cerebral amyloidosis. Neurological examinations and laboratory data showed no abnormality. A neuropsychological examination revealed moderately severe disorders in attention, comprehension, severe impairment in episodic memory and executive function with a score of 23 out of 30 in the MoCA, of 21 out of 30 in the MMSE, and 12 out of 19 in the CAM-S LF. Since there was no suggestive evidence of brain metastasis, the patient was diagnosed with substance intoxication delirium combinated with psychotic symptoms and mania-episodes, according to Diagnostic and Statistical Manual of Mental Disorders, 4th Edition, Text Revision (DSM-IV-TR).
Pharmaceutically, a therapy with Zyprexa and Stilnox was initiated. The increasement of olanzapine dosage from 5 to 10 mg/day failed to control the patient’s symptoms. Owing to the acute state of confusion with elevated predisposition to walk away and the need for treatment, a medical FU was issued on 24.05.19 by the colleagues of psychiatry. Due to the increasingly agitated and disoriented patient, a psychiatric reconcile was performed and therefore the medication was switched to the haloperidol in combination with diazepam and quetiapine. After a dose adjustment, at 4 mg/day of Haldol, 5 mg/day of Valium and 75 mg/day of Quetiapin, her symptoms were spectacularly improved. By the third week of hospitalization, elated mood, agitation, pressured speech, and grandiose delusion had remitted and memory had bettered. Although the light episodic memory dysfunction with temporal disorientation remained, the patient had recovered her independence in ADL and IADL and could return home.
4. Discussion
Oncological patients often develop multiple complications, including psychiatric disorders, due to the direct impact of cancer on metabolic processes and immune system, resulting in inflammatory and neurochemical changes, the appearance of brain metastasis, vascular disorders, paraneoplastic and autoimmune syndromes, in addition to secondary effects of related chemotherapy [19].
In our case report, we described a patient with a new diagnosed multiple myeloma affected by a severe, fulminant and persisting mixed psychiatric disorder, including hyperactive delirium with psychotic symptoms and mania-episodes after the first cycle of Velcade-Revlimid-Dexamethasone-based treatment. The neurological and psychiatric manifestations with an important impact on ADL and social life appeared progressively after the first week of chemotherapy initiation. Our patient presented total disorientation to place and time and severe cognitive impairment in episodic memory and executive function, i.e. the ability to learn and retain new information. This acute cognitive decline was evidenced by poor performance in the MoCA, MMSE, CAM-S LF, and in the 3D-CAM.
The therapy of multiple myeloma comprises the combination of different anti-neoplastic agents, leading to multiple side effects that occur during and can persist long after therapy, causing serious physical, psychic and cognitive problems [9, 10, 16, 20]. Patients with isolated delirium usually take a few days to one week to get better, whereas patients with psychosis, mania, or mixed conditions typically recuperate longer [21]. Moreover, elderly vulnerable patients are more likely than younger fit ones to develop chemotherapy-induced cognitive disorders, also known as “chemobrain”, reflecting age- and disease-associated changes in the immune system and brain neurochemistry [22, 23]. However, the pathogenic mechanisms underlying the development of temporal and long persisting chemotherapy-related neuropsychiatric adverse effects remain poorly understood.
Recent studies have shown that frank psychosis, mania alone and/or in combination with minor psychiatric disturbances (like irritability, insomnia, anxiety, labile mood, etc.), and even delirium, are possible systemic corticosteroid-induced adverse effects, appearing early, mostly in females, without evident correlation between administrated doses and severity of side-effects, but typically reversible [15, 21, 24]. Glucocorticoids increase the brain sensitivity to neurotoxic effects of drugs and aggravate cognitive deficits via activation of transactive response DNA-binding protein 43 (TDP-43) and its 25 kDa C-terminal fragment (TDP-25) [25]. Novel antineoplastic agents trigger molecular changes on gene expression levels and chromatin remodelling [23, 26], protein and lipids levels [27, 28], brain-derived neurotrophic factor levels [29], oxidative stress via accumulation of the reactive oxygen species (ROS) [30, 31], cytokine dysregulation [20], epigenetic alterations [32, 33], and vascular abnormalities [34]. Moreover, they provoke direct structural abnormalities in the various brain region including the hippocampus, frontal, parietal and parietal regions, and prefrontal cortices, and functional changes in the central nervous system (CNS), producing transit and persistent psychiatric side effects [20, 23, 35-39].
The comprehensive geriatric assessment and geriatric complex treatment, including objective evaluation of the health status of older people, focusing on somatic, functional, and psychosocial spheres, is a more sensitive and successful method in the healing of elderly patients with multiple myeloma. Different neuropsychological tests provide a comprehensive evaluation of cognitive function and carefully estimate the seriousness of chemotherapy-induced impairment [20, 40-42]. Recent studies have schown that regular physical activity together with psychiatric medication significantly improves cognitive function and quality of live in patients under chemotherapy [43, 44]. Occurrence and severity of chemotherapy-induced delirium often depend on the age, genetic risk factors and premorbid personality organization [22, 45], whose role cannot be excluded in the pathogenesis of fulminant manifestation of psychiatric symptoms. However, several lines of evidence suggest that dexamethasone in combination with lenalidomide caused the cognitive disorders noticed in our patient. Initially, there was a temporal correlation between the observed symptomes and medicaments’ administration. Our patient had an insignificant psychiatric history and her family did not remark any of cognitive problems before starting the VRD-treatemnt. The following chemotherapy neuropsychiatric deterioration was very acute and unexpected. Normally, a patient with neoplasia, who develops psychiatric disorders, should be evaluated for potential organic aetiologies, especially brain metastasis. Despite wide-ranging investigations performed in our clinic no infectious, vascular, metabolic disorders or metastases were found. The signs of depression were also excluded. The medication-induced anticholinergic and neuroleptic malignant syndromes were also excluded. Although the patient had been taking oxycodone 5 mg/d for some time, the diagnosis of serotonin syndrome was also improbable. Finally, we observed a considerable improvement of symptoms after dexamethasone-lenalidomide withdraw, following by specific psychiatric medication. Our experience indicates that the combination of haloperidol, quetiapine and diazepam could be used for good management of complexe, combaines psychiatric disorders.
The present rapport underlines the significance of chemotherapy-related side effects for life quality of elderly polymorbid individuals concerning all physicians, and providing a significant challenge to patient management, increasing hospital stays and health care costs. The available data on incidence and prevalence of chemotherapy-induced psychiatric adverse events in geriatric patients are poor, and there is a lack of clinical trial studies evaluating the structural and functional impact of chemotherapy on the brain and the efficacy of proposed treatment in these patients. Thus, additional studies on the pharmacotherapy of chemotherapy-related psychiatric disorders are needed in order to clarify underlying mechanisms and to develop brain repair strategies in order to improve clinical outcomes.
Conflict of Interest
The authors declare no conflict of interest to report.
Acknowledgments
We would like to thank the patient, the doctors, the nurses and laboratory technicians of HFR Fribourg the cantonal hospital and Inselspital (Bern) whose works made this report possible.
Author Contributions
OP conceived of the paper, researched the literature, and wrote the manuscript. All authors reviewed and approved the paper.
References
- Al-Farsi K. Multiple myeloma: an update. Oman Med J 28 (2013): 3-11.
- Gerecke C, Fuhrmann S, Strifler S, et al. The Diagnosis and Treatment of Multiple Myeloma. Dtsch Arztebl Int 113 (2016): 470-476.
- Michels TC, Petersen KE. Multiple Myeloma: Diagnosis and Treatment. Am Fam Physician 95 (2017): 373-383.
- Fairfield H, Falank C, Avery L, et al. Multiple myeloma in the marrow: pathogenesis and treatments. Ann N Y Acad Sci 1364 (2016): 32-51.
- Rajkumar SV, Kumar S. Multiple Myeloma: Diagnosis and Treatment. Mayo Clin Proc 91 (2016): 101-119.
- Girnius S, Munshi NC. Challenges in multiple myeloma diagnosis and treatment. Leuk Suppl 2 (2013): S3-S9.
- Collins CD. Problems monitoring response in multiple myeloma. Cancer Imaging. 5 Spec No A (2005): S119-S126.
- Kumar SK, Rajkumar SV. The multiple myelomas - current concepts in cytogenetic classification and therapy. Nat Rev Clin Oncol 15 (2018): 409-421.
- Munshi NC, Anderson KC. New strategies in the treatment of multiple myeloma. Clin Cancer Res 19 (2013): 3337-3344.
- Tuchman SA, Shapiro GR, Ershler WB, et al. Multiple myeloma in the very old: an IASIA conference report. J Natl Cancer Inst 106 (2014).
- Kyle RA, Rajkumar SV. Treatment of multiple myeloma: a comprehensive review. Clin Lymphoma Myeloma 9 (2009): 278-288.
- Merchionne F, Perosa F, Dammacco F. New therapies in multiple myeloma. Clin Exp Med 7 (2007): 83-97.
- Remes K, Anttila P, Silvennoinen R, et al. Real-world treatment outcomes in multiple myeloma: Multicenter registry results from Finland 2009-2013. PLoS One 13 (2018): e0208507.
- Field-Smith A, Morgan GJ, Davies FE. Bortezomib (Velcadetrade mark) in the Treatment of Multiple Myeloma. Ther Clin Risk Manag 2 (2006): 271-279.
- Brown ES, Chandler PA. Mood and Cognitive Changes During Systemic Corticosteroid Therapy. Prim Care Companion J Clin Psychiatry 3 (2001): 17-21.
- Meyers CA. How chemotherapy damages the central nervous system. J Biol. 2008;7(4):11.
- Ahmed S, Leurent B, Sampson EL. Risk factors for incident delirium among older people in acute hospital medical units: a systematic review and meta-analysis. Age Ageing 43 (2014): 326-333.
- Filonzi G, Mancuso K, Zamagni E, et al. A Comparison of Different Staging Systems for Multiple Myeloma: Can the MRI Pattern Play a Prognostic Role? AJR Am J Roentgenol 209 (2017): 152-158.
- Nolan C, DeAngelis LM. The confused oncologic patient: a rational clinical approach. Curr Opin Neurol 29 (2016): 789-796.
- Pearre DC, Bota DA. Chemotherapy-related cognitive dysfunction and effects on quality of life in gynecologic cancer patients. Expert Rev Qual Life Cancer Care 3 (2018): 19-26.
- Warrington TP, Bostwick JM. Psychiatric adverse effects of corticosteroids. Mayo Clin Proc 81 (2006): 1361-1367.
- Chari D, Ali R, Gupta R. Reversible dementia in elderly: Really uncommon? Journal of Geriatric Mental Health 2 (2015): 30-37.
- Briones TL, Woods J. Chemotherapy-induced cognitive impairment is associated with decreases in cell proliferation and histone modifications. BMC Neurosci 12 (2011): 124.
- Ling MH, Perry PJ, Tsuang MT. Side effects of corticosteroid therapy. Psychiatric aspects. Arch Gen Psychiatry 38 (1981): 471-477.
- Caccamo A, Medina DX, Oddo S. Glucocorticoids exacerbate cognitive deficits in TDP-25 transgenic mice via a glutathione-mediated mechanism: implications for aging, stress and TDP-43 proteinopathies. J Neurosci 33 (2013): 906-913.
- Asor E, Ben-Shachar D. Gene expression dynamics following mithramycin treatment: A possible model for post-chemotherapy cognitive impairment. Clin Exp Pharmacol Physiol 45 (2018): 1028-1037.
- Evans AR, Miriyala S, St Clair DK, et al. Global effects of adriamycin treatment on mouse splenic protein levels. J Proteome Res 11 (2012): 1054-1064.
- Mohammadi AS, Li X, Ewing AG. Mass Spectrometry Imaging Suggests That Cisplatin Affects Exocytotic Release by Alteration of Cell Membrane Lipids. Anal Chem 90 (2018): 8509-8516.
- Zimmer P, Mierau A, Bloch W, et al. Post-chemotherapy cognitive impairment in patients with B-cell non-Hodgkin lymphoma: a first comprehensive approach to determine cognitive impairments after treatment with rituximab, cyclophosphamide, doxorubicin, vincristine and prednisone or rituximab and bendamustine. Leuk Lymphoma 56 (2015): 347-352.
- Gaman AM, Uzoni A, Popa-Wagner A, et al. The Role of Oxidative Stress in Etiopathogenesis of Chemotherapy Induced Cognitive Impairment (CICI)-"Chemobrain". Aging Dis 7 (2016): 307-317.
- Aluise CD, Sultana R, Tangpong J, et al. Chemo brain (chemo fog) as a potential side effect of doxorubicin administration: role of cytokine-induced, oxidative/nitrosative stress in cognitive dysfunction. Adv Exp Med Biol 678 (2010): 147-156.
- Shi DD, Huang YH, Lai CSW, et al. Chemotherapy-Induced Cognitive Impairment Is Associated with Cytokine Dysregulation and Disruptions in Neuroplasticity. Mol Neurobiol 56 (2019): 2234-2243.
- Wang XM, Walitt B, Saligan L, et al. Chemobrain: a critical review and causal hypothesis of link between cytokines and epigenetic reprogramming associated with chemotherapy. Cytokine 72 (2015): 86-96.
- Abdel-Aziz AK, Mantawy EM, Said RS, Helwa R. The tyrosine kinase inhibitor, sunitinib malate, induces cognitive impairment in vivo via dysregulating VEGFR signaling, apoptotic and autophagic machineries. Exp Neurol 283 (2016): 129-141.
- Li M, Caeyenberghs K. Longitudinal assessment of chemotherapy-induced changes in brain and cognitive functioning: A systematic review. Neurosci Biobehav Rev 92 (2018): 304-317.
- Nokia MS, Anderson ML, Shors TJ. Chemotherapy disrupts learning, neurogenesis and theta activity in the adult brain. Eur J Neurosci 36 (2012): 3521-3530.
- Christie LA, Acharya MM, Parihar VK, et al. Impaired cognitive function and hippocampal neurogenesis following cancer chemotherapy. Clin Cancer Res18 (2012): 1954-1965.
- Ponto LL, Menda Y, Magnotta VA, et al. Frontal hypometabolism in elderly breast cancer survivors determined by [(18)F]fluorodeoxyglucose (FDG) positron emission tomography (PET): a pilot study. Int J Geriatr Psychiatry 30 (2015): 587-594.
- Silverman DH, Dy CJ, Castellon SA, et al. Altered frontocortical, cerebellar, and basal ganglia activity in adjuvant-treated breast cancer survivors 5-10 years after chemotherapy. Breast Cancer Res Treat 103 (2007): 303-311.
- Vardy J, Wong K, Yi QL, et al. Assessing cognitive function in cancer patients. Support Care Cancer 14 (2006): 1111-1118.
- Cheung YT, Foo YL, Shwe M, et al. Minimal clinically important difference (MCID) for the functional assessment of cancer therapy: cognitive function (FACT-Cog) in breast cancer patients. J Clin Epidemiol 67 (2014): 811-820.
- Vasunilashorn SM, Guess J, Ngo L, et al. Derivation and Validation of a Severity Scoring Method for the 3-Minute Diagnostic Interview for Confusion Assessment Method--Defined Delirium. J Am Geriatr Soc 64 (2016): 1684-1689.
- Hillman CH, Erickson KI, Kramer AF. Be smart, exercise your heart: exercise effects on brain and cognition. Nat Rev Neurosci 9 (2008): 58-65.
- McNeely ML, Campbell KL, Rowe BH, et al. Effects of exercise on breast cancer patients and survivors: a systematic review and meta-analysis. Cmaj 175 (2006): 34-41.
- Dietrich J, Prust M, Kaiser J. Chemotherapy, cognitive impairment and hippocampal toxicity. Neuroscience 309 (2015): 224-232.
- Punke AP, Waddell JA, Solimando DA, Jr. Lenalidomide, Bortezomib, and Dexamethasone (RVD) Regimen for Multiple Myeloma. Hosp Pharm 52 (2017): 27-32.


 Impact Factor: * 5.3
Impact Factor: * 5.3 Acceptance Rate: 75.63%
Acceptance Rate: 75.63%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 
