Monitoring the Clinical Evolution of A Psychotic Patient Presenting A First-Schizophrenic Episode Thanks to Bimodal Oddball-P300 Event-Related Potentials: First Evidence from A Single-Case Study
Hendrik Kajosch, Florence Hanard, Geerke Steegen, Georgios Persefonis, Agnieszka Cimochowska, Steve Michel, Charles Kornreich, Salvatore Campanella*
Laboratoire de Psychologie Médicale et d’Addictologie, ULB Neuroscience Institute (UNI), CHU Brugmann-Université Libre de Bruxelles (U.L.B.), Belgium
*Corresponding Author: Salvatore Campanella, Research Associate, The Belgian Fund for Scientific Research (F.N.R.S.), CHU Brugmann, Psychiatry Secretary, 4, Place Vangehuchten - B-1020 Brussels, Belgium
Received: 20 October 2020; Accepted: 03 November 2020; Published: 07 December 2020
Article Information
Citation:
Hendrik Kajosch, Florence Hanard, Geerke Steegen, Georgios Persefonis, Agnieszka Cimochowska, Steve Michel, Charles Kornreich, Salvatore Campanella. Monitoring the Clinical Evolution of A Psychotic Patient Presenting A First-Schizophrenic Episode Thanks to Bimodal Oddball-P300 Event-Related Potentials: First Evidence from A Single-Case Study. Archives of Clinical and Medical Case Reports 4 (2020): 1194-1207.
View / Download Pdf Share at FacebookAbstract
Objective: Previous studies showed that a bimodal oddball design allows to increase the sensitivity of the P300 in subjects with anxious-depressive tendencies and patients presenting an adjustment disorder. In this case report we illustrate the increased sensitivity of a bimodal oddball task in the clinical follow-up of a patient presenting a psychosis in the context of a first onset schizophrenia.
Method: The patient was instructed to detect deviant stimuli in three separate oddball tasks by hitting a button. Tasks comprised an auditory, a visual and a synchronized and congruent audio-visual block of stimuli. The peak-latency and the amplitude of the P300 evoked response were compared and confronted to scores of the clinical assessment.
Results: Only the bimodal task mirrored the clinical evolution of the patient, as the reduction of psychotic symptoms was accompanied by an increase of the bimodal P300 amplitude. However, this was not true for both unimodal tasks (auditory and visual).
Conclusions: A bimodal oddball design combined with unimodal ones could be necessary to monitor the joined clinical/neurophysiological evolution of a patient presenting a first onset psychosis.
Significance: Further longitudinal studies applying the bimodal P300 paradigm should be designed in order to verify whether the oddball-bimodal P300 component could be used as “state” and/ or “trait” biological marker of the disease.
Keywords
<p>P300; Bimodal; Oddball; Schizophrenia; Sensitivity</p>
Article Details
1. Introduction
This case report aims at testing the clinical efficiency of a bimodal event-related potential (ERP) P300 paradigm to monitor the clinical evolution of a patient presenting a first psychotic decompensation in the context of a first onset schizophrenia.
The Electroencephalography (EEG) is an important, non-invasive and cost-efficient medical technique allowing the investigation of electrical activity in the brain. It is a useful tool in the differential diagnosis of psychiatric, neurological and medical conditions that can help monitor and evaluate the clinical and/or therapeutic course of psychiatric disorders [1]. ERPs like the P300 are time-related voltage fluctuations linked to internal or external events (e.g. physical properties of stimuli, decisions and responses linked to these stimuli) and can be isolated from the current EEG by a signal averaging method. They are of major clinical interest in psychiatry because of their capacity to indicate cortical neuronal dysfunction in different neuropsychiatric disorders [2]. It is the physiological relationship between ERPs and specific, well-defined cognitive functions that gives them a complementary interest compared to the classical clinical approach based on medical examinations and a behavioral analysis. Indeed, ERPs are true indicators of dysfunctional, cognitive processes, which are not accessible to the classical clinical approach [2]. As ERPs reflect postsynaptic effects of released neurotransmitters and neuromodulators that have an effect on the cortical neuronal function, they can be considered as excellent candidates to monitor millisecond –resolution measured alterations of the cortical function induced by psychotropic substances [2, 3]. Thanks to their closeness to the underlying (patho)physiological mechanisms of psychiatric disorders, ERPs might serve as endophenotypic markers that present a higher sensitivity and sensibility in this context than clinical symptoms or neuropsychological testings [3]. Because ERPs are intrinsically dynamic, they have the potential to follow functional brain changes over short periods of time and thus might deliver biological markers of progressive neurophysiological decline in schizophrenia [4]. In his attempt to highlight the pathophysiological mechanisms of schizophrenia, Mathalon describes the visual P300 as a marker of state which tracks the effects of antipsychotic medication and the auditory P300 as a marker indicating trait as well as state effects [5]. Recently, the visual and auditory P3b component of the P300 has been identified as a potential vulnerability marker of an imminent risk of psychosis [6]. A state marker is defined as an indication of a P300 abnormality only present during an acute disease exacerbation, whereas a trait marker displays a P300 abnormality present during an acute exacerbation of the disease and persisting after a remission of the acute symptomatology (state or trait marker) [5, 7]. A vulnerability marker is specified as an abnormality which is detectable even before the emergence of disease symptoms in subjects with an increased risk to develop a pathology [8].
The P300 is an evoked potential that can be elicited by a classical two-stimulus oddball task (presenting an infrequent target in the background of a frequent stimulus [9]), with a late positive waveform between 300 and 700 ms and a maximal amplitude for visual stimuli at parietal sites [10]. In addition, the P300 is functionally related to high level cognitive processes such as short-term memory updating [11] and cognitive closure mechanisms [12].
The research using ERP and especially the P300 already exists for more than 50 years and generated very precious considerations that can be applied in clinical issues as a complementary use (like the diagnostic process and the follow-up of the evolution of a psychopathological symptomatology). Considering that schizophrenia is a disease characterized by a disturbance of thinking as a result of pathologic neural connectivity, and that the P300 represents important cognitive components like short-term memory updating [6] and cognitive closure mechanisms [12], the P300 seems to be an excellent candidate to trace and illustrate clinical aspects of the pathophysiology of the disease. In this view, we would first like to outline some important statements resulting from previous research on schizophrenic patients that illustrate the relevance of the use of the P300 components in the clinical context:
- The P300 amplitude is definitely reduced in patients suffering from schizophrenia. This is the result of many cross-sectional studies asserting that trait effects can be disclosed by comparing patients to healthy controls and that state effects can be uncovered by illustrating a negative correlation between the severity of negative symptoms and the amplitude of the auditory P300 [5]. In a longitudinal retrospective study, Mathalon concluded that auditory and possibly visual P300 amplitudes track fluctuations in clinical state, but that only the auditory P300 amplitude has to be considered as a trait marker of schizophrenia [5]. The visual P300 is likely to be more sensitive to track clinical improvement due to administered medication. A recent study of Kim et al. confirms the reduction of the amplitude of the P300 as an endophenotype for schizophrenia. Indeed, the study analyzes the inter-trial variability of the P300 in patients suffering from schizophrenia, subjects at genetic high risk, subjects with an increased clinical risk and healthy controls with the aim to disentangle aspects of genetic predisposition and current clinical status [13]. In the context of this case report, a meta-analysis realized in 2014 confirms that a disturbed information processing occurs in first episode schizophrenia patients who manifest a decreased amplitude and a delayed latency of the P300 [14].
- When we are talking about state and trait aspects of biomarkers, it is important to take into account that biological abnormalities (such as a decreased amplitude and a longer latency of the P300 component in mental disorders like schizophrenia) may reflect both static (stable attributes of the schizophrenia phenotype disclosing abnormal brain development and/or discrete brain lesions) and dynamic (neurodevelopmental and neurodegenerative processes and non-progressive fluctuations in specific symptom domains) aspects of the disease. Given the dynamic nature of schizophrenia (in its evolution), data need to be collected at various times over the clinical course in the context of prospective longitudinal studies. A study design of these aspects should also allow to disentangle state and trait influences [5].
- The P300 component – and especially the auditory P3b component which is considered to be a trait marker of schizophrenia – represents both state and trait aspects of the pathophysiology of this disease. This means that, even if the P300 presents a stable alteration in amplitude in a group of patients suffering from schizophrenia, it also indicates alterations of the clinical state due to an increasing or decreasing symptomology in this same group of patients [4]. Therefore the significance of trait and state should not be used in absolute terms.
- In their discussion about the role of ERPs in the creation of subgroups of schizophrenic patients, Luck and colleagues underlined the importance of the methodological development of single subject data in order to characterize an individual within a group. This seems particularly important in a clinical context in order to support a health care practitioner to define the potential treatment targets of a patient suffering from schizophrenia [3].
- Another aspect of particular importance and interest is that schizophrenia is in its very heterogeneous presentation and evolution a brain disease characterized by a “disturbance in thinking” as a result of a process of pathologic neural connectivity [15]. From this point of view, the characterizing properties of ERPs like the P300, that particularly represent this aspect of neural connectivity, make them once again an excellent tool for the investigation of the pathophysiology of this disease on the one hand, and for the clinical follow-up of a singular subject suffering from schizophrenia on the other hand. Mathalon resumes this in his considerations about state and trait aspects reflected by the P300 component, concluding that the P300 amplitude as marker of state and trait is particularly suitable to illustrate the progressive neuropathological processes of schizophrenia over the long term of the illness [4].
To summarize the essential aspects of the relevance of the P300 in a clinical context for patients suffering from schizophrenia, we can state that only its auditory component presents a trait aspect in comparison with healthy controls, and that the auditory P300 and probably the visual P300 have the capacity to track fluctuations in the clinical state. State and trait aspects should not be used in absolute terms when we are thinking and talking about the characteristics of the P300. The P300 represents state and trait aspects in a dynamic way at the same time. Considering that schizophrenia is a disease characterized by a disturbance of thinking as a result of pathologic neural connectivity, the P300 seems to be an excellent candidate to trace and illustrate clinical aspects of the pathophysiology of the disease, thanks to its important cognitive components such as short-term memory updating [6] and cognitive closure mechanisms [12]. Indeed, the cognitive correlates of the described (positive and negative) symptoms and probably their evolution could be investigated to determine whether the recording of the signal is realized by an adapted technical methodology that allows the description of single subject data (and this, even if the idea of the collection of normative data for ERPs seems to keep a certain interest for the clinical context).
In the present study, we suggest that a bimodal paradigm of the P300, which has already disclosed an improved sensitivity, could help to track more reliably the clinical evolution of the symptoms of a single patient suffering from schizophrenia (concept of personalized medicine [16]). The specific motivation to apply a bimodal paradigm in comparison to unimodal paradigms lies in the fact that schizophrenia is essentially a pathology of neural connectivity. Hence, the bimodal concept should allow to more closely approach the supposed pathophysiology of the disease by accessing integrative processes and then by augmenting its sensitivity compared to unimodal paradigms. Indeed, earlier studies demonstrated an augmented sensitivity of the bimodal design of the P300 [17, 18] to record a more powerful signal of the P300 in response to psychopathological traits. The idea that the concept of a bimodal stimulation seems to be a more ecological form of stimulation than unimodal ones results from the finding that multisensory convergence already exists in low level cortical structures [19]. The application of an audiovisual bimodal design of the P300 allowed us to discriminate a group of healthy subjects presenting subclinical tendencies of anxiety and depression from a group of healthy subjects which were free free of such tendencies. In contrast, the unimodal conditions did not unveil any differences between the two groups [17]. The augmented sensitivity of the P300 in a bimodal design has been confirmed by a subsequent study in a clinical setting with patients presenting an adjustment disorder [20]. This bimodal interactions seem to be disturbed in patients suffering from schizophrenia as shown by de Jong [21] which is another time in line with one of the main hypotheses of Andreasen, stating that schizophrenia is a “disease of neuronal connectivity”[15].
We would like to illustrate that this augmented sensitivity should unveil the possibility to implement the acquisition of the bimodal P300 in the clinical context of the individual follow up of a patient suffering from schizophrenia. To do so, we confronted this patient to the recording of auditory, visual and bimodal P300 oddball components at three moments: at the beginning of his hospitalization in a state of acute psychotic decompensation (t0), at the end of the hospitalization assuming that the patient presents a clinical evolution allowing him to leave the hospital ward (t1) and six months following the discharge of the hospital ward provided that he presented a clinical evolution which did not demand a hospitalization in this period (t2). At the three moments of acquisition, the patient was also submitted to an evaluation of the different categories of clinical symptoms (psychotic, mood and anxiety symptoms). Our main idea was to monitor whether the clinical evolution of the patient could be mirrored at the neurophysiological level by the auditory and/or visual and/or bimodal P300 component. Our main hypothesis was that (1) because schizophrenia is a connectivity disorder; and (2) because bimodal P300 involved specific integrative processes compared to unimodal P300, the bimodal P300 would be better suited than unimodal P300s to mirror the clinical evolution of this single patient.
2. Methods
2.1 Clinical case
In this case report, we illustrate the evolution of the P300 at different clinical stages by studying the clinical evolution at three different moments in the case of a 30 year old man presenting a first onset psychosis without any preliminary medical history neither in the personal nor in the family context. The first psychotic symptoms were reported by hetero-anamnesis (family) one year before the first non-voluntary hospitalization after a pathological journey and a car accident without physical injury in a paranoid delusional context. Previously he also presented a deterioration of social functioning: he lost his work in a public transport service because of paranoid interpretations concerning his work environment, he got progressively isolated even in the family context, presenting strange religious ideas and an increasingly aggressive attitude towards others. At the beginning of the hospitalization there was also a complete unconsciousness of his illness. Ideas of reference towards television and radio, a disturbed bizarre contact, soliloquies with religious content and an anxious fidgetiness have been reported in the hospital ward. The patient confirmed also the presence of auditory hallucinations. His behavior and discourse presented elements of disorganization. A treatment with olanzapine was initialized and titrated to a daily dose of 20 mg. A temporary treatment of Lorazepam was admitted for the treatment of anxiety symptoms. The oral treatment of olanzapine was switched to olanzapine-pamoate depot with a dose of 300 mg in a biweekly treatment rhythm. The patient presented a progressive positive evolution in a period of seven weeks: the delusional ideas cleared up and the social contact notably improved, whereas auditory hallucinations and a certain level of disorganization persisted until the end of the hospitalization. The treatment setting continued by the admission in a day hospital for six months, followed by a discharge of the hospital context and a further follow-up in a ambulant setting of a psychiatric consultation. At that moment the patient presented a further improvement of his psychotic symptoms and his self-awareness of the presence of a psychotic disorder even when mild levels of auditory hallucinations and discrete signs of behavioral disorganization persisted. Following the wish of the patient, the depot application of the treatment was then switched again to an oral treatment of olanzapine with a daily dose of 10 mg one month before the third and last assessment. The diagnosis of paranoid schizophrenia was confirmed by the application of the Structured Clinical Interview for DSM IV axis I Disorders (SCID) [22].
The clinical assessment as well as the recording of the electrophysiological data took place at the beginning of the hospitalization (t0), at the moment of discharge as inpatient (t1) and at the moment when the patient left the day hospital, six months after t1 (t2).The patient signed an informed consent which has been approved by the local ethical committee of the hospital. Psychotic symptoms have been quantified by the Positive and Negative Syndrome Scale (PANSS) [23], whereas levels of depressed mood and anxiety have been measured by the mean of the 13-item Beck Inventory Depressive Scale [24; 25] and the Spielberger Trait Anxiety Inventory (STAI) [26].
2.2 ERP task and procedure
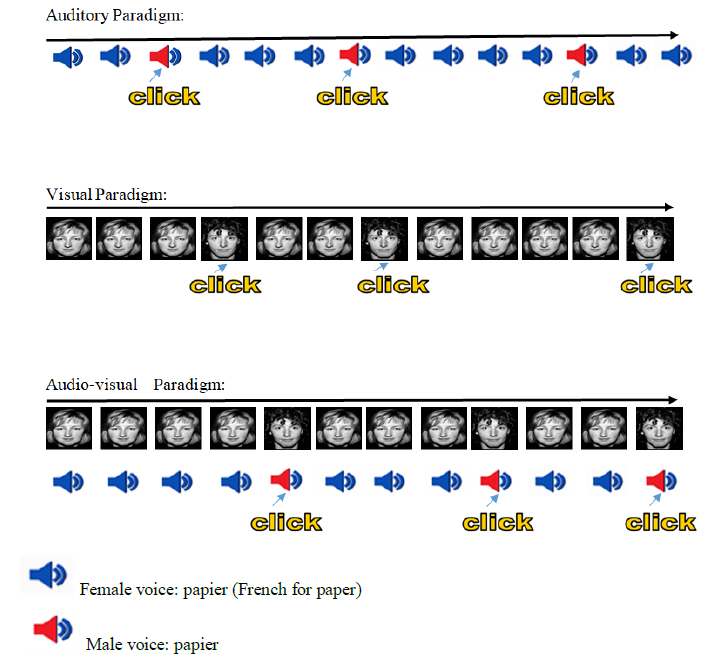
Task and procedure are comparable with those of previous studies of our team [17, 18, 20, 27]. The patient was confronted with three kinds of oddball tasks (visual (V), auditory (A), and bimodal, i.e. auditory and visual at the same time (AV)) and received the same instructions for all three different tasks: as quickly as possible the deviant stimuli (i.e. targets) needed to be detected by hitting a button with the right forefinger among the frequently repeated standard stimuli. He was told that speed was important, but not at the cost of accuracy, because response times and error rates were recorded. During the visual oddball task, the frequent stimulus was a female face and the deviant one was a male face. The faces were all neutral and selected from Ekman and Friesen’s set of standardized pictures [28]. During the auditory oddball task, the frequent stimulus was a female voice pronouncing the word “papier” (French for ‘paper’) and the deviant one was a male voice pronouncing the same word “papier”. For the deviant as well as for the frequent stimulus the voices with an emotional neutral prosody were chosen from the validated battery of vocal emotional expressions [29].
During the bimodal AV oddball task, pairs of congruent and simultaneous auditory and visual stimuli were presented to the patient (the frequent stimulus: a female face with a female voice; the deviant stimulus: a male face with a male voice). In all three tasks (visual, auditory and the bimodal combining auditory and visual), the patient had to complete one unit including a total number of 170 stimuli with 30 deviant (P=18%) and 140 frequent (P=82%) stimuli. In the short breaks, the patient was informed in regard to what kind of block he would encounter next (V, A, AV). As already conceived in the studies of Campanella and colleagues [17, 18, 30], the visual stimuli engraved a visual angle of 3 x 4°; the auditory stimuli were presented via headphones binaurally (60 dB tone); and each stimulus (face alone; voice alone; synchronized face-voice) has been presented for 700 ms. Between all the stimuli a black screen was presented for a random duration between 500 and 1000 ms. So the patient had at least 1200 ms of time to react after the stimulus onset (see Figure 1 for an illustration).
During the ERP recording, he was sitting in a darkened room on a comfortable chair placed one meter from the screen. The person who instructed the patient and managed the recording stayed alongside the patient during the complete examination and registration procedure. The order of presentation of the three tasks (A, V, AV) was changed for the three different acquisitions (t0, t1, t2).
Female voice: papier (French for paper)
Male voice: papier
2.3 EEG recording and analysis
The set-up used for the EEG recording is comparable to the one that is applied in previous studies [17, 18, 20, 30]. Only correct answers (i.e., correctly identified target stimuli) were taken into account in the subsequent analyses. The EEG was recorded by 19 electrodes fixed in an electrode Quick-Cap (Fpz, Fp1, Fp2, Fz, F3, F7, F4, F8, Cz, C3, C4, P3, Pz, P4, O1, Oz, O2, M1, M2). The data-recordings were realized with a linked mastoid physical reference. The amplification of the EEG was realized by a battery-operated A.N.T.® amplifier with a gain of 30,000 and a band-pass of 0.01–100 Hz. The impedance of the electrodes was below 10 kΩ during the complete experiment. The recording of the EEG was continuously (sampling rate 256 Hz, A.N.T.® Cognitrace software) and all trials which had been contaminated by EOG or movement artifacts were cut out off-line. Only artifact-free trials were kept for further analysis. A 30 Hz low-pass filter was applied to filter the data. The response type (keypress for deviant stimuli and no keypress for neutral stimuli) as well as the type of stimulus (deviant vs. frequent) and the modality of the task (V, A, AV) were encoded for every stimulus. The main component of interest (the P300) was analyzed and computed on the classical electrodes which are considered to define the P300 component and on which the maximum amplitudes were recorded for this component (i.e., P3, Pz, and P4) for each modality. We identified the peak by taking the first maximal amplitude value (and its corresponding latency) observable on P3, Pz, and P4 electrodes in the subsequent (250–500 ms) time interval. The Usual ‘Deviant minus Frequent’ subtractions were applied for each condition but used only for illustration purposes (e.g., [31]).
3. Results
3.1 Clinical evolution
The patient featured a progressive favorable clinical evolution in a time span of seven months (t1 was realized 1 month and t2 7 months after t0) and this as well for the three categories of psychotic symptoms assessed by the PANSS (with a very prominent improvement of the positive symptoms by about 65% and an important reduction of negative symptoms and global psychopathology by about 50%) as for symptoms of anxiety quantified by the STAI. The stagnating level of depressive symptoms can probably be explained by the mourning process due to the development of insight of his mental condition and life situation [32, 33].
|
t0 |
t1 |
t2 |
|
|
PANSS (P) |
26 |
21 |
9 |
|
PANSS (N) |
29 |
24 |
15 |
|
PANSS (G) |
48 |
40 |
25 |
|
Beck |
20 |
19 |
17 |
|
Stai-T |
59 |
46 |
39 |
Table 1: Clinical Data.
3.2 Behavioral data
In the analysis of the behavioral data of the oddball paradigm we see a progressive favorable evolution in a shortening of the reaction time in the bimodal condition which is in line with the clinical evolution assessed by the scales. On the contrary, there is no evolution of the reaction time in the unimodal oddball paradigms. Remarkable for the behavioral data is further that the patient does not make any mistake in the bimodal condition while he makes nearly systematically one or two omissions in the unimodal condition (there is only in t2 in the visual condition no mistake). The difference of behavioral data between the bimodal and the unimodal condition could be interpreted in the logic of the more ecologic and integrative aspects of the bimodal condition. The bimodal paradigm seems to permit a higher level of functional attention for the task than the unimodal does.
|
Modality |
Auditory (A) |
Visual (V) |
Audio-Visual (AV) |
|
|
t0 |
Performance/ 30 |
28 |
29 |
30 |
|
Reaction Time (ms) |
722 |
491 |
746 |
|
|
t1 |
Performance/ 30 |
28 |
29 |
30 |
|
Reaction Time (ms) |
728 |
442 |
709 |
|
|
t2 |
Performance/ 30 |
28 |
30 |
30 |
|
Reaction Time (ms) |
735 |
496 |
605 |
Table 2: Mean performance and reaction times (RT) per condition (Auditory: A, Visual: V, Audio-Visual: AV).
3.3 ERP data
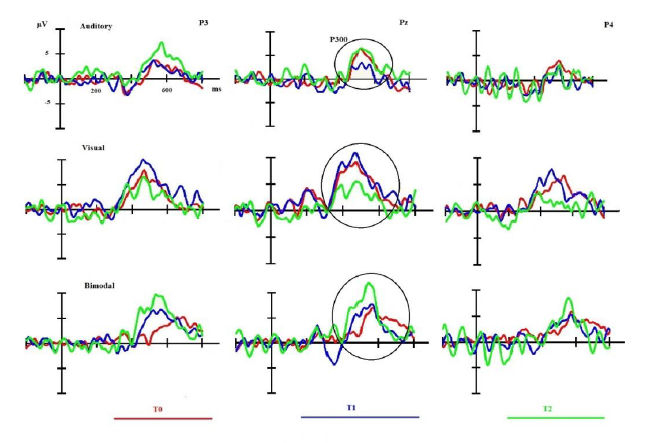
Concerning the electrophysiological data only the bimodal condition of the oddball task seems to mirror the clinical evolution: there is a reduction of the latency between t0 and t1, which is even more pronounced when comparing the latencies between t1 and t2. The amplitude of the P300 in the bimodal condition features a marked increment between t0 and t2.
4. Discussion
The main aim of the present study was to compare the bimodal paradigm of the P300 to the unimodal auditory and visual paradigms concerning its sensitivity in a patient suffering from schizophrenia. The main result is that the bimodal paradigm of the P300 seems to present an adequate sensitivity to track the clinical evolution of psychotic symptoms of the patient, whereas the unimodal paradigms (auditory and visual) do not.
This specific improvement of the bimodal P300 in latency and amplitude could be linked to the recuperation of neuronal resources which were enfeebled by the pathology of the patient. Indeed, prospective longitudinal study of P300 amplitude and latency realized by Molina et al. disclosed an increase of the P300 amplitude with olanzapine and an improvement in negative symptoms [36]. This is in agreement with behavioral data, as suggested by the shortening of the reaction time for the bimodal condition that could reflect a task facilitation [37]. Other variables also probably impacted these behavioral and neurophysiological results. First, the progressive and marked reduction of anxiety between t0 and t1 and also the augmentation of the amplitude between t1 and t2 can be interpreted in the same line of reflection. The reduction of the latency between t0 and t1 seems to be linked to the introduction of the psychopharmacological treatment [37] whereas the increase of the amplitude between t1 and t2 accompanied by an important shortening of the reaction time coincides with the switch and reduction of the psychopharmacological treatment [34, 35]. The increased amplitude between t0 and t1 seems to reflect the improvement of attentional resources due to a reduction of positive symptoms joining the introduction of a neuroleptic treatment [34, 35]. The more marked rise of the amplitude between t1 and t2 can be interpreted in the same tenor and coincides with a more marked reduction of negative symptoms and global psychopathology and this is remarkably coinciding with a reduction of the neuroleptic treatment. This differentiated evolution of the amplitude seems to reflect the hypothesis that a neuroleptic treatment reduces positive symptoms but not negative symptoms and has little impact on cognitive function improvement [34, 35].
The two unimodal conditions of the P300 are not at all corresponding to the clinical findings and do not seem to track the clinical evolution: for the auditory condition the amplitude at t1 is lower than the amplitude of t0 and there is no difference neither in latency nor in amplitude between t0 and t2; in the visual condition there is no difference for the three moments of testing concerning the latency and for the amplitude there is no difference between t0 and t1 and even a decreased amplitude at t2.
5. Conclusion
This case report seems to indicate a greater sensitivity of the bimodal P300 compared to the unimodal paradigms in order to track the clinical evolution of a patient who presents psychotic symptoms in the context of a first-onset schizophrenia. A combination of the bimodal with the unimodal (auditory and visual) paradigm of the P300 could allow us to improve our possibilities to track the individual clinical evolution of the disease. The bimodal oddball task seems to have a higher sensitivity because it reflects not only the classical, unimodal processes, but also the integrative processes which are necessary to connect simultaneous congruent information coming from different sensory modalities. Therefore it can be considered as a useful tool to monitor the clinical evolution of the disease, rather as a state marker than as a trait marker. A longitudinal study concept should permit to understand its capacities as a trait marker taking into account that the P300 paradigm simultaneously represents state as well as trait aspects of the pathophysiology of schizophrenia [4, 5]. In addition, it should also allow us to replicate these first findings presented in this case report. One of the main questions coming up in this context is if the movement to a bimodal from a unimodal paradigm of the P300 allows us to track in a more sensible way the clinical evolution of a patient presenting schizophrenia and to give clinical electrophysiology a consistent complementary place to the clinical follow up of a patient suffering from schizophrenia.
In this context, we have to take into account that the presented data of this case report have been acquired on a patient with a first onset process of schizophrenia, which probably has to be distinguished from data acquired from a patient presenting a chronical state of the illness [4]. The prospective longitudinal study design should allow to also monitor the dynamic and neurodevelopmental aspects of schizophrenia and could help to disentangle changes in clinical states from effects of antipsychotic medication. These single subject data indeed need to be completed and validated by a group of patients presenting a similar pathology as part of a longitudinal study.
Role of the Funding Source and Conflicts of Interest
The authors report no competing financial interests or potential conflicts of interest, as well as no financial relationships with commercial entities. The last author was funded by the Belgian Fund for Scientific Research (F.N.R.S., Belgium), but this fund did not exert any editorial direction or censorship on any part of this article.
References
- Boutros N, Galderisi S, Pogarell O, et al. Standard eElectroencephalography In Clinical Psychiatry: A Practical Handbook. John Wiley & Sons (2011).
- Pogarell O, Mulert C, Hegerl U. Event Related Potentials In Psychiatry. Clin EEG Neurosci 38 (2007): 25-34.
- Luck SJ, Mathalon DH, O'Donnell BF, et al. A Roadmap For The Development And Validation Of Event-Related Potential Biomarkers In Schizophrenia Research. Biol Psychiatry 70 (2011): 28-34.
- Mathalon DH, Ford JM, Rosenbloom M, et al. P300 Reduction And Prolongation With Illness Duration In Schizophrenia. Biol Psychiatry 475 (2000): 413-427.
- Mathalon DH, Ford JM, Pfefferbaum A. Trait And State Aspects Of The P300 Amplitude Reduction In Schizophrenia: A Retrospective Longitudinal Study. Biol Psychiatry 47 (2000): 434-449.
- Hamilton HK, Woods SW, Roach BJ, et al. Auditory And Visual Oddball Stimulus Processing Defecits in Schizophrenia And The Psychosis Risk Syndrome: Forecasting Psychosis Risk With P300. Schizophr Res 191 (2018): 87-94.
- Karaaslan F, Gonul AS, Oguz A, et al. P300 changes in major depressive disorders with and without psychotic features. J Affect Disord 73 (2003): 283-287.
- Hill SY, Shen S, Locke J, et al. Developmental delay in P300 production in children at high risk for developing alcohol-related disorders. Biol Psychiatry 46 (1999): 970-981.
- Polich J. Updating P300: an integrative theory of P3a and P3b. Clin Neurophysiol 118 (2007): 2148-2148.
- Campanella S, Quinet P, Bruyer R, et al. Categorical perception of happiness and fear facial expressions: an ERP study. J Cognitive Neurosci 14 (2002): 210-227.
- Polich J, Herbst KL. P300 as a clinical assay: rationale, evaluation, and findings. Int J Psychophysiol 38 (2000): 3-19.
- Verleger R. Event-related potentials and cognition: A critique of the context updating hypothesis and an alternative interpretation of P3. Behav Brain Sci 11 (1988): 343-356.
- Kim M, Lee TH, KIM JH, et al. Decomposing P300 into correlates of genetic risk and current symptoms in schizophrenia: An inter-trialvariability analysis. Schizophr Res 192 (2018): 232-239.
- Qiu YQ, Tang YX, Chan RC, et al. P300 aberration in first-epeisode schizophrenia patients: a meta-analysis. PloS One 9 (2014): e97794.
- Andreasen NC. Schizophrenia: the fundamental questions. Brain Res Rev (2000): 106-112.
- Lydiard J, Nemeroff CB. Biomarker-Guided Tailored Therapy. Adv Exp Med Biol 1192 (2019): 199-224.
- Campanella S, Bruyer R, Froidbise S, et al. Is two better than one? A cross-modal oddball paradigm reveals greater sensitivity of the P300 to emotional face-voice associations. Clin Neurophysiol 121 (2010): 1855-1862.
- Campanella S, Delle-Vigne D, Kornreich C, et al. Greater sensitivity of the P300 component to bimodal stimulation in an event-related potentials oddball task. Clin Neurophysiol 123 (2012): 937-946.
- Schroeder CE, Foxe J. Multisensory contributions to low-level, ‘unisensory’ processing. Curr Opin Neurobiol 15 (2005): 454-458.
- Kajosch H, Gallhofer B, Corten P, et al. The bimodal P300 oddball component is decreased in patients with an adjustment disorder: An event-related potentials study. Clin Neurophysiol 127 (2016): 3209-3216.
- de Jong JJ, Hodiamont PP, Van den Stock J, et al. Audiovisual emotion recognition in schizophrenia: reduced integration of facial and vocal affect. Schizophr Res 107 (2009): 286-293.
- Kay SR, Fszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull 13 (1987): 261-276.
- Gorgens KA. Structured Clinical Interview For DSM-IV (SCIDI/SCIDII). In: Kreutzer JS, DeLuca J, Caplan B. (eds) Encyclopedia of Clinical Neuropsychology. Springer, NY (2011).
- Beck AT, Beck RW. Screening depressed patients in family practice. A rapid technic. Postgrad Med 52 (1972): 81-85.
- Beck AT, Steer RA. Beck depression inventory manual. Psychological Corporation, San Antonio (1987).
- Spielberger CD, Gorsuch RL, Lusthene RE. Manual for the state and trait anxiety inventory. Consulting Psychologist Press, Palo Alto (1983).
- Delle-Vigne D, Kornreich C, Verbanck P, et al. Front Hum Neurosci 8 (2014): 10.
- Ekman P, Friesen WV. Pictures of facial affect. Consulting Psychologists Press, Palo Alto, CA (1976).
- Maurage P, Joassin F, Philippot P, et al. A validated battery of vocal emotional expressions. Neuropsychological Trends 2 (2007): 63-74.
- Delle-Vigne D, Kornreich C, Verbanck P, et al. The P300 component wave reveals differences in subclinical anxious-depressive states during bimodal oddball tasks: An effect of stimulus congruence. Clin Neurophysiol 126 (2015): 2108-2123.
- Polich J. Clinical application of the P300 event-related brain potential. Phys Med Rehabil Clin N Am 15 (2004): 133-161.
- Wittman D, Keshavan M. Grief and mourning in schizophrenia. Psychiatry 70 (2007): 154-166.
- Lewis L. Mourning, insight, and a reduction of suicide risk in schizophrenia. Bull Menninger Clin 68 (2004): 231-244.
- Owen MJ, Sawa A, Mortensen PB. Schizophrenia: Lancet 388 (2016): 86-97.
- Kaplan & Sadock’s comprehensive textbook of psychiatry. Editors, Sadock BJ, Sadock VA. edtion:8th. Publisher: Lippincott Williams & Wilkins (2004): ISBN: 0781734347
- Molina V, Muñoz F, Martín-Loeches M, et al. Long-Term Olanzapine Treatment and P300 Parameters in Schizophrenia. Neuropsychobiology 50 (2004): 182-188.
- Iwanami A, Okajima Y, Isono H, et al. Effects of risperidone on event-related potentials in schizophrenic patients. Pharmacopsychiatry 34 (2001): 73-79.


 Impact Factor: * 5.3
Impact Factor: * 5.3 Acceptance Rate: 75.63%
Acceptance Rate: 75.63%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 
