The Efficacy of a Plant Based Formulation in the Symptomatic Management of Mild COVID-19 Cases: A Double Blind, Randomized Controlled Trial
Sanjib kumar Das1, Durga Prasad Dash2, Pradip Kumar Panda3, Sandhya Sadana4, Reddy M Ravi Kumar5, Venkatesh Hari K6, Divya Kanchibhotla7*
1Consultant, deptartment of Kayachikitsa, Sri Sri College of Ayurvedic Science & Research Hospital, Sri Sri University, India
2Medical Superintendent, Sri Sri College of Ayurvedic Science & Research Hospital, Sri Sri University, India
3Dean, Sri Sri College of Ayurvedic Science & Research Hospital, Sri Sri University, India
4CAO, Sri Sri College of Ayurvedic Science & Research Hospital, Sri Sri University, India
5Chief Science Officer, Sri Sri Tattva, India
6Head, Research & Development – Healthcare, Sri Sri Tattva, India
7Executive director, Sri Sri Institute for Advanced Research, India
*Corresponding Author: Divya Kanchibhotla, Sri Sri Institute for Advanced Research, 21st Km Kanakapura Rd, Udaypura, Bangalore, Karnataka 560082, India.
Received: 11 July 2022; Accepted: 30 December 2022; Published: 18 January 2023
Article Information
Citation: Sanjib kumar Das, Durga Prasad Dash, Pradip Kumar Panda, Sandhya Sadana, Reddy M Ravi Kumar, Venkatesh Hari K, Divya Kanchibhotla. The Efficacy of a Plant Based Formulation in the Symptomatic Management of Mild COVID-19 Cases: a Double Blind, Randomized Controlled Trial. Archives of Clinical and Medical Case Reports 7 (2023): 05-12.
View / Download Pdf Share at FacebookAbstract
Background: The COVID-19 pandemic has infected millions of people globally. There is a need for integrated modern and traditional systems of medicine to work together to find effective solutions quickly.
Methods: NAOQ19 is a plant based formulation of 13 herbs. 50 COVID-19 patients were enrolled in the study. RT-PCR analysis was done on day 1, day 5 and day 10. Clinical symptoms were noted daily.
Result: NAOQ19 arm showed a higher population with RT-PCR negative compared to the placebo arm on Day 5 (76% vs 0%) On Day 5, NAOQ19 arm showed a complete recovery from few symptoms while by Day 10, they showed complete recovery from all symptoms, unlike the placebo arm which showed only 10.5% of the population with clinical recovery on day 10.
Conclusion: NAOQ19 facilitated a faster recovery from all clinical features of COVID-19, when compared to the placebo group. No side effects were observed during the entire study duration.
CTRI Registration: CTRI/2021/08/036025
Keywords
<p>COVID; Clinical Trial; NAOQ19; Traditional Medicine</p>
Article Details
1. Introduction
The COVID-19 pandemic caused by the SARS-CoV-2 virus has infected millions of people around the globe in over 200 countries. Thousands have been hospitalized and even more have required health care support [1]. The high rate of transmission, quick mutations and rapidly evolving variants of the virus have hindered the discovery of a therapeutic cure [2-3]. The modern pharmaceutical and health care system are overwhelmed. The pandemic has highlighted the need for modern and traditional systems of medicine to support each other. The current system of medicine is primarily oriented towards symptomatic treatment of disease, whereas several traditional systems of medicine advocate prevention, as well as treatment. Compared to conventional pharmaceuticals, bioactive compounds found in plants tend to have good biocompatibility and bioavailability, less toxicity and have ample phytoconstituents such as phenols, steroids and flavonoid molecules for the treatment of COVID-19 symptoms [4]. An earlier study explored the immunomodulatory and anti-SARS-CoV-2 effects of Withania somnifera, Tinospora cordifolia and Asparagus racemosus using network pharmacology and docking [5]. A retrospective observational study was conducted to evaluate the impact of other such naturally available plant based compounds in early clinical recovery of COVID-19 patients [6]. The present study investigates the efficacy of a plant based formulation called NAOQ19 in the management of COVID-19. NAOQ19 contains several herbs like Withania somnifera, Adhatoda vasica, Tinospora cordifolia, and Glycyrrhiza glabra. A robust immune system plays an important role in protecting against communicable diseases, such as COVID-19 [7]. Several constituents of NAOQ19 have been demonstrated to have immunomodulatory, antiviral, anti-inflammatory, and antioxidant properties [8]. Earlier studies on individual constituents have investigated the herbs and their phyto-constituents individually. Withania somnifera alone has about 69 (39 preclinical and 30 clinical) studies related to its safety and efficacy [9]. Withania somnifera is a prime medicinal plant of indigenous medicine. It is an immune booster and has hepatoprotective, anti-inflammatory, antioxidant and immunomodulatory properties [10]. A molecular docking study on Withania somnifera, Tinospora cordifolia and Ocimum sanctum (all three are present in NAOQ19) showed a promising effect against COVID-19 [11]. Another constituent of NAOQ19, Glycyrrhiza glabra is potent in treating respiratory tract infections, dry cough and hoarseness. It is reported to have antiviral effects as well [12]. A molecular docking study revealed that various phytochemicals found in Glycyrrhiza glabra have significant binding affinity with SARS-CoV-2 proteins [13-14]. An earlier study showed the antimicrobial, antioxidant and cytotoxic properties of the alkaloid from Adhatoda vasica. Adhatoda vasica is recommended to treat chronic fever, cough, and asthma because of its antitussive activity. In acute stage of bronchitis, Adhatoda vasica may give relief, especially when the sputum is thick and sticky [15]. Its antispasmodic and expectorant properties make it effective in treating respiratory conditions [16]. The extracts and compounds isolated from Phyllanthus fraternus, also present in NAOQ19, show a wide spectrum of pharmacological activities including antiviral, antibacterial, antispasmodial, anti-inflammatory, antioxidant, hepatoprotective and diuretic properties [17]. Andrographis paniculata, another ingredient of NAOQ19, commonly known as Kalamegha, is reported to have hepatoprotective, antiulcer, anti-inflammatory and antipyretic properties. Clinically, it has shown significant result in viral hepatitis, common cold, vitiligo and upper respiratory tract infections [18]. It is traditionally used for the treatment of common cold, diarrhoea, fever secondary to infection, and as a health tonic for the liver and heart [19]. Curcuma longa, another constituent of NAOQ19, has been shown to benefit inflammatory conditions and reduce pain [20]. Ocimum sanctum, another constituent of NAOQ19, is one of the plant used in ancient Indian system of medicine. It is considered a potent adaptogen that promotes wellbeing and resilience. It is antimicrobial, antioxidant, anti-inflammatory, neuro-protective, cardio-protective, analgesic, anti-pyretic and immunomodulatory in nature [21]. An in vitro study conducted on the efficacy and safety of NAOQ19 showed a 100% antiviral efficacy of NAOQ19 in Vero E6 cells [22]. An in vivo study in Syrian golden hamsters demonstrated a greater than 78% reduction in viral load when treated with NAOQ19 [23]. Analysis of a pilot study on NAOQ19 demonstrated that 74% of patients turned RTPCR negative within 5 days of taking NAOQ19. Additionally, 98% of the subjects turned RTPCR negative on day 10 after taking NAOQ19 along with standard care of treatment including Vitamin. C, Zinc and antipyretic as needed. No participants reported adverse events. The objective of this clinical trial is to investigate the safety and efficacy of NAOQ19, provided in addition to the standard therapy, for patients with mild COVID-19, by specifically measuring the symptomatic parameters.
2. Materials and Methods
2.1 Study Design
The study was a double blind, randomized placebo-controlled clinical trial. The trial was conducted at Sri Sri College of Ayurvedic Science & Research Hospital, Sri Sri University, Cuttack, Odisha. The study was approved by the Institutional Ethics Committee, Sri Sri University (IEC Number- SSCASRH/IEC/001/21) and registered with CTRI No. CTRI/2021/08/036025. The duration of the study was from 1st to 25th September 2021. The participants were recruited during the entire study period, as and when they reported to the hospital. The intervention period for an individual patient was 10 days.
2.2 Participants
All patients who reported to inpatient (IPD) and outpatient (OPD) sections of COVID Care Centre at Sri Sri College of Ayurvedic Science & Research Hospital, Sri Sri University, Cuttack, Odisha, were invited to participate in the trial. Each patient with a confirmed symptomatic or asymptomatic case of COVID-19 was informed about the purpose of the clinical trial. Informed consent was obtained from those who wished to enroll in the study. Participants were informed about the insurance details, and were made aware that they could withdraw from the study at any time. Their written informed consent was obtained via a study specific proforma prepared for this trial, which included demographic information and baseline study parameters. 50 RTPCR positive COVID-19 patients were enrolled after receiving their written consent. A total of 6 Patients were excluded from the study due to noncompliance. 25 patients in the NAOQ19 arm and 19 patients in the placebo arm completed the clinical trial.
2.2.1 Inclusion Criteria
- Asymptomatic or symptomatic RT-PCR positive mild COVID-19 cases (without comorbidities) as per the GOI norms
- History of fever of >98.60F
- Cough
- Dysponea (shortness of breath) or Tachypnea
- Patients of both genders between the ages of 18-75 years
- Indian Nationals
- Willingness to participate in the study with a written consent
2.2.2 Exclusion Criteria
- Not willing to give consent/ participate in the clinical trial
- Age less than 18 years or more than 75 years
- Patients with comorbidities such as uncontrolled hypertension, diabetes, thyroid disorder, Heart/Lung/Liver/Renal disorder, moderate to severe anaemia/hematological disorders, Malignancy, Stroke: ischaemic stroke & intracerebral haemorrhage,
- Patients on immunosuppressive therapy
- Pregnant women or lactating mothers
- Any major surgery in the last six months or hospitalized for more than three days for any condition.
2.3 Intervention
Each patient was monitored for 10 days. Follow up assessments were conducted on day 5 and day 10. Nasopharyngeal sample for RT-PCR was obtained on day 5 and 10 through an ICMR accredited laboratory. The patients were advised to inform the hospital staff immediately in case of any adverse events and/or aggravation of symptoms. Compliance with the treatment was assessed and subjects were counseled for dosage compliance in person.
2.3.1 NAOQ19 Preparation: NAOQ19 is a plant based formulation with 19 ingredients from 13 herbs. They are Withania somnifera powder and extract, Aegle marmelos, Glycyrrhiza glabra powder and extract, Pluchea lanceolata, Adhatoda vasica powder and extract, Piper longum, Curcuma longa, Cissampelos pareira, Phyllanthus fraternus powder and extract, Andrographis paniculata powder and extract, Alstonia scholaris, Ocimum sanctum Tinospora cordifolia) powder and extract. NAOQ19 was procured from Sriveda Sattva Pvt Ltd, Bangalore (Sri Sri Tattva). The drug was licensed by Ministry of AYUSH, Govt. of India with the license number- AUS782. All the herbs which constituted NAOQ19 were subjected to quality control. All the ingredients were blended with excipients followed by granulation, drying and compression. Once the Tablet passed the QC tests, it was packed in bottles following standard procedure.
2.3.2 NAOQ19 Group: Participants enrolled in the NAOQ19 arm were given the NAOQ19 tablets. The dosage for NAOQ19 arm was 2 tablets (500 mg each) thrice a day after food for 10 days.
2.3.3 Comparator Group: The placebo control group was provided with placebo tablets made of starch. They were packed in bottles identical to the NAOQ19 bottles and also color matched with the NAOQ19 tablets. The dosage for placebo arm was similar to the NAOQ19 arm, 2 tablets (500 mg each) thrice a day after food for 10 days.
2.3.4 Standard Treatment: In addition to receiving NAOQ19 or placebo, both groups received the standard treatment according to the government guidelines. The standard treatment included Azithromycin (500mg, once a day for 5 days) or Doxycycline (100mg, twice a day for 5 days), Levocetrizine (5mg once a day for 5 days), Vitamin C (10 days), Zinc (10 days) and Paracetamol (500mg, as required).
2.4 Outcome Measurements
Clinical history was obtained from each participant enrolled in the study, followed by clinical examination and baseline investigations. The primary outcome measured was the percentage of population turning RT-PCR negative by Day 5 and 10 in both study arms. Two nasopharyngeal swabs were collected from each patient and subjected to the PCR analysis to identify the viral load in patients. The secondary outcomes included the assessment of subjective parameters such as sore throat, cough, runny nose, shortness of breath, joint pain, fatigue, headache, loss of smell and loss of taste in both groups on Day 5 and Day 10 (Supplementary Table 1). The presence or absence of subjective outcomes was noted for each patient, in person, at each follow up time point, based on their baseline clinical history. The study also observed clinical recovery among the patients. Taking Singh et al [25] as the reference, clinical recovery was defined as the absence of any clinical symptoms of COVID-19. Symptoms measured in the study were those commonly associated with COVID-19 like fever, weakness, cough, shortness of breath, loss of smell, loss of taste and running nose. Recovery or clinical improvement of patients was in par with the discharge policy provided by the Government of India [24]. Observations of adverse events or side effects were also included in the secondary outcome. The assessment form has been provided for reference at the end of the paper as a supplementary table.
2.5 Sample Size Calculation
A total sample size of 50 patients was targeted. The sample size was calculated based on the study of Devapura G [25], et al, where the treatment group witnessed 100% recovery by day 7, while it was 60% in the placebo group. Taking these values as reference, the minimum required sample size at 95% power of study and 5% level of significance 20 patients in each study group were done. Considering margin of error 50 (25 in each group) male or female volunteers who were mild symptomatic positive patients of COVID-19 were enrolled in the study.
2.6 Randomization and Blinding
The study participants were allocated into different study arms with the help of a computer generated randomization code. The allocation was sequentially distributed. Since both NAOQ19 and placebo were packaged in identical bottles, the participants were blinded to the respective intervention. The participants, data collector and the laboratory technician were blinded to the study group allocation.
2.7 Statistical Analysis
The baseline characteristics of both study groups were reported as proportion/ mean (SD). Data analysis was conducted using per protocol approach. The categorical variables were listed as number and percentage (%). The quantitative data was presented as the mean (SD). The data normality was checked using kolmogorov-Smirnov test. Wherever the data was not normal, non- parametric tests were used. Paired t test was used for comparison across follow up time points. The variables which were qualitative in nature were compared and analyzed using Chi-Square test. If any cell had an expected value of less than 5 then Fisher’s exact test was used. The data entry was done in the Microsoft EXCEL spreadsheet and the final analysis was done with the use of Statistical Package for Social Sciences (SPSS) software, IBM manufacturer, Chicago, USA, version 2.0. For statistical significance, p value of less than 0.05 was considered statistically significant.
3. Results
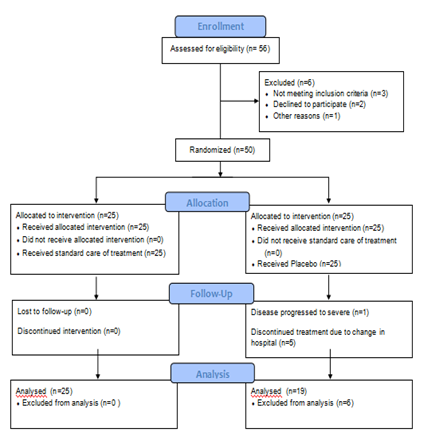
Consort Flow Diagram.
A total of 50 participants were enrolled in the study, 25 in each arm. Those who did not adhere to the protocol or could not comply were excluded from the data analysis. No significant difference was found in the age and gender distribution or co-morbidities between the two groups. The duration between the onset of symptoms, positive COVID-19 test and the enrollment in NAOQ19 or control arm group was also similar. Both the groups were predominantly male. Table 1 lists the demographic characteristics of the study population.
|
Demographic characteristics |
NAOQ19 (n=25) |
Placebo (n=19) |
Total |
p value |
|
IPD (n) |
25 |
19 |
44 |
NA |
|
Age Range (yrs) |
18-70 |
18-50 |
18-70 |
|
|
Age (Mean ± SD) |
38.96 ± 16.90 |
28.11 ± 9.28 |
34.27 ± 15.00 |
0.0156 |
|
Female (n %) |
10 (40%) |
7 (37%) |
17 (38.6%) |
1.00 |
|
Male (n %) |
15 (60%) |
12 (63%) |
27 (61.4%) |
|
|
SPO2 (Mean ± SD) |
97.56 ± 0.70 |
97.58 ± 0.69 |
97.57 ± 0.69 |
0.91 |
|
Temp (Mean ± SD) |
98.51 ± 1.15 |
98.52 ± 1.15 |
98.54 ± 1.13 |
0.97 |
|
Pulse Rate (Mean ± SD) |
82.93 ± 5.16 |
83.11 ± 10.45 |
83.00 ± 7.68 |
0.93 |
|
Respiratory Rate (Mean ± SD) |
20.08 ± 1.26 |
19.11 ± 0.88 |
19.67 ± 1.21 |
0.006* |
*<0.05-significant
Table 1: Demographic Characteristics of the study population.
Table 1 shows no significant difference in the age and gender distribution between the two groups. The duration between the onset of symptoms, positive COVID-19 test and the enrollment in NAOQ19 or placebo arm was also similar.
3.1 Primary Outcome - Rate RT-PCR Negativity
19 out of the 25 patients (76%) in the NAOQ19 arm turned RT-PCR negative after 5 days of treatment. By Day10 all patients in both arms were RT-PCR negative.
|
RTPCR |
NAOQ19 (n=25) |
Placebo (n=19) |
|
|
Day 0 |
RTPCR Negative |
0 (0%) |
0 (0%) |
|
Day 5 |
RTPCR Negative |
19 (76%) |
0 (0%) |
|
p value |
<0.001** |
||
*<0.05-significant **<0.001-very significant
Table 2: Rate of RT-PCR negativity at Day 05.
Table 2 shows that NAOQ 19 arm had a 76% increase in patients who turned RTPCR negative on Day 5, while the placebo arm showed no improvement. Patients in the NAOQ 19 arm showed an early and strong recovery from COVID-19.
3.2 Secondary Outcome - Symptomatic Recovery
The secondary outcomes included subjective parameter assessments of sore throat, cough, runny nose, shortness of breath, joint pain, fatigue, malaise, headache, loss of smell and loss of taste in both groups on Day 5 and Day 10.
*<0.05-significant **<0.001-very significant
Table 3: Rate of symptom recovery at Day 5 and Day 10.
Table 3 depicts the improvement in the symptomatic clinical features of COVID-19. There was a negligible difference in the severity of clinical features between the two groups on Day 1. On Day 5, the population in the NAOQ19 arm showed a complete recovery from sputum formation, sore throat, headache and fever. By Day 10, 90-100% of the population in NAOQ19 arm showed a complete recovery from all symptoms, unlike the control arm.
|
Time Points |
NAOQ19 |
Placebo |
p value |
|
Day 5 |
11(44%) |
1(5.2%) |
0.0047* |
|
Day 10 |
25(100%) |
2(10.5%) |
<0.001** |
*<0.05-significant **<0.001- very significant
Table 4: Proportion of patients with clinical Recovery between Both the Arms.
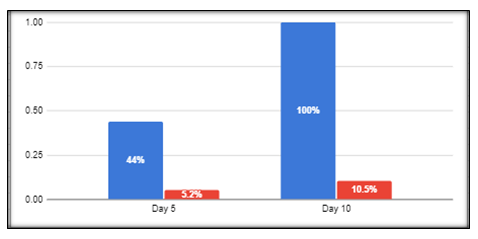
Figure 1: Proportion of patients with clinical Recovery between Both the Arms.
Table 4 and figure 1 demonstrates the percentage of the population recovering from all the clinical symptoms between the two groups. The NAOQ19 group demonstrated a significantly larger population recovering from the disease on Day 5 compared to those in the placebo group. The clinical recovery reached up to 100% in the NAOQ19 group while only 10.5% had recovered in the placebo arm. Since day 10 was the last time point of assessment, 89.5% of the patients in the placebo group did not attain clinical recovery.
4. Discussion
COVID-19 is a highly communicable disease that can cause severe pneumonia and acute respiratory distress syndrome [26]. The results of the study demonstrate a significantly faster recovery from clinical symptoms of COVID-19 in the NAOQ19 arm, both for the time taken to experience clinical recovery and the time taken for RT PCR to turn negative from positive. The patients in NAOQ19 arm recovered faster than the placebo arm, as depicted in Table 3. 76% of the patients in NAOQ19 arm turned RTPCR negative by day 5. The results of this clinical study are supported by earlier studies on NAOQ19. An in vitro study conducted on the efficacy and safety of NAOQ19 showed 100% antiviral efficacy of NAOQ19 in Vero E6 cells [22]. An in vivo study in the Syrian golden hamsters demonstrated a greater than 78% reduction in viral load when treated with NAOQ19 [23]. None of the participants reported any adverse events. This study investigated the impact of NAOQ19 on the common clinical features of COVID-19 and found significant improvement in almost all of them, compared to the placebo arm. One of the common syndromes associated with COVID-19 are its post Covid complications. A review on several studies on post COVID complications demonstrate that these complications persist for longer than 24 weeks and few others can lead to several fatal complications in long run [27]. These symptoms vary depending on the severity of illness [28-29]. Our study demonstrated a speedy recovery of symptoms like fatigue also among COVID-19 infected patients. Patients report acute respiratory problems like chronic cough, fibrotic lung disease, bronchiectasis and pulmonary vascular disease. These long term respiratory distress syndrome decrease the lung capacity and quality of life [30]. Fast recoveries with absence of shortness in breath, as discovered in the NAOQ19 group can help patients cope the distress and improve patients’ lung capacity. Previous pre-clinical studies on Adhatoda vasica demonstrate an anti-hypoxic effect of the drug on the mice model [31]. Tinospora cordifolia has been previously used in the management of bronchial asthama [32]. Presence of the above mentioned herbs in NAOQ19 may be a contributor of associated symptom recovery and absence of breathlessness in patients. Presence of Withania somnifera and Glycyrrhiza glabra, as demonstrated by many in-silico studies may contribute to inhibhition of viral protein attachment to lung ACE-2 cell receptors, that may reduce the viral load [33-34]. Withania somnifera a popular Indian drug has been investigated for its role in decreasing fatigue as also noted in patients from NAOQ19 arm [35]. The study has certain limitations. The study period saw a decline in the number of COVID-19 patients; hence the sample size was small. Another limitation was the non-inclusion of patients with comorbidities, as well as absence of blood biomarker assessment. Similar trials can be conducted on a larger population, including patients with comorbidities, to confirm the results of the present study. Most of the studies on polyherbal formulation are done in silico. This study is significant in observing the changes in viral load and clinical symptoms for the COVID-19 patients. It opens a new dimension to look towards the easily available and growing components in the nature as one of the important options to cure COVID patients.
5. Conclusion
In this study, NAOQ19 was seen to provide a complete recovery from most of the clinical features of COVID-19 faster, when compared to the control. Conversion of RTPCR from positive to negative was seen in 5 days only. Significant improvements were seen in the clinical symptoms of sputum formation, sore throat, headache, joint pains, fatigue, runny nose, loss of smell and loss of taste, after 5 days and 10 days of treatment with NAOQ19, in comparison to placebo with standard treatment. No side effects were observed during the entire study duration.
Disclosure Section
Acknowledgements
We would like to acknowledge Mr. Prateek Harsora for coordinating the study. We would also like to acknowledge Dr. Somya Ramrakhyani for language edits.
Author Contributions
Conceptualization: Pradip Kumar Panda, Sandhya Sadana, Divya Kanchibhotla; Data curation: Dr. Sanjib kumar Das, Durga Prasad Dash; Formal Analysis: Divya Kanchibhotla. Funding acquisition: NA; Investigation: Dr. Sanjib kumar Das , Durga Prasad Dash, Sandhya Sadana; Methodology: Divya Kanchibhotla, Pradip Kumar Panda, Sandhya Sadana; Project administration: Dr. Sanjib kumar Das , Durga Prasad Dash, Sandhya Sadana; Resources: Reddy M. Ravi Kumar, Venkatesh Hari K.; Supervision: Divya Kanchibhotla, Pradip Kumar Panda; Validation: Divya Kanchibhotla, Pradip Kumar Panda; Visualization: Divya Kanchibhotla, Pradip Kumar Panda; Writing – original draft: Dr. Sanjib kumar Das; Writing – review & editing: Pradip Kumar Panda, Durga Prasad Dash, Divya Kanchibhotla.
Conflict of Interest
The test resources were provided by Sri Sri Tattva, /Sriveda Sattva Pvt Ltd, India. Dr Ravi Reddy is the chief scientific officer of Sriveda Sattva Pvt. Ltd., In addition Dr. Hari Venkatesh is the Research and Development head at Sriveda Sattva Pvt. Ltd. Besides providing the Intervention materials, Sriveda Sattva Pvt Ltd. was not involved in any aspect of this study. All the other authors have no conflicts of interest to declare.
Funding
This research was supported by Cooper Family Foundation, Australia.
Ethical Approval
The study was approved by the Institutional Ethics Committee, Sri Sri University (IEC Number- SSCASRH/IEC/001/21) and registered with CTRI No. CTRI/2021/08/036025.
References
- WHO Dashboard (04th October, 2021).
- Li W, Zhang B, Lu J, et al. Characteristics of household transmission of COVID-19. Clin. Infect. Dis 71 (2020): 1943-1946.
- Atkeson AG, Kopecky K, Zha T. Behavior and the Transmission of COVID-19. InAEA Papers and Proceedings 111 (2021): 356-360.
- Kashyap VK, Dhasmana A, Yallapu MM, et al. Withania somnifera as a potential future drug molecule for COVID-19. Future drug discovery 2 (2020): 2020-2024.
- Borse S, Joshi M, Saggam A, et al. Ayurveda botanicals in COVID-19 management: An in silico multi-target approach. PLoS One 16 (2021): e0248479.
- Thakar A, Panara K, Patel F, et al. Add-on Ayurveda treatment for early stage COVID-19: a single center retrospective cohort study from Gujarat, India. J. Evid.-Based Integr. Med (2021).
- Chowdhury MA, Hossain N, Kashem MA, et al. Immune response in COVID-19: A review. J. Infect. Public Health 13 (2020): 1619-1629.
- Tandon N, Yadav SS. Safety and clinical effectiveness of Withania Somnifera (Linn.) Dunal root in human ailments. J. Ethnopharmacol 255 (2020): 112768.
- Khanal P, Chikhale R, Dey YN, et al. Withanolides from Withania somnifera as an immunity booster and their therapeutic options against COVID-19. J. Biomol. Struct. Dyn 26 (2020): 1-4.
- Shree P, Mishra P, Selvaraj C, et al. Targeting COVID-19 (SARS-CoV-2) main protease through active phytochemicals of ayurvedic medicinal plants–Withania somnifera (Ashwagandha), Tinospora cordifolia (Giloy) and Ocimum sanctum (Tulsi)–a molecular docking study. J. Biomol. Struct. Dyn 40 (2022): 190-203.
- Armanini D, Fiore C, Bielenberg J, et al. Coronavirus-19: possible therapeutic implications of spironolactone and dry extract of Glycyrrhiza glabra L.(Licorice). Front. Pharmacol 11 (2020): 558418.
- Maurya DK. Evaluation of Yashtimadhu (Glycyrrhiza glabra) active phytochemicals against novel coronavirus (SARS-CoV-2). Preprint, Research square (2020).
- Karthick R, Adithya K, Hariharaprasath C, et al. Evaluation of mechanical behavior of banana fibre reinforced hybrid epoxy composites. Mater. Today: Proc 5 (2018): 12814-12820.
- Duraipandiyan V, Al-Dhabi NA, Balachandran C, et al. Antimicrobial, antioxidant, and cytotoxic properties of vasicine acetate synthesized from vasicine isolated from Adhatoda vasica L. BioMed Res. Int (2015).
- Hossain MT, Hoq MO. Therapeutic use of Adhatoda vasica. Asian J. Med. Biol. Res 2 (2016): 156-163.
- Gangwar AK, Ghosh AK. Medicinal uses and pharmacological activity of Adhatoda vasica. Int. J. Herb. Med 2 (2014): 88-91.
- Patel JR, Tripathi P, Sharma V, et al. Phyllanthus amarus: ethnomedicinal uses, phytochemistry and pharmacology: a review. J. Ethnopharmacol 138 (2011): 286-313.
- Kumar P, Naik R, Karra N. Experimental and clinical evidence of Andrographis paniculata (Roxb.) Wall. Ex Nees (Bhunimba)—a review. Int. j. pharm. biol. sci. arch 4 (2013): 1086-1093.
- Hossain MD, Urbi Z, Sule A, et al. Andrographis paniculata (Burm. f.) Wall. ex Nees: a review of ethnobotany, phytochemistry, and pharmacology. Sci. World J (2014): 1-28.
- Hewlings SJ, Douglas S. Kalman. Curcumin: A Review of Its’ Effects on Human Health. Foods 6 (2017): 10-92.
- Cohen MM. Tulsi-Ocimum sanctum: A herb for all reasons. J. Ayurveda Integr. Med 5 (2014): 251.
- Kanchibhotla D, Subramanian S, Reddy R, et al. An In-vitro evaluation of a polyherbal formulation, against SARS-CoV-2. 2021, Researchsquare. Preprint (2021).
- Kanchibhotla D, Subramanian S, KR HV. To study the in-vivo efficacy and safety of AYUSH polyherbal formulation among COVID-19 infected Syrian gold hamsters. 2021, Researchsquare. Preprint (2021).
- Discharge Policy, Govt. of India for COVID patients.
- Devpura G, Tomar BS, Nathiya D, et al. Randomized placebo-controlled pilot clinical trial on the efficacy of ayurvedic treatment regime on COVID-19 positive patients. Phytomedicine 84 (2021): 153494.
- Bchetnia M, Girard C, Duchaine C, et al. The outbreak of the novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2): A review of the current global status. J. Infect. Public Health 13 (2020): 1601-1610.
- Suvvari TK, Kutikuppala LS, Tsagkaris C, et al. Post-COVID-19 complications: Multisystemic approach. J. Med. Virol (2021).
- Kamal M, Abo Omirah M, Hussein A, et al. Assessment and characterisation of post-COVID-19 manifestations. Int. J. Clin. Pract 75 (2021): e13746.
- Fernández-de-Las-Peñas C, Palacios-Ceña D, Gómez-Mayordomo V, et al. Defining post-COVID symptoms (post-acute COVID, long COVID, persistent post-COVID): an integrative classification. Int. J. Environ. Res. Public Health 18 (2021): 2621.
- Fraser E. Long term respiratory complications of covid-19. BMJ (2020).
- Gheware A, Panda L, Khanna K, et al. Adhatoda vasica rescues the hypoxia-dependent severe asthma symptoms and mitochondrial dysfunction. Am. J. Physiol. Lung Cell Mol. Physiol 320 (2021): L757-769.
- Kapadiya PR, Dave AR, Harisha CR, et al. Pharmacognostical and pharmaceutical analysis of amrutadi vati in the management of tamaka shwasa wsr to bronchial asthma. Pharm. Glob 5 (2014): 1.
- Maurya DK. Evaluation of Yashtimadhu (Glycyrrhiza glabra) active phytochemicals against novel coronavirus (SARS-CoV-2). Research square. Preprint (2020).
- Tripathi MK, Singh P, Sharma S, et al. Identification of bioactive molecule from Withania somnifera (Ashwagandha) as SARS-CoV-2 main protease inhibitor. J. Biomol. Struct. Dyn 39 (2021): 5668-5681.
- Lopresti AL, Smith SJ. Ashwagandha (Withania somnifera) for the treatment and enhancement of mental and physical conditions: A systematic review of human trials. J. Herb. Med 28 (2021): 100434.
Supplementary Files
|
ILLNESS |
SEVERITY |
GRADE |
|
|
Fever |
A febrile |
0 |
|
|
Mild fever (98.6°F-100°F) |
1 |
||
|
Moderate fever (100°F- 103°F) |
2 |
||
|
Severe (>103°F) |
3 |
||
|
Cough |
Dry |
Absent |
0 |
|
Mild dry cough only at morning and evening time |
1 |
||
|
Dry cough at any time |
2 |
||
|
Unable to sleep due to severe dry cough at night |
3 |
||
|
Sputum |
Absent |
0 |
|
|
Thin and scanty |
1 |
||
|
Expectoration of thick sputum during coughing |
2 |
||
|
Expectoration of thick & yellow sputum |
3 |
||
|
Haemoptysis |
Absent |
0 |
|
|
Mild (<30ml) |
1 |
||
|
Moderate(30 -100ml) |
2 |
||
|
Severe(>100ml) |
3 |
||
|
Sore throat |
Absent |
0 |
|
|
Complaints of sore throat only on asking |
1 |
||
|
Complaints of sore throat on his /her own |
2 |
||
|
Change of voice associated with throat pain |
3 |
||
|
Runny nose |
Absent |
0 |
|
|
Sometimes watery secretion from nose |
1 |
||
|
Most of the times watery secretion from nose |
2 |
||
|
Always watery secretion from nose, and patient feels discomfort |
3 |
||
|
Shortness of breath |
Absent |
0 |
|
|
Present in resting condition |
1 |
||
|
Present after little work |
2 |
||
|
Present after moderate to heavy work |
3 |
||
|
Headache |
No Headache |
0 |
|
|
Occasional headache |
1 |
||
|
Can be tolerated without medication |
2 |
||
|
Uncontrolled headache |
3 |
||
|
Fatigue |
Patient can do his /her normal work |
0 |
|
|
Patient is able to do routine work with weakness |
1 |
||
|
Patient is able to do routine interfere the physical working |
2 |
||
|
Patient cannot do his normal work |
3 |
||
|
Loss of smell (Anosmia) |
Absent (Normal Smell) |
0 |
|
|
Mild Hyposmia |
1 |
||
|
Moderate Hyposmia |
2 |
||
|
Anosmia |
3 |
||
|
Loss of Taste (Ageusia) |
Normal Taste |
0 |
|
|
Mild Ageusia |
1 |
||
|
Moderate Ageusia |
2 |
||
|
Ageusia |
3 |
||
|
Joint Pain |
No pain in walking, sitting, standing and slipping) |
0 |
|
|
Pain in going up and down stair |
1 |
||
|
Pain in sitting and standing |
2 |
||
|
Pain in walking, sitting, standing and slipping) |
3 |
||
Supplementary Table 1: Assessment of Specific Clinical Features of COVID-19 patients.


 Impact Factor: * 5.3
Impact Factor: * 5.3 Acceptance Rate: 75.63%
Acceptance Rate: 75.63%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 
