Changes in Blood Thallium Concentration during and after Prussian Blue Administration
Makoto Onodera1*, Yuji Fujita2, Yasuhisa Fujino3, Yoshihiro Inoue3
1Department of Regional Emergency Medicine, Fukushima Medical University, Japan
2Department of Critical Care and Disaster Medicine, School of Medicine, Iwate Medical University, Japan
3Iwate Prefectural Advanced Critical Care and Emergency Center, Iwate Medical University, Japan
*Corresponding Author: Makoto Onodera, Fukushima Medical University, Japan. .
Received: 12 May 2023; Accepted: 19 May 2023; Published: 30 May 2023
Article Information
Citation: Makoto Onodera, Yuji Fujita, Yasuhisa Fujino, Yoshihiro Inoue. Changes in Blood Thallium Concentration during and after Prussian Blue Administration. Archives of Clinical and Medical Case Reports. 7 (2023): 244-247.
View / Download Pdf Share at FacebookAbstract
Prussian blue is an antidote for acute thallium poisoning that binds to thallium in the gastrointestinal tract, thereby increasing thallium fecal excretion. However, the effects of Prussian blue monotherapy in humans remain unknown. A 66-year-old male ingested approximately 50 mL of liquid thallium rodenticide (thallium sulfate: 2%), a lethal dose, and presented to our hospital 40 hours after ingestion. The initial laboratory test results were normal. On the second day of admission, oral Prussian blue ordered from outside the hospital was started at a dose of 3 g every eight hours, and the whole blood thallium concentration was measured on consecutive days using inductively coupled plasma-mass spectrometry. On the fourth day, however, he complained of respiratory discomfort, and moderate acute respiratory distress syndrome was diagnosed via chest computed tomography (CT) scan and PaO2/FiO2 ratio. Endotracheal intubation was performed, and Prussian blue was discontinued because of medical costs. The patient died of respiratory failure on the ninth day after admission. The half-life of thallium in whole blood was 53 h under Prussian blue administration.
After discontinuing the administration of Prussian blue, the whole-blood thallium concentration decreased very slowly, with a half-life of thallium in whole blood of 330 h. The half-life of thallium in the whole blood was reduced approximately six-fold after Prussian blue therapy. Prussian blue should thus be considered the drug of choice in case of acute thallium poisoning.
Keywords
<p>Acute Thallium Poisoning; Liquid Thallium Rodenticide; Blood Thallium Concentration; Prussian Blue Administration; Acute Respiratory Distress Syndrome</p>
Article Details
1. Background
Acute thallium poisoning is a rare occurrence but is serious and even fatal with a human lethal dose of 10-15 mg/kg [1]. Currently, patients with acute thallium poisoning are usually treated with Prussian blue (PB; Radiogardase®, Haupt Pharma, Berlin, Germany) combined with blood purification methods, such as hemodialysis, hemoperfusion, or plasma exchange [2]. However, changes in the blood concentration of thallium using PB monotherapy have not been reported [3]. Herein, we report the blood thallium concentration during and after PB administration in a case of acute thallium poisoning.
2. Case Presentation
A 66-year-old male ingested approximately 50 mL of liquid thallium rodenticide (thallium sulfate: 2%) in an attempt to commit suicide. The estimated amount of thallium ingested was 14.2 mg/kg, a lethal dose. Thirty hours after thallium ingestion, the patient developed epigastric pain and vomiting and presented at our hospital 40 hours after ingestion. He was a known hypertensive and had been treated with antihypertensive agents for 10 years. On arrival, he was awake and alert; however, he complained of epigastric pain and the tips of the toes of both feet. Physical examination revealed a blood pressure of 176/83 mmHg, a pulse rate of 68 beats per minute, a temperature of 37.1 °C, and a respiratory rate of 22 breaths per minute.
The results of the initial laboratory tests, including general blood tests, biochemical tests, and a blood gas analysis (pH 7.484, PaCO2 34.3 mmHg, PaO2 85.0 mmHg, PaO2 / FiO2 ratio 404.8 mmHg) were normal. There were no abnormalities on the electrocardiogram, chest and abdominal X-rays, and computed tomography (CT) scans of the brain, chest, abdomen, and pelvis. The initial treatment included intravenous fluids and multiple-dose activated charcoal (MDAC) at a dose of 50 g every four hours. On the second day of admission, the patient’s symptoms (epigastric pain and pain at his toe tips) resolved. Oral PB ordered from outside the hospital was started at a dose of 3 g every eight hours, and the whole blood thallium concentration was measured on consecutive days using inductively coupled plasma-mass spectrometry (ICP-MS).
However, on the fourth day, he complained of respiratory discomfort, and a repeat chest CT showed the development of bilateral alveolar infiltrates (Figure 1). The PaO2/FiO2 ratio had decreased to 206.7 mmHg, and a diagnosis of mild acute respiratory distress syndrome (ARDS) was made according to the Berlin definition [4]. Consequently, endotracheal intubation was performed, and mechanical ventilation was initiated. At the same time, his family requested that PB administration be stopped owing to medical costs. As a result, PB administration was discontinued within 48 hours.
In addition, sivelestat sodium hydrate was administered continuously at a dose of 300 mg/day in addition to piperacillin sodium at a dose of 4 g/day; however, the PaO2/FiO2 ratio remained around 100 mmHg, and the patient died of respiratory failure on the ninth day after admission.
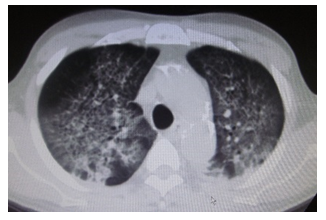
Figure 1: Chest CT scan showing the development of bilateral alveolar infiltrates.
The serum creatinine levels ranged from 1 to 2 mg/dL, and the 24-hour urinary output was kept at 2,000 -3,000 mL during hospitalization. The test results for sputum culture, urine culture, and arterial blood culture submitted on the fourth day of hospitalization were negative. No alopecia or Mees’ lines were observed during the patient’s clinical course, which are typical symptoms of thallium poisoning. We measured the whole blood thallium concentration on consecutive days from day 2 to day 8. The logarithmic whole blood thallium concentrations were 8.284 μg/L on day 2 and 7.634 μg/L 48 h after PB administration. The half-life of thallium in whole blood was 53 h under PB administration.
After the discontinuation of PB administration, the whole blood thallium concentration decreased very slowly, and the logarithmic whole blood thallium concentration was 7.459 μg/L 144 h after admission. The half-life of thallium in whole blood was 330 h after the PB treatment was discontinued (Figure 2).
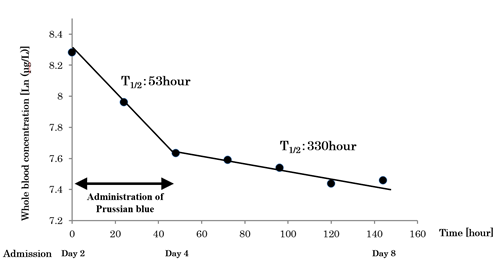
Figure 2: Logarithmic whole-blood concentration of thallium measured using inductively coupled plasma-mass spectrometry (ICP-MS). The half-life of thallium was 53 hours during Prussian Blue administration, as compared to 330 hours after stopping Prussian Blue administration.
3. Discussion
The clinical course of this case suggests two important clinical issues. First, the half-life of thallium in the whole blood was reduced by approximately six-fold because of PB therapy. When administered orally, PB binds to thallium in the gastrointestinal tract, thereby increasing thallium excretion into feces and decreasing the thallium concentration in the blood [5]. PB also inhibits the enterohepatic circulation of thallium and decreases thallium accumulation in cells [6]. In a rat experiment, Mulkey et al. [6] demonstrated a significant decrease in blood thallium concentrations in rats treated with PB as compared with those in the untreated group. In addition, Rìos et al. [7] reported that PB administration decreased thallium content in rat organs. The present case ingested a lethal dose of thallium, and 40 h had passed before the initiation of treatment. We started treatment with MDAC and oral PB on the second day of hospitalization. The half-life of thallium in the blood was 53 h during PB administration, suggesting that PB may be useful in reducing blood thallium concentrations. However, administering PB, which has not been approved by the Ministry of Health, Labor, and Welfare of Japan, is not covered by medical insurance; thus, all medical expenses will be shouldered by the patients [8]. As a result, the family requested that the treatment be terminated along with the worsening of his respiratory condition. Until now, the effect of PB monotherapy in humans remains unknown because no controlled clinical trials have been conducted. Coincidentally, we found that the half-life of thallium in the blood was prolonged by approximately 6-fold after PB administration was discontinued. Therefore, PB should be considered the drug of choice for cases of acute thallium poisoning. Furthermore, prompt administration of PB would be beneficial for patients with acute thallium poisoning.
Second, the patient developed ARDS on the fourth day of ingestion, did not respond to treatment, and expired. It has been reported that symptoms of thallium poisoning range from nerve palsy to respiratory muscle paralysis [1, 9]. However, only a few cases of thallium poisoning causing ARDS have been reported. Roby et al. [10] reported two cases of patients with acute thallium poisoning who developed ARDS. There was no abnormality on their admission X-rays, but both developed ARDS and expired. Similarly, our case did not have abnormal X-rays on admission, and the patient’s PaO2/FiO2 ratio was normal. However, the patient’s respiratory status deteriorated on day 4, and was eventually diagnosed with mild ARDS. Although therapy for ARDS was initiated, the patient’s condition progressed to severe ARDS, and he expired. Generally, thallium is absorbed from the respiratory tract, gastrointestinal tract, or skin and is widely distributed in organs and tissues [6]. However, the thallium concentration in the lungs at autopsy has been reported to be lower than that in other organs [11].
In a case report by Roby et al. [10], the lungs of their patients during autopsy had pleural effusion, diffuse interstitial and alveolar inflammation, and hyaline membrane formation consistent with a diagnosis of ARDS. Similar findings were also observed in acute thallium poisoning when the blood concentration was high [11]. Thus, it remains elusive whether ARDS is associated with acute thallium poisoning; however, ARDS may occur in patients with high blood thallium concentrations, which should be noted as a respiratory complications to thallium.
In recent studies, the combined use of PB and hemodialysis, hemoperfusion, or plasma exchange enhanced the elimination of thallium in animals and humans and improved the survival of patients, especially those whose hospital admissions were delayed [2, 12, 13]. However, the EXTRIP Workgroup reported that thallium has low dialyzability and limited evidence; therefore, extracorporeal removal is recommended only in cases of severe thallium poisoning [14]. In this case, hemodialysis was not considered because the patient did not exhibit renal failure or oliguria. However, in retrospect, we think that hemodialysis should have been considered because the blood thallium concentration was high.
4. Conclusion
The half-life of thallium in the whole blood was reduced by approximately six-fold due to PB therapy. Thus, PB should be considered the drug of choice in cases of acute thallium poisoning. However, it should be noted that in some regions, PB may be difficult to obtain or may not be covered by health insurance.
Disclosure Statement
No financial support or other potential conflicts of interest relevant to this article are reported.
Acknowledgements
We would like to thank Editage (www.editage.com) for English language editing.
References
- Cvjetko P, Cvjetko I, Pavlica M. Thallium toxicity in humans. Arh Hig Rada Toksikol 61 (2010): 111-119.
- Lin G, Yuan L, Peng X, et al. Clinical characteristics and treatment of thallium poisoning in patients with delayed admission in China. Medicine (Baltimore) 98 (2019): e16471.
- Hoffman RS. I. Metals. A31. Prussian blue. Goldfrank’s Toxicologic emergencies. 11th edition, Nelson LS, et al. McGraw Hill Educations, New York (2019): 1357-1361.
- ARDS Definition Task Force, Ranieri VM, Rubenfeld GD, et al. Acute respiratory distress syndrome: the Berlin Definition. JAMA 307 (2012): 2526-2533.
- Mercurio-Zappala M, Hoffman RS. I. Metals. 99. Thallium. Goldfrank’s Toxicologic emergencies. 11th edition, Nelson LS, et al. McGraw Hill Educations, New York (2019): 1350-1356.
- Mulkey JP, Oehme FW. Are 2, 3-dimercapto-1-propanesulfonic acid or prussian blue beneficial in acute thallotoxicosis in rats? Vet Hum Toxicol 42 (2000): 325-329.
- Rìos C, Monroy-Noyola A. D-penicillamine and prussian blue as antidotes against thallium intoxication in rats. Toxicology 74 (1992): 69-76.
- The Ministry of Health, Labour and Welfare of Japan. Outline of Scope and Method of Estimation of National Medical Care Expenditure (2016).
- Osorio-Rico L, Santamaria A, Galvan-Arzate S. Thallium toxicity: general issues, neurological symptoms, and neurotoxic mechanisms. Adv Neurobiol 18 (2017): 345-353.
- Roby DS, Fein AM, Bennett RH, et al. Cardiopulmonary effects of acute thallium poisoning. Chest 85 (1984): 236-240.
- Hologgitas J, Ullucci P, Driscoll J, et al. Thallium elimination kinetics in acute thallotoxicosis. J Anal Toxicol 4 (1980): 68-75.
- Huang C, Zhang X, Li G, et al. A case of severe thallium poisoning successfully treated with hemoperfusion and continuous veno-venous hemofiltration. Hum Exp Toxicol 33 (2014): 554-558.
- Wang TT, Wen B, Yu XN, et al. Early diagnosis, treatment, and outcomes of five patients with acute thallium poisoning. World J Clin Cases 9 (2021): 5082-5091.
- Ghannoum M, Nolin TD, Goldfarb DS, et al. Extracorporeal Treatment for thallium poisoning: recommendations from the EXTRIP Workgrooup. Clin J Am Soc Nephrol 7 (2012): 1682-1690.


 Impact Factor: * 5.3
Impact Factor: * 5.3 Acceptance Rate: 75.63%
Acceptance Rate: 75.63%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 
