Metastatic Primitive Neuroectodermal Tumor Arising from Stomach: A Case Report
Song Yi Yu1, Chang-Hoon Lee1, So-Yeon Jeon1,2, Ho-Young Yhim1,2, Na-Ri Lee1,2, Jae-Yong Kwak1,2, Chang-Yeol Yim1,2, Ae Ri An3, Ho Sung Park2,3, Eun-Kee Song1,2*
1Department of Internal Medicine, Jeonbuk National University Medical School, Jeonju, Republic of Korea
2Research Institute of Clinical Medicine of Jeonbuk National University-Biomedical Research Institute of Jeonbuk National University Hospital, Jeonju, Republic of Korea
3Department of Pathology, Jeonbuk National University Medical School, Jeonju, Republic of Korea
*Corresponding Author: Eun-Kee Song, Department of Internal Medicine, Jeonbuk National University Medical School, 20 Geonji-ro, Deokjin-gu, Jeonju, Republic of Korea
Received: 28 February 2020; Accepted: 11 March 2020; Published: 24 March 2020
Article Information
Citation:
Song Yi Yu, Chang-Hoon Lee, So-Yeon Jeon, Ho-Young Yhim, Na-Ri Lee, Jae-Yong Kwak, Chang-Yeol Yim, Ae Ri An, Ho Sung Park, Eun-Kee Song. Metastatic Primitive Neuroectodermal Tumor Arising from Stomach: A Case Report. Archives of Clinical and Medical Case Reports 4 (2020): 259-265.
View / Download Pdf Share at FacebookAbstract
Gastric primitive neuroectodermal tumor (PNET) is extremely rare and treatment of metastatic gastric PNET is not established. Herein, we report a case of a 54-year-old male who had undergone total gastrectomy with D2 lymph node dissection, distal pancreatectomy, and splenectomy for the curative intent of primary gastric PNET initially and received multimodal treatment including chemotherapy, radiotherapy, and palliative metastasectomy after recurrence and metastasis. He died 5.6 years after first diagnosis and 4.2 years after relapse due to disease progression. Although no effective chemotherapy agents and standard treatments have been determined, multimodality treatment approach applied to this patient has helped to achieve relatively long-term survival. This case report will be helpful to identify the disease progress and prognosis of gastric PNET and to know the effects of various treatment methods.
Keywords
<p>Stomach; Primitive Neuroectodermal Tumor; Chemotherapy; Radiotherapy</p>
Article Details
1. Introduction
Primitive neuroectodermal tumor (PNET) is a malignant tumor composed of small round cells of neuroectodermal origin that affect soft tissue and bone. PNET exhibits great diversity in their clinical manifestations and pathologic similarities with other small round cell tumors. Batsakis et al. divided this kind of tumors into the following three groups based on the tissue of origin: CNS PNETs, neuroblastoma, and peripheral PNETs [1]. Peripheral PNETs are also classified as part of the Ewing family of tumors (so called Ewing’s sarcoma) and often referred to interchangeably in the literature. The pathologic and cytogenetic understanding of these tumors has significantly advanced after the first description of PNETs by Arthur Purdy Stout in 1918 [2]. Based on molecular cytogenetic analysis, both Ewing family of tumors and peripheral PNETs are characterized by chromosomal translocation resulting in fusion of EWS gene with FLI-1 gene; t(11;22) (q24;q12). Although PNET has been documented in visceral organs, PNET arising in the stomach is extremely rare. As far as we know, there are only 7 cases reported in the literature [3-9]. Herein, we report a case of primary gastric PNET of 54-year-old man. The patient had a far advanced disease of metastasis and recurrence, but we could treat the patient successfully using multimodality approaches.
2. Case Report
A 54-year-old male was admitted to the hospital with melena and anemia for one month. He was taking several medicines because of previous history of paroxysmal atrial fibrillation and hypertension and had a coronary artery stent placed for ischemic heart disease three years ago. Endoscopic examination showed an ulcerofungating mass in the upper body of stomach. Pathologic findings of the biopsy taken by endoscopy showed proliferation of small round cells with some degree of artifactual shrinkage, giving the classic appearance of small cell carcinoma, that is, nuclear molding, elongation of the nuclei, and deformation, clumping, and diffusion of the chromatin (Figure 1A). Abdominal computed tomography (CT) scan revealed focal wall thickening in the cardia of stomach with multiple perigastric lymph nodes enlargement and tumor thrombus in left gastric vein. F-18 FDG positron emission tomography-computed tomography (PET-CT) also showed focal hypermetabolic lesions in the cardia of stomach and perigastric areas without the evidence of distant metastasis. The initial diagnosis according to the endoscopic biopsy was primary gastric neuroendocrine carcinoma. Total gastrectomy with D2 dissection, distal pancreatectomy, and splenectomy were performed. According to the postoperative pathologic report, the tumor, sized 6.5 × 6.3 × 3.4 cm, was located in the posterior wall of upper third of stomach. It was penetrating the serosa without involving pancreas and the resection margins were not involved. Lymphovascular and perineural invasion were found and 8 out of 26 lymph nodes dissected were metastatic lesions. Tumor cells were strongly expressing CD99 and Ki-67 labeling index was 60%. EWS-FLI1 fusion transcripts [t(11;22)(q24;q12)] were detected by RT-PCR in the tumor cells and the final diagnosis was changed to primary gastric PNET. Because the postoperative pathologic findings suggested poor prognosis and high probability of recurrence, 6 cycles of adjuvant chemotherapy with etoposide and cisplatin were applied.
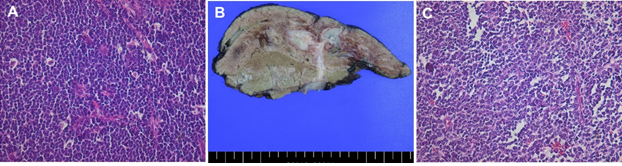
Figure 1: Microscopic and macroscopic findings. (A) Microscopic finding of the biopsy taken by endoscopy showed proliferation of small round cells with some degree of artifactual shrinkage, giving the classic appearance of small cell carcinoma, that is, nuclear molding, elongation of the nuclei, and deformation, clumping, and diffusion of the chromatin (H&E, 400x); (B) Cut section of fixed specimen from right hemihepatectomy showed relatively homogenous white two masses connected with each other in segment 7; (C) Microscopic finding of the metastasectomy of liver mass was identical with the primary gastric lesion indicating metastatic PNET (H&E, 400x).
Despite of above treatment, liver metastasis was found on 15 months after surgery (11 months after completion of adjuvant chemotherapy). Abdominal CT scan showed multiple huge hypervascular masses in right lobe of liver and an enlarged lymph node in portocaval area (Figure 2A). We confirmed the recurrence and metastasis by liver biopsy and tumor cells were immunohistochemically strong positive to CD99 and negative to CD56 similar to the previous stomach lesion. PET-CT finding was similar to the outcome of CT scan (Figure 2B). Palliative chemotherapy was decided and cyclophosphamide, doxorubicin, and vincristine (CAV) combination chemotherapy was applied. After 4 cycles of CAV chemotherapy, partial response with complete resolution of metastatic lymph node in portocaval area was achieved (Figure 2C).
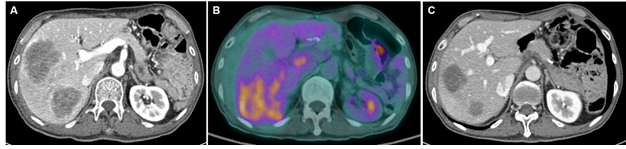
Figure 2: Imaging studies before hepatectomy. (A) Abdominal CT scan showed two huge hypervascular masses in right lobe of liver and an enlarged lymph node in portocaval area; (B) F-18 FDG positron emission tomography-computed tomography (PET-CT) showed FDG-avid huge mass with central low attenuation in right lobe of liver; (C) Abdominal CT scan revealed partial response of liver metastases with complete resolution of metastatic lymph node in portocaval area after 4 cycles of CAV chemotherapy.
E, 400x).
There were two residual lesions in the liver after 10 cycles of CAV chemotherapy for 6 months, but the cumulative dose of doxorubicin reached to the level of high incidence of chronic cardiotoxicity (550 mg/m2) and the general condition of the patient was deteriorated. We decided to stop CAV chemotherapy and give him a chemotherapy holiday until progression. The disease progression was noted after 8 months of the discontinuation of chemotherapy. Through the systemic evaluation including abdominal CT, chest CT, and endoscopy, metastatic lesions were found to be localized in right lobe of liver and right hepatectomy with cholecystecomy was performed. Pathologic finding of the resected mass was identical with the primary gastric lesion indicating metastatic PNET (Figure 1B, 1C). Despite of metastasectomy, regrowing nodules were found in the site of hepatectomy after 7 months (Figure 3A). Although the recurrence was localized to the margin of previous operation site, reoperation was not amenable due to the risk of massive bleeding and possibility of incomplete resection. Radiotherapy with gross tumor volume of 50 gray in 25 fractions over a period of 5 weeks was undergone. The size of nodular lesions was decreased after radiotherapy, but multiple lung nodules and regrowth of nodular lesions in liver were noted after 6 months (Figure 3B, 3C).
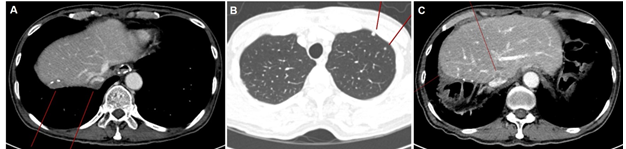
Figure 3: Imaging studies after hepatectomy and radiotherapy. (A) Abdominal CT scan showed regrowing nodules at the boundary of previous hepatectomy site; (B) Chest CT scan showed multiple nodules in the upper lobe of left lung; (C) Abdominal CT scan showed multiple new metastases in liver.
Six cycles of second line chemotherapy with ifosfamide and etoposide were applied and partial response was achieved, but lung metastases were aggravated and new pancreatic metastasis was found after 7 months. Fms-related tyrosine kinase 3 (FLT3) gene and growth arrested-specific 6 (GAS6) gene amplifications were found by next generation sequencing using metastatic specimen acquired metastasectomy of liver, but there was not suitable target agent for this variations. So, third line chemotherapy with bleomycin and cyclophosphamide and forth line immunotherapy with pembrolizumab were attempted but both failed. The patient died of disease progression 68 months (5.6 years) after first diagnosis and 51 months (4.2 years) after relapse.
3. Discussion and Conclusions
Here we reported a very rare case of primary gastric PNET with multiple metastases. Although several cases have already been reported, most of the cases were treated with surgery alone and there was no report of palliative chemotherapy as far as we know. This case was successfully treated with multimodality combining surgery, chemotherapy, and radiation in the metastatic setting and we think it is worthwhile to be reported. PNETs are members of the Ewing family of tumors, which comprises skeletal and extra-skeletal malignancies originating from the neural crest and sharing distinct alterations. They mainly occur in adolescents or young adults, but are quite rare in elderly. PNETs have been reported to originate from a number of extra-skeletal sites, such as kidney, ureter, urinary bladder, uterus, and cervix [10]. However, PNETs originating from gastrointestinal tract are extremely rare. According to the literatures, only 7 cases of primary gastric PNET were reported until now [3-9]. The median age with primary gastric PENT was 35 years (14 ~ 68 years). All patients received gastrectomy and 5 patients were treated with (neo) adjuvant chemotherapy, but recurrence and metastasis were found in three patients and all of the three patients were dead within 2 years.
Histologic features, immunopheynotypes, and molecular studies are needed for the differential diagnosis and most important diseases that requires discrimination are small cell carcinoma and gastrointestinal neuroectodermal tumor (GNET). Histologically, PNET is composed of small round cells that are usually rack in glycogen, and the neuroepithelial morphologic differentiation. The neuroendocrine phenotype is confirmed by positivity to CD99 immunohistochemically. RT-PCR or FISH testing for the presence of t(11;12) translocation can be particularly useful when the tumor occurs in older patients or in a unusual site, as well as to differentiate from other small round cell tumors. Small cell carcinoma and PNET share similar histologic features such as sheet-like infiltrative growth pattern, frequent rosette-like arrangement, and inconspicuous nucleoli. However, our case showed additional heterogeneous histologic features including frequent multinucleated giant cells and some light areas composed of tumor cells with amphophilic cytoplasm. In addition, strong positive of CD99 and FLI1 and negative of TTF-1 on immunohistochemistry were not consistent for small cell carcinoma.
Another important differential diagnosis is GNET, which has been previously referred to as clear cell sarcoma-like gastrointestinal tumor [11, 12] or clear cell sarcoma-like tumor with osteoclast-like giant cells of the gastrointestinal tract [13-15]. It was first described in 2003 by Zambrano et al. who reported six cases as malignant mesenchymal neoplasm of gastrointestinal tract that is characterized by the presence of osteoclast-like multinucleated giant cells histologically, and expresses S100 protein and is negative for CD117 and melanocytic markers, immunohistochemically [13]. At the molecular genetic level, GNET is associated with EWSR1 gene rearrangements, which results in the fusion of EWSR1 and ATF1 or CREB1 [11]. In our case, the tumor cells strongly expressed CD99 and FLI1, but did not express S-100 protein, Melan-A, HMB45, WT1, and desmin and EWS-FLI1 fusion could be another difference between PNET and GNET. Surgical resection with negative margins is gold standard for treating PNET. However, complete resection may not be possible in some cases including recurrence or metastasis. Chemotherapy and radiation are necessary in such cases. Chemotherapy has significantly improved outcomes in patients with PNET and the adjuvant or neoadjuvant chemotherapy is also recommended. Carvajal and Meyers, in a comprehensive review of the chemotherapeutic regimens in the treatment of PENTs and Ewing family of tumors, recommended a combination regimens such as vincristine, doxorubicin, and cyclophosphamide or ifosfamide and etospodie [16]. For the treatment of gastric PNET, same strategies can be applied. In our case, combination chemotherapy combining cyclophosphamide, doxorubicin, and vincristine (CAV regimen) for first line chemotherapy and ifosfamide and etoposide for second line chemotherapy were applied and good response was acquired. Radiation was also applied after local recurrence after surgical resection of liver metastasis.
As in other cancers, multidisciplinary approach is very important in recurrent or metastatic PNETs. The treatment of metastatic disease includes chemotherapy and radiotherapy or metastasectomy if the disease is localized. While outcomes vary based on disease status, patients with metastatic PNET have poor outcome ranging from 0~25% of 5 year survival rates compared to 40~79% for those with localized diasese [17]. Because gastric PNET is extremely rare, it is unknown what kind of treatment sequence and combination will be optimal. So, various clinical experiences are likely to be useful for determining the best treatment. Here, we report a rare case of recurrent and metastatic gastric PNET, which was successful controlled with multimodality treatments with long term survival.
Compliance with Ethical Standards
This study was approved by the Institutional ethical committee of Jeonbuk National University Hospital, Jeonju, Republic of Korea.
Consent for Publication
Written informed consent was obtained from the patient for publications of this case reports and any accompanying images.
Conflicts of Interest
All authors declare that they have no conflicts of interest.
Acknowledgements
This paper was supported by fund of Biomedical Research Institute, Jeonbuk National University Hospital.
References
- Batsakis JG, Mackay B, el-Naggar AK. Ewing's sarcoma and peripheral primitive neuroectodermal tumor: an interim report. Ann Otol Rhinol Laryngol 105 (1996): 838-843.
- Sout AP. A tumor of the ulnar nerve. Proc NY Pathol Soc 12 (1918): 2-12.
- Colovic RB, Grubor NM, Micev MT, et al. Perigastric extraskeletal Ewing's sarcoma: a case report. World J Gastroenterol 15 (2009): 245-247.
- Inoue M, Wakai T, Korita PV, et al. Gastric Ewing sarcoma/primitive neuroectodermal tumor: A case report. Oncol Lett 2 (2011): 207-210.
- Kim HS, Kim S, Min YD, et al. Ewing's Sarcoma of the Stomach; Rare Case of Ewing's Sarcoma and Suggestion of New Treatment Strategy. J Gastric Cancer 12 (2012): 258-261.
- Rafailidis S, Ballas K, Psarras K, et al. Primary Ewing sarcoma of the stomach--a newly described entity. Eur Surg Res 42 (2009): 17-20.
- Song MJ, An S, Lee SS, et al. Primitive Neuroectodermal Tumor of the Stomach: A Case Report. Int J Surg Pathol 24 (2016): 543-547.
- Soulard R, Claude V, Camparo P, et al. Primitive neuroectodermal tumor of the stomach. Arch Pathol Lab Med 129 (2005): 107-110.
- Czekalla R, Fuchs M, Stölzle A, et al. Peripheral primitive neuroectodermal tumor of the stomach in a 14-year-old boy: a case report. Eur J Gastroenterol Hepatol 16 (2004): 1391-1400.
- Khoury JD. Ewing sarcoma family of tumors. Adv Anat Pathol 12 (2005): 212-220.
- Stockman DL, Miettinen M, Suster S, et al. Malignant gastrointestinal neuroectodermal tumor: clinicopathologic, immunohistochemical, ultrastructural, and molecular analysis of 16 cases with a reappraisal of clear cell sarcoma-like tumors of the gastrointestinal tract. Am J Surg Pathol 36 (2012): 857-868.
- Kosemehmetoglu K, Folpe AL. Clear cell sarcoma of tendons and aponeuroses, and osteoclast-rich tumour of the gastrointestinal tract with features resembling clear cell sarcoma of soft parts: a review and update. J Clin Pathol 63 (2010): 416-423.
- Zambrano E, Reyes-Mugica M, Franchi A, et al. An osteoclast-rich tumor of the gastrointestinal tract with features resembling clear cell sarcoma of soft parts: reports of 6 cases of a GIST simulator. Int J Surg Pathol 11 (2003): 75-81.
- Friedrichs N, Testi MA, Moiraghi L, et al. Clear cell sarcoma-like tumor with osteoclast-like giant cells in the small bowel: further evidence for a new tumor entity. Int J Surg Pathol 13 (2005): 313-318.
- Huang W, Zhang X, Li D, et al. Osteoclast-rich tumor of the gastrointestinal tract with features resembling those of clear cell sarcoma of soft parts. Virchows Arch 448 (2006): 200-203.
- Carvajal R, Meyers P. Ewing's sarcoma and primitive neuroectodermal family of tumors. Hematol Oncol Clin North Am 19 (2005): 501-525.
- Scurr M, Judson I. How to treat the Ewing's family of sarcomas in adult patients. Oncologist 11 (2006): 65-72.


 Impact Factor: * 5.3
Impact Factor: * 5.3 Acceptance Rate: 75.63%
Acceptance Rate: 75.63%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 
