Prolonged SARS-CoV-2 Infection Successfully Treated with a Consecutive Combined Scheme Therapy in an HIV-Positive Patient with AIDS
Alessandra Vergori1*, Francesco Baldini1, Carmela Pinnetti1, Susanna Grisetti1, Annalisa Mondi1, Giulia Matusali2, Marta Camici1, Fabrizio Maggi2, Andrea Antinori1
1HIV/AIDS Unit, National Institute for Infectious Diseases L. Spallanzani, IRCCS, Rome, Italy
2Laboratory of Virology, National Institute for Infectious Diseases L. Spallanzani, IRCCS, Rome, Italy
*Corresponding Author: Alessandra Vergori, HIV/AIDS Unit, National Institute for Infectious Diseases L. Spallanzani, IRCCS, Via Portuense, 292, 00149 Rome, Italy.
Received: 21 February 2023; Accepted: 01 March 2023; Published: 21 November 2023
Article Information
Citation: Alessandra Vergori, Francesco Baldini, Carmela Pinnetti, Susanna Grisetti, Annalisa Mondi, Giulia Matusali, Marta Camici, Fabrizio Maggi, Andrea Antinori. Prolonged SARS-CoV-2 Infection Successfully Treated with a Consecutive Combined Scheme Therapy in an HIV-Positive Patient with AIDS. Archives of Clinical and Medical Case Reports. 7 (2023): 409-413.
View / Download Pdf Share at FacebookAbstract
Purpose: Cases of persistent infection have already been widely described with some proposals for combination or extended course therapies in immunocompromised subjects, but nothing has been addressed in AIDS patients. We present a case of prolonged, mild SARS-CoV-2 infection that was successfully treated with a consecutive combined scheme of therapy.
Methods/Results: A prolonged shedding of SARS-CoV-2 was observed up to 92 days and the COVID-19 clinical manifestation was mild without evidence of pneumonia and/or acute respiratory insufficiency. The infection was not cleared after the first treatment with remdesivir IV as early treatment (for 3 days) suggesting a limited effect on SARS-CoV-2 in an immunocompromised individual. Several weeks later, a second therapeutic attempt was made with tixagevimab/cilgavimab 300/300 IM but SARS-CoV-2 RNA was still detected for further 5 weeks. A third attempt with nirmatrelvir/ritonavir determined the definitive viral clearance of SARS-CoV-2 after 92 days since the first detection.
Conclusion: Our data indicate that certain immunocompromised individuals may shed infectious virus longer and need a tailored and valuable therapeutics approach. Additional data from clinical trials are required to support a feasible approach to managing this vulnerable group of patients.
Keywords
<p>AIDS; Prolonged SARS-CoV-2 infection; COVID-19; Tixagevimab/cilgavimab; Nirmatrelvir/ritonavir</p>
Article Details
1. Introduction
Since the onset of the SARS-CoV-2 pandemic, there have been numerous randomized clinical trials that have, due to their high quality, enabled the development of strategies for the management of patients with COVID19 very rapidly. However, these trials have focused on prevention of severe disease in patients with very early infection and on the treatment of patients with moderate/severe COVID19. Although this strategy is appli-cable to most patients, it is now known that specific categories of patients, such as severely immunocompromised, not included in these studies, do not benefit in the same way. One of the most significant problems of the immunocompromised patients is that since they are unable to develop an adequate antibody and neutralizing response, they are at risk of incurring in prolonged SARS-CoV-2 infections and exhibiting clinical manifestations from asymptomatic to severe disease, and also recurrence of symptoms that can last for months [1-6]. It’s therefore known that patients with highly impaired B- and T-cell adaptive immunity can prevent COVID-19 progression to severe complications, even if they are unable to clear SARS-CoV-2 infection. This condition can result in a long-term status of asymptomatic SARS-CoV-2 carriage, with an intra host viral evolution with potential development of resistance mutations and subsequent further immunological escape to available drugs [1, 5, 6]. Cases of persistent infection have already been widely described with some proposals for combination or extended course therapies in immunocompro-mised subjects [7-9], but nothing has been addressed in AIDS patients. We present a case of prolonged, mild SARS-CoV-2 infection that was successfully treated with a consecutive combined scheme of therapy.
2. Method and Results
2.1 Case description
On August 1, 2022, a 54-year-old man with a 9-year history of HIV-1 infection, off therapy from antiretroviral treatment, presented to our department with vomiting, severe as-thenia, frontal headache, subjective dizziness, and difficulty walking. He had previously received 2 doses of mRNA anti-SARS-CoV-2 vaccination, completed on October 2021. He was diagnosed with HIV-1 infection in July 2013 as an AIDS presenter with Pneumocystis jirovecii pneumonia complicated by spontaneous pneumomediastinum and oral candidiasis [CD4 6/mmc (1%), HIV RNA pre-therapy 386502 copies/mL]. He began antiretroviral therapy with tenofovir/emtricitabine (TDF/F) and efavirenz (EFV) in the same month and subsequently switched to TDF/F/Rilpivirine (RPV) and Tenofovir alafena-mide/F/RPV (TAF/F/RPV) with stable viral suppression and immunological recovery. However, in September 2019, he discontinued therapy by his own choice and was unable to follow up. On August 1, 2022, he was admitted to the hospital in critically ill conditions with a mental and motor slowdown, oral mucositis, skin dehydration and nuchal stiffness. After being diagnosed with SARS-CoV-2, he was placed in isolation measures and we immediately performed a diagnostic lumbar puncture, which confirmed the diagnosis of Cryptococcal meningitis, thus, specific therapy was initiated with liposomal Amphotericin B IV at dosage of 4 mg/Kg IV q24h and fluconazole 800 mg IV (due to flucytosine’s temporary unavailability to be administered). Moreover, having verified the absence of pneumonia by Chest Computed tomography (CT) scan and the onset of SARS-CoV-2 related symptoms (possibly severe asthenia) for less than 5 days, we promptly proceeded with antiviral therapy with Remdesivir intravenously (IV), carried out from August 3 to August 5, 2022, at standard dosage. Anti-Spike IgG were 251 BAU/mL, anti-nucleocapsid was not detected. Anti-Toxoplasma gondii IgG/IgM, HCVAb, Quantiferon test and CMV-DNA originally resulted negative; Cryptococcus neoformans antigen resulted positive on plasma sample and on Cerebro-Spinal Fluid (CSF) (titres 1:1000 on plas-ma/CSF); among the other agents investigated on CSF: EBV, CMV, JCV, HSV-1/2, VZV, SARS-CoV-2, M. tuberculosis and non-tuberculous mycobacteria were negative. HIV-RNA was 304432 copies/ml (49343 cp/mL in CSF), the CD4 count was 22/mm3 (3.6%), the CD8 count was 357/mm3 (58%) and the CD4/CD8 ratio was 0.06. The patient's course was complicated by another intercurrent opportunistic infection such as a disseminated CMV infection with ocular localization, for which specific therapy with ganciclovir was given. A progressive worsening of the neurological picture with the impending onset of almost total deafness and blindness as a complication of cryptococcal meningitis was observed. Antifungal therapy was modified after about 8 weeks with the addition of flucytosine (25 mg/Kg po q6h) and then, after a proper induction period of 21 days, continued with maintenance therapy with fluconazole 800 mg. Since the change in an-tifungal therapy, the patient has experienced progressive clinical improvement. After eleven weeks from diagnosis of Cryptococcal meningitis antiretroviral therapy with TAF/F/Bictegravir (BIC) was initiated. The clinical picture related to COVID-19 remains essentially stable and mild, but over the course of the next 6 weeks, he was tested for SARS-CoV-2 on naso-pharingeal swab (NPS) another 8 times, remaining positive. As a result of immunocompromised clinical condition and our Regulatory Agency’s indications approval, we decided to initiate Tixagevimab/cilgavimab 300/300 mg IM monoclonal antibody therapy. Subsequent NPS (N= 5) results all positive for 5 weeks with no sub-stantial modification of the cycle threshold values (CT). Given the concurrent clinical improvement of the opportunistic infections and to perform assessments for cochlear implant placement and visuo-spatial rehabilitation, after about 5 weeks from the ad-ministration of Tixagevimab/cilgavimab, we prescribed Nirmatrelvir/ritonavir at a dosage of 300/100 mg BID for 5 days, paying close attention to any pharmacological in-teractions. The patient has been tested again 5 times in 2 weeks, observing a progressive increase in CT until November 1, 2022, exactly 3 months after the first viral detection, the day we found viral clearance with a negative RT-PCR for SARS-CoV-2 on NPS (the timeline of clinical presentation, cycle threshold values, and treatments is shown in Figure 1). Currently, the patient is in fair general condition, blind and deaf, persistently negative for SARS-CoV-2, stably on antiretroviral therapy and on primary prophylaxis with co-trimoxazole. Soon he will undergo to bilateral cochlear implantation and visuo-spatial and emotional rehabilitation for blind persons.
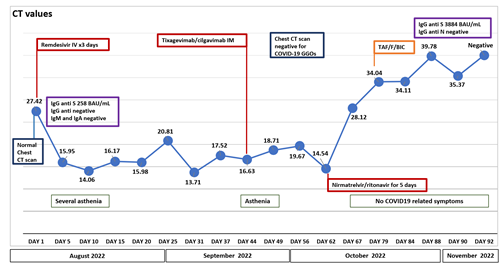
Figure 1: Timeline of clinical Presentation, cycle treshold values, and treatments
3. Discussion
To the best of our knowledge, this is the first report of a successful treatment of persistent SARS-CoV-2 prolonged infection in an immunocompromised individual with AIDS using a combined consecutive scheme therapy with remdesivir, tixagevimab/cilgavimab and nirmatrelvir/ritonavir. We describe long-term SARS-CoV-2 shedding in an AIDS-patient for 92 days after the initial positive test, with a positive PCR and a cycle threshold con-sistently <30 on the Abbot Alinity platform. Although the exact timepoint when the in-dividual acquired SARS-CoV-2 is unknown, the patient remained paucisymptomatic throughout the course of infection. This clinical aspect could be expected since the patient has profound T-cell immunocompromisation, considered to have a dominant role in the control and clearance of SARS-CoV-2 infection [10, 11]. The management of immunocompromised patients with persistent COVID-19 infection remains poorly investigated and is not currently being considered in randomized clinical trials. Convalescent plasma, monoclonal antibody, and multiple courses of remdesivir as well as extended courses of antivirals have been described with variable success [7-9]. The patient eventually cleared the SARS-CoV-2 infection from the upper respiratory tract after taking the oral antiviral Nirmatrelvir/ritonavir or, marginally, after receipt of neutralizing antibody titers of Tixagevimab/cilgavimab. When admitted in August 2022, his presentation with frontal headache, difficulty in walking, and physical and motor slowing, placed the focus on the neurological aspect, and the finding of NPS positive for SARS-CoV-2 in the absence of pneumonia was considered as a mild form, deserving of early and brief treatment with remdesivir, with the sole aim of facilitating viral clearance and preventing the worsening of the respiratory function, which was not achieved even by a second therapeutic attempt with these monoclonal antibodies, proven effective, at least in vitro, in neutralizing Omicron subvariants such as BA.4-BA.5, predominantly circulating at the time of patient hospitalization [12]. At this point, we postulated that using a con-secutive treatment scheme that takes advantage of different mechanisms of action to in-hibit viral replication - SARS-CoV-2’s RNA-dependent RNA polymerase (remdesivir), monoclonal antibodies with their neutralizing activity and boosted protease (nirma-trelvir/ritonavir) - may be beneficial for their potential additive effect [13] and especially to allow the patient to continue his or her treatment for opportunisitic infection, HIV-1 infection and rehabilitation.
Having no certainty about the intervention that allowed viral clearance in this patient and in light of the initial impairment of T immunity as mirrored by his AIDS-related op-portunistic infections, the hypothesis of gradual immunologic recovery and possible spontaneous clearance, as already reported [14] cannot be ruled out. We observed a marked increase in CTs with a negative NPS after 92 days of continuous viral detection especially after administration of the antiviral nirmatrelvir/ritonavir; therefore, we speculate that it was effective in inhibiting viral replication despite the presence of high levels of passive neutralizing monoclonal antibodies. Antiretroviral therapy has been introduced late in the course of SARS-CoV-2 infection, after eleven weeks from the admission to hospital for cryptococcal meningitis, according with available evidences that despite the low certainty, it appears that initiating antiretroviral therapy (ART) within four weeks of cryptococcal meningitis diagnosis increases the risk of mor-tality compared to delaying ART beyond four weeks [15]. For this reason it is unlikely that such immunological T recovery has occurred to contribute to spontaneous viral clearance. One of the major limitations of this report is the lack of genotyping of the virus and also the lack of cell T immunity and pro-inflammatory cytokines in order to better explore the mild maniifestation of COVID-19 in AIDS. Furthermore, looking back on the case critically and rerospectively, it may have made more sense to use tixagevimab/cilgavimab and nirmatrelvir/ritonavir simultaneously as a dual therapy, with the goal of shortening the time to positivity and reducing the opportunity of the virus evading treatment through mutations providing resistance to the medications used.
4. Conclusions
In summary, we present a case of persistent SARS-CoV-2 shedding and mild COVID19, over a 3-month period in an immunocompromised individual with AIDS that was suc-cessfully treated with a consecutive scheme therapy which uses different mechanisms of action in inhibiting viral replication. Additional data from clinical trials are required to support a feasible approach to managing this vulnerable group of patients.
Conflicts of Interest
The authors declare no conflict of interest t that are directly or indirectly related to the work submitted for publication.
Informed Consent Statement
Written informed consent was obtained from patients relatives to publish this paper.
Funding
This work was supported by Line 1 and 2—Ricerca Corrente and by Progetto COVID-2020- 12371675, both funded by Italian Ministry of Health (Ministero della Salute).
Institutional Review Board Statement
Not applicable.
Author Contributions
AV contributed to data collection and manuscript writing. AV, FB, CP, SG, AM and AA have taken charge, managed the patient and contributed to manuscript revision; FM and GM contributed to RT-PCR and manuscript revision.
References
- Choi B, Choudhary MC, Regan J, et al. Persistence and evolution of SARS-CoV-2 in an immunocompromised host. N Engl J Med 383 (2020): 2291-2293.
- Aydillo T, Gonzalez-Reiche AS, Aslam S, et al. Shedding of viable SARS-CoV-2 after immunosuppressive therapy for cancer. N Engl J Med 383 (2020): 2586-2588.
- Monrad I, Sahlertz SR, Nielsen SSF, et al. Persistent severe acute respiratory syndrome coronavirus 2 infections in immunocom-promised host displaying treatment induced viral evolution. Open Forum Infect Dis 8 (2021): ofab295.
- Avanzato VA, Matson MJ, Seifert SN, et al. Case Study: Prolonged Infectious SARS-CoV-2 Shedding from an Asymptomatic Immunocompromised Individual with Cancer. Cell 183 (2020): 1901-1912.
- Spinicci M, Mazzoni A, Coppi M, et al. Long-term SARS-CoV-2 Asymptomatic Carriage in an Immunocompromised Host: Clinical, Immunological, and Virological Implications. J Clin Immunol 42 (2022): 1371-1378.
- Leung WF, Chorlton S, Tyson J, et al. COVID-19 in an immunocompromised host: persistent shedding of viable SARS-CoV-2 and emergence of multiple mutations: a case report. Int J Infect Dis 114 (2022): 178-182.
- Martinez MA, Chen TY, Choi H, et al. Extended remdesivir infusion for persistent coronavirus disease 2019 infection. Open Fo-rum Infect Dis 9 (2022): ofac382.
- Helleberg M, Niemann CU, Moestrup KS, et al. Persistent COVID-19 in an immunocompromised patient temporarily responsive to two courses of remdesivir therapy. J Infect Dis 222 (2020): 11030-11037
- Trottier CA, Wong B, Kohli R, et al. Dual antiviral therapy for persistent COVID-19 and associated organizing pneumonia in an immunocompromised host. Clin Infect Dis (2022): ciac847.
- Sette A, Crotty S. Adaptive immunity to SARS-CoV-2 and COVID-19. Cell 184 (2021): 861-880.
- Spinicci M, Mazzoni A, Borchi B, et al. AIDS patient with severe T cell depletion achieved control but not clearance of SARS-CoV-2 infection. Eur J Immunol 52 (2022): 352-355.
- Tuekprakhon A, Nutalai R, Dijokaite-Guraliuc A, et al; OPTIC Consortium; ISA-RIC4C Consortium, Fry EE, Huo J, Mongkolsapaya J, Ren J, Stuart DI, Screaton GR. Antibody escape of SARS-CoV-2 Omicron BA.4 and BA.5 from vaccine and BA.1 serum. Cell 185 (2022): 2422-2433.
- Schultz DC, Johnson RM, Ayyanathan K, et al. Pyrimidine inhibitors synergize with nucleoside analogues to block SARS-CoV-2. Nature 604 (2022): 134-140.
- Fallet B, Kyburz D, Walker UA. Mild Course of COVID-19 and Spontaneous Virus Clearance in a Patient with Depleted Pe-ripheral Blood B Cells Due to Rituximab Treatment. Arthritis Rheumatol 72 (2020): 1581-1582.
- Eshun-Wilson I, Okwen MP, Richardson M, et al. Early versus delayed antiretroviral treatment in HIV-positive people with cryptococcal meningitis. Cochrane Database Syst Rev 7 (2018): CD009012.


 Impact Factor: * 5.3
Impact Factor: * 5.3 Acceptance Rate: 75.63%
Acceptance Rate: 75.63%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 
