Longitudinal Performance Validation of Siemens SARS-CoV-2 Spike Protein Serologic Assay
Stewart Comer MD MA FCAP*
Cottage Health - Pacific Diagnostics Laboratories, Santa Barbara, CA, USA
*Corresponding Author: Stewart Comer MD FCAP, Cottage Health - Pacific Diagnostics Laboratories, 400 W. Pueblo St. Santa Barbara, CA 93110, USA
Received: 20 October 2021; Accepted: 03 November 2021; Published: 08 November 2021
Article Information
Citation:
Stewart Comer. Longitudinal Performance Validation of Siemens SARS-CoV-2 Spike Protein Serologic Assay. Archives of Clinical and Medical Case Reports 5 (2021): 807-810.
View / Download Pdf Share at FacebookKeywords
<p>SARS-CoV-2; COVID-19; IgG Antibody assay; Vaccination</p>
Article Details
1. Introduction
The Siemens Centaur® SARS-CoV-2 IgG Antibody assay, which received Food and Drug Administration Emergency Use Authorization (FDA EUA), serologically detects the presence of circulating IgG antibodies to SARS-CoV-2 in human serum or plasma using the Siemens Centaur® Immunoassay Systems. This new semiquantitative test was validated in February 2021 at the Core facility of Pacific Diagnostics Laboratories (PDL), the largest clinical reference laboratory in coastal California between Los Angeles and San Francisco. It is recognized that this commercially available test correlates well with circulating neutralizing antibody (nAb) titers [1-3] and is capable of assessing specific antibody production without any current claim by the FDA regarding immunity to COVID-19 infection. This was reaffirmed when the FDA issued their safety communication on 19 May 2021; however, many recent studies [4, 5] reported during the summer of 2021 have clearly demonstrated waning levels of circulating antibodies to SARS-CoV-2 over the course of several months post vaccination. This is the rationale for the FDA [6] authorizing a booster dose for select populations at least 6 months after completion of the primary series of Pfizer BioNTech COVID-19 vaccine. At the present time, neutralizing antibodies are the primary and determinative correlate “to protective immunity to SARS-CoV-2 induced either through natural infection or through vaccination” [3]. Commercially available enzyme-linked immunoassays (EIA) similar to this Siemens assay do not directly measure nAb; however, recently published studies [3] have demonstrated that this Siemens EIA demonstrated the highest correlation for commercial assays with the gold-standard Plaque Reduction Neutralization Test (PRNT) and “best agreement” with the newly FDA EUA approved cPass Neutralization Antibody Assay.
2. Methods
This validation study was conducted utilizing a traditional laboratory correlation protocol (as prescribed under CFR Title 42 requirements) that is designed to assess the performance metrics of any new clinical assay in comparison to the FDA Instructions for Use (IFU). In this context, 57 of 57 uninfected laboratory staff, who were tested in March 2020, prior to vaccination, were resulted negative with an index value < 1.00. In February 2021, we validated the new semiquantitative assay and 28 of the original members with no prior COVID-19 infection and who were at least 14 days post 2nd dose of the Pfizer BioNTech (BNT162b2) COVID-19 vaccine, were retested.
3. Results
Of this longitudinal cohort (spanning a time interval of 6-8 months), 25 of the 28 who were tested 2-4 weeks after second vaccine dose, demonstrated maximal response with an index value > 150 (limit of the analytic measurement range instituted by the manufacturer at that time) and then retested at 6 months. 3 of the 28, who had values less than this maximal value, were vaccinated early and thus were actually tested 6-7 weeks after their second dose and thus their retest time interval essentially represents an expanded (7- 8 month) time span. This same group of three were clustered into the lowest tier on the August 2021 retest. These observations clearly appear to demonstrate that circulating antibodies start to decline sometime after the 2-4-week post vaccination period and continue to show larger quantitative decrements as the months progress. Individuals tested during the 7-8-month time frame had noticeably lower index values than index values for those tested at the 6-month interval. Of note, the largest single 6-month decrement was in an individual older than 60 that is similar to the observations published in the large Israeli study [7].
Although this sample size is small, the 6-month average index value decrement was large enough to be considered highly statistically significant (p < 0.001) as illustrated in Figure 1. None of the 28 individuals dipped below the index value of 1.00, which is qualitatively resulted as negative. Specifically, this means that the level of circulating Spike (S1 RBD) antibody cannot be analytically distinguished from the cohort of 57 uninfected individuals tested as part of the original PDL validation studies, performed prior to the release of vaccine, who demonstrated index values < 1.00 consistent with the performance characteristics as submitted to the FDA. The Siemens SARS-CoV-2 IgG Spike (S1 RBD) Antibody performed on the Siemens Centaur® must generate an index value ≥ 4.8 in order to meet the FDA requirement to be categorized as a high-titer COVID-19 Convalescent Plasma donor [2]. Although none of 28 individuals dropped below an index value of 1.00, 6 of the 28 (21%) after 6 months and 3 of 3 (100%) after 7 months demonstrated index values < 4.8, which is a numerical cutoff recognized as an immunity transference milestone.
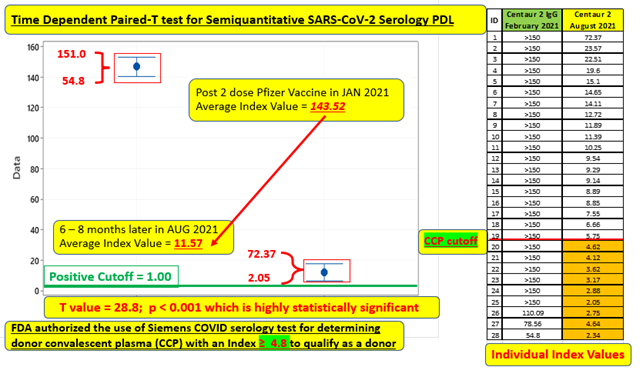
Figure 1: Time Dependent Paired-T test of average index value for 28-member cohort 6-8 months post vaccination.
4. Conclusion
The intent of this longitudinal validation study was to corroborate the temporal pattern in waning circulating antibodies as recently reported in multiple studies [3, 4, 6, 7] using a commercially available assay, which has been recognized [3] as having demonstrated excellent agreement with FDA-EUA approved SARS-CoV-2 nAb and PRNT assays. Although limited in sample size, the decrement is highly statistically significant and the time interval of our longitudinal validation was 180-225 days, which exceeds those time intervals of 70, 146 and 150 days previously reported [4, 7, 8]. Our time interval and eventual results tends to parallel the decay interval as recently reported in an expansive and comprehensive predictive study [5] that specifically evaluated multiple vaccine and convalescent studies and mathematically modeled “the decay of neutralization titers over the first 250 days after immunization.”
Acknowledgements
The PDL Clinical Laboratory Scientists, particularly Paola Rubio MBA, MLS (ASCP)CM, DLM CM, MB CM who provided invaluable support for this longitudinal laboratory validation study.
Conflict of Interest Disclosures
None
Funding/Sponsor
There is no specific funding beyond the normal and customary costs associated with performing required laboratory validation studies, which is supported by the performing laboratory (Cottage Health - Pacific Diagnostics Laboratories).
References
- US Food and Drug Administration (FDA). Coronavirus Disease (COVID-19) Emergency Use Authorizations for Medical Devices - In Vitro Diagnostics EUAs for SARS-CoV-2, on US FDA (2020).
- Zhen W, Smith E, Manji R, et al. Clinical Evaluation of Three Sample-To-Answer Platforms for the Detection of SARS-CoV-2. J Clin Microbiol (2020).
- Stein R. Study Raises Questions About False Negatives from Quick COVID-19 Test, p In National Public Radio (NPR). (2020).
- Hanson KE, et al. Infectious Diseases Society of America Guidelines on the Diagnosis of COVID-19 (IDSA) (2020).
- Kim C, Ahmed JA, Eidex RB, et al. Comparison of Nasopharyngeal and Oropharyngeal Swabs for the Diagnosis of Eight Respiratory Viruses by Real-Time Reverse Transcription-PCR Assays. PLoS ONE 6 (2011): e21610.
- Interim study by Tu YP and O’Leary TJ. Sample Collection and Molecular Diagnosis of SARS-CoV-2 Infection presented at the Association of Molecular Pathology webinar (2020).
- Basu A, et al. Performance of the rapid Nucleic Acid Amplification by Abbott ID NOW COVID-19 in nasopharyngeal swabs transported in viral media and dry nasal swabs, in a New York City academic institution. bioRxiv/Cold Spring Harbor Laboratory (2020).
- Puranik A, et al. Comparison of two highly-effective mRNA vaccines for COVID-19 during periods of Alpha and Delta prevalence. medRxiv (2021).


 Impact Factor: * 5.3
Impact Factor: * 5.3 Acceptance Rate: 75.63%
Acceptance Rate: 75.63%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 
