A Case Report of Inherited DYRK1A Neurodevelopmental Syndrome
Iman S Abumansour1,2, Mohammed H Almatrafi1, Mahmoud N Almutadares3, Asim A Khogeer4,5*
1Department of Medical Genetics, Faculty of Medicine, Umm Al-Qura University, Makkah, Saudi Arabia
2Neurogenetic section, Department of Pediatrics, King Faisal Specialist Hospital and Research Center, Jeddah, Saudi Arabia
3Department of Genetic Medicine, Faculty of Medicine, King Abdulaziz University, Jeddah, Saudi Arabia
4Plan and Research Department, General Directorate of Health Affairs of Makkah Region, Ministry of Health, Makkah, Saudi Arabia
5Medical Genetics Unit, Maternity & Children Hospital, Makkah Healthcare Cluster, Ministry of Health, Makkah, Saudi Arabia
*Corresponding Author: Asim A Khogeer, Medical Genetics Unit, Maternity & Children Hospital, Makkah Healthcare Cluster, Ministry of Health, Makkah 24382, Saudi Arabia.
Received: 12 December 2022; Accepted: 23 December 2022; Published: 31 January 2023
Article Information
Citation: Iman S Abumansour, Mohammed H Almatrafi, Mahmoud N Almutadares, Asim A Khogeer. A Case Report of Inherited DYRK1A Neurodevelopmental Syndrome. Archives of Clinical and Medical Case Reports 7 (2023): 39-41
View / Download Pdf Share at FacebookAbstract
DYRK1A disorder is among the most frequent monogenic forms of intellectual disability (ID). The majority of cases have been reported to be due to de novo pathogenic variants in DYRK1A gene. This report describes the second case of inherited DYRK1A syndrome that had created issues around variant classification due to its inheritance from an apparently healthy mosaic mother.
Keywords
<p><em>DYRK1A</em>; Intellectual Disability; Mosaicism; Neurodevelop mental Syndrome</p>
Article Details
Abbreviation:
Brain MRI- Brain Magnetic Resonance Imaging; DYRK1A- Dual-specificity-tyrosine-phosphorylation-regulated-kinase-1A; NGS- Next Generation Sequencing; NMD- Nonsense-Mediated Decay
1. Background
DYRK1A syndrome is a rare neurodevelopmental disorder that was first described in 2008, and characterized by peculiar facial gestalt and neurological manifestations including microcephaly, global developmental delay, mild to severe intellectual disability, speech impairment, behavioral issues such as autism spectrum disorder, and seizures. Other frequent clinical features include short stature, musculoskeletal defects, ocular, cardiac, and urogenital anomalies [1]. As far as we know, all previously reported cases of DYRK1A syndrome were mostly due to de novo pathogenic variant in DYRK1A gene except for one reported case of transmission through a parent [2,3]. Here, we report another case of a child with inherited DYRK1A syndrome due to maternal mosaicism.
2. Case Report
A six-year-old girl was born to consanguineous parents after an uneventful pregnancy and had a normal birth history. She presented with global developmental delay and autistic behavior. She was non-verbal and had no vocabulary. However, she understood simple commands. She had a history of simple febrile seizures but no seizure disorder that required anti-seizure medications. On physical examination, she looked alert, active and followed one-step commands. Her head circumference was 45 cm (below 3rd%ile; -4.7 SD). She had distinct dysmorphic features in form of a low anterior hairline, deep-set eyes, thick eyebrows, slightly up-slanted palpebral fissures, smooth short philtrum, prominent nasal root, a thick helix of ears and microretrognathia (Figure 1). She had a wide-based unsteady gait. She had normal muscle tone, power, and deep tendon reflexes in all four limbs. She had an abnormal finger to nose test partly due to difficulty in understanding commands with no tremor. Chest, cardiac and abdominal exams were within normal. Brain MRI was not performed in her case. Mother of the child was a 23-year-old woman, who was healthy and had normal intellect. She completed her bachelor’s degree and had an excellent academic performance. She denied any history of learning difficulties or medical issues. Her physical examination revealed a head circumference of 52.5 cm (at 4th%ile), and minor dysmorphic features in form of prominent nasal root and micrognathia but otherwise rest of the exams were unremarkable. Whole exome sequencing of the child was performed in a clinical molecular diagnostic laboratory. It showed a heterozygous novel likely pathogenic variant (NM_001396.4) (c.1560del; p.T521Qfs*71) in DYRK1A gene. Parental studies, which sanger sequencing tests were performed in a different clinical molecular diagnostic laboratory, identified the proband’s mother as heterozygous for the same variant (c.1560del; p.T521Qfs*71); and reclassified by the laboratory as a variant of unknown significance. The variant was not detected in the father’s sample. Given the lack of neurological manifestations related to DYRK1A syndrome in the mother, Next Generation Sequencing (NGS) based analysis of DYRK1A gene was performed again in the mother using blood and buccal swab samples. This variant was detected at an allele frequency of 14% (total number of reads was 117) in the blood sample (Figure 2). NGS analysis on buccal swab detected allele frequency of 16.1% (total number of reads 87) confirming somatic mosaicism in mother. This null variant (c.1560del; p.T521Qfs*71) in DYRK1A gene is predicted to cause nonsense-mediated decay (NMD) as it is not located in the last exon. Confirmation of NMD by mRNA study would further validate the pathogenicity of this variant however we were unable to pursue such study due to a lack of research laboratory resources.
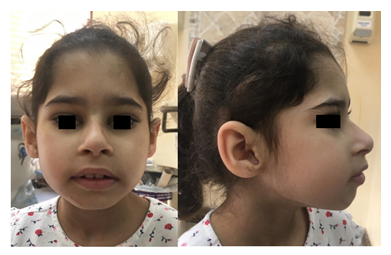
Figure 1: Front picture (Left) of child shows low anterior hairline, slightly up-slanted palpebral fissures, and prominent nose. Profile picture (Right) shows deep set eyes, prominent nasal bridge, microretrognathia and large ear with thick helix. Permission for publication of facial photos has been obtained from parents with the covered eyes.
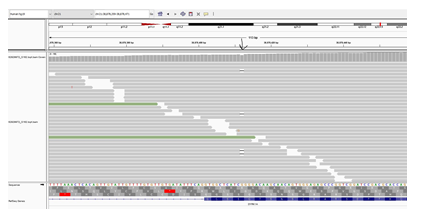
Figure 2: Next generation sequencing reads of the mosaic variant c.1560del (Chr21:38,878,359-38,878,471) in DYRK1A gene is displayed. This analysis was performed on mother’s blood sample. The location of variant is indicated by the black arrow. The deleted nucleotide appears as (-) within the parallel vertical lines. Allele frequency was 14% at ≥20x targeted nucleotide coverage.
3. Discussion
The DYRK1A (dual-specificity-tyrosine-phosphorylation-regulated-kinase-1A) gene is located within the critical region of Down syndrome on chromosome 21 [1]. It encodes a protein that has a kinase domain, leucine zipper motif, nuclear targeting signal sequence, and a conserved 13-consecutive-histidine repeat. This enzyme plays a role in phosphorylation of serine and threonine residues in targeted proteins in addition to autophosphorylation of tyrosine residues in its own activation loop [4,5]. DYRK1A deficnet mice (Dyrk1a+/− mutants) display a smaller brain size (30%) compared to wild-type mice, and they show growth retardation, behavioral defects, and altered motor activity due to dopaminergic dysfunction (Fotaki, et al., 2002) (Fotaki V. M., 2004) (Martinez de Lagran, 2007). In humans, DYRK1A has been proposed to be linked mental retardation, given its localization to the minimal overlapping region observed in affected individuals with partial monosomy (Matsumoto, 1997). Moreover, A de novo truncation of DYRK1A gene due to balanced translocations was detected in two unrelated patients with prenatal onset intrauterine growth retardation, microcephaly, developmental delay, and seizure disorder [6]. The phenotypic spectrum of DYRK1A syndrome has become more delineated after subsequent case report descriptions of similar manifestations due to different pathogenic variants in DYRK1A gene. Common clinical features observed in these cases include peculiar dysmorphic facies, speech impairment, intellectual disability with a variable frequency of microcephaly, and epilepsy [1,5,7-10]. Based on a previous review study, eighty-four people with DYRK1A variants were reported; 60.7% had loss of function variants, 17.9% had missense variants, 11.9% had splice-site variants, and 8.3% had larger deletions. The mode of inheritance in these patients was de novo in 84.5% ; and unknown in 15.5%.[2]. Also, there was only one reported case of inherited DYRK1A from a mosaic father whose clinical features were not reported [3]. Reduced penetrance is not thought to be a feature of this gene given the lack of reported asymptomatic individuals harboring germline pathogenic variants in this gene. Our case was the second to be described as inherited DYRK1A syndrome. The phenotype of the proband was strongly correlated with DYRK1A syndrome; and WES revealed a heterozygous null frameshift variant (c.1560del; p.T521Qfs*71) in DYRK1A gene that has not been reported previously. The variant was found to be inherited from the mother, who was healthy from a neurological perspective with normal cognitive functions, and this led to the reclassification of the variant to a variant of unknown significance by the molecular laboratory. Given the proband’s phenotype and what is known about DYRKA1 syndrome, we pursued further testing for possible mosaicism in the mother using blood and buccal swab samples. After all, the NGS-based analysis of DYRKA1 gene in the mother plus the maternal transmission of the variant to proband suggested the presence of gonadosomatic mosaicism of this variant in the mother. The normal intellect of the mother is then attributed to the variable level of mosaicism in different organ tissues and probably the absence of this variant in her central nervous system. That being said, there were limitations in the mosaicism analysis. Depth of coverage encompassing the DYRK1A gene in the mother’s NGS analysis was limited in blood and buccal swab samples (the number of reads were 117x and 87x respectively) which might not reflect the exact rate of mosaicism. Regardless, the reported low level of mosaicism in the mother is highly correlated with the lack of neurological manifestations in her. In conclusion, DYRK1A syndrome could be inherited from a mosaic parent. Thus, if a likely pathogenic variant is detected in a parent who lacks the clinical features of this syndrome, determining the level of mosaicism could be helpful before downgrading the class of variant.
Acknowledgments
The authors thank the family for their valuable permission to publish the case. Parents agreed on publishing the facial pictures of the child with blurring eyes. Also, the authors would like to thank the Deanship of Scientific Research at Umm Al-Qura University (UQU) for supporting this publication through Grant code:19-Med-1-03-0013.
Ethical Approval
This manuscript is a product of an approved project (IRB 2021-55) by King Faisal Specialist Hospital and Research Center (KFSH&RC), Jeddah, Saudi Arabia.
Funding
This work was funded by the Deanship of Scientific Research at Umm Al-Qura University, Makkah, Saudi Arabia under Grant code:19-Med-1-03-0013.
Conflict of Interest
The authors declare no conflict of interest.
Author Contribution
- initiated this research project and supervised progress. AK. edited and reviewed the manuscript. Mo.A. did the literature review and wrote the manuscript. Ma.A. contributed to manuscript editing and critically reviewed clinical data of case.
References
- Van Bon BW, Coe BP, de Vries BBA, et al. DYRK1A Syndrome. GeneReviews® (2021).
- Hanly C, Shah H, Billie Au PY, et al. Description of neurodevelopmental phenotypes associated with 10 genetic neurodevelopmental disorders: A scoping review. Clinical genetics 99 (2021): 335-346.
- Courraud J, Chater-Diehl E, Durand B, et al. Integrative approach to interpret DYRK1A variants, leading to a frequent neurodevelopmental disorder. Genetics in medicine: official journal of the American College of Medical Genetics 23 (2021): 2150-2159.
- Méjécase C, Way CM, Owen N, et al. Ocular Phenotype Associated with DYRK1A Variants. Genes 12 (2021): 1-14.
- Ji J, Lee H, Argiropoulos B, et al. DYRK1A haploinsufficiency causes a new recognizable syndrome with microcephaly, intellectual disability, speech impairment, and distinct facies. European journal of human genetics: EJHG 23 (2015): 1473-1481.
- Møller RS, Kübart S, Hoeltzenbein M, et al. Truncation of the Down syndrome candidate gene DYRK1A in two unrelated patients with microcephaly. American journal of human genetics 82 (2008): 1165-1170.
- Bronicki LM, Redin C, Drunat S, et al. Ten new cases further delineate the syndromic intellectual disability phenotype caused by mutations in DYRK1A. European journal of human genetics: EJHG 23 (2015): 1482-1487.
- Ruaud L, Mignot C, Guët A, et al. DYRK1A mutations in two unrelated patients. European journal of medical genetics 58 (2015): 174.
- LE M, Macnamara EF, D'Souza P, et al. DYRK1A pathogenic variants in two patients with syndromic intellectual disability and a review of the literature. Molecular genetics & genomic medicine 8 (2020).
- Van Bon BWM, Coe BP, Bernier R, et al. Disruptive de novo mutations of DYRK1A lead to a syndromic form of autism and ID. Molecular psychiatry 21 (2016): 126-132.


 Impact Factor: * 5.3
Impact Factor: * 5.3 Acceptance Rate: 75.63%
Acceptance Rate: 75.63%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 
