Manifestation of High Endogenous Heparinization in Postpartum Hemorrhage Patient using Thromboelastography: New Avenue of Coagulopathy Monitoring
Tian Yan1,3#, He Fei2#, Ji Changfu1,3, Lai Dong1,3*, Ching-Feng Weng3,4*
1Department of Transfusion, the Second Affiliated Hospital of Xiamen Medical College; Xiamen361021, Fujian, China
2Intensive Care Unit, the Second Affiliated Hospital of Xiamen Medical College; Xiamen361021, Fujian, China
3Medical Research Center, Xiamen Medical College, Xiamen361023, Fujian, China
4Department of Physiology, School of Basic Medical Science, Xiamen Medical College, Xiamen 361023, Fujian, China
*Corresponding Author: Dong Lai and Ching-Feng Weng, Department of Physiology, School of Basic Medical Science, Xiamen Medical College, Xiamen361023, Fujian, China.
Received: 17 January 2023; Accepted: 31 January 2023; Published: 16 February 2023
Article Information
Citation: Tian Yan, He Fei, Ji Changfu, Lai Dong, Ching-Feng Weng. Manifestation of High Endogenous Heparinization in Postpartum Hemorrhage Patient using Thromboelastography: New Avenue of Coagulopathy Monitoring. Archives of Clinical and Medical Case Reports 7 (2023): 82-88.
View / Download Pdf Share at FacebookAbstract
Background: Postpartum hemorrhage (PPH) is a leading cause of severe maternal morbidity and mortality worldwide which leads to massive blood loss. Coagulation abnormalities in response to severe trauma or infection are a latent cause that might aggravate PPH.
Case Presentation: A 39-week menolipsis of a 26-year-old puerpera appeared lacking amniotic fluid and uterine infection after examination. During the cesarean section, the patient manifested fever, massive hemorrhage, and shock. The low coagulation of the PPH patient was diagnosed by thromboelastography (TEG) guided with heparinase (type I). According to the sequential monitoring via the TEG guided assay, the coagulopathy and hyper-heparinization were obviously shown. Concurrent protamine correction for the patient’s coagulation abnormality gradually resulted in a stable condition after 4 hours of emergent treatment. This setting revealed that TEG-guided determination of endogenous heparin and subsequent infusion of protamine effectively reverted the syndrome of PPH.
Conclusions: This is an investigation of the PPH syndrome with infection patient suggests that hyper-endogenous heparinization should be clinically taken into consideration for low coagulation.
Keywords
<p>Coagulopathy; Hyper-Heparinization; Postpartum Hemorrhage; Protamine; Thromboelastography</p>
Article Details
Highlights
The appliance of TEG and hmTEG on the coagulation assessment can specifically detect the heparinization of patients to assist resuscitation and transfusion. High endogenous heparinization should be accurately identified and appropriate intervention given the PPH patients. Timely use of protamine significantly reverses the occurrence and progress of coagulation disorder in the momentous treatment for the PPH patients. Infection, ischemia-reperfusion and low platelets level might be considered into the risk of high endogenous heparinization.
Abbreviations: PPH- Postpartum Hemorrhage; TEG- Thromboelasmogram; HS- Heparin Sulfate; TM- Thrombomodulin; TPAI-C- Plasminogen Activator Inhibitor-1; GAG- Glycosaminoglycan; TNF-α- Tumor Necrosis Factor-α; IL- Interleukin; APTT- Activated Partial Thromboplastin Time; TT- Thrombin Time; hmTEG- Heparinase-modified TEG; DIC- Disseminated Intravascular Coagulation.
1. Background
Postpartum hemorrhage (PPH) is a symptom attributed to loss more than 500 mL within 24 h after delivery; furthermore, if lost more than 1000 mL within 24 h or sustained concomitantly losing over 2500 mL are called massive blood loss [1]. According to occurrence statistical analysis, about 11.5% of pregnancy-related deaths in the United States are caused by PPH from 2011 to 2013 [2]. In China, accumulative evidence demonstrated that the incidence of PPH has increased since 2016 [1]. Notably, coagulation dysfunction is one of the imperative factors of PPH. During pregnancy, the increase of pro-coagulant factors and the decrease of anti-coagulant factors will lead to the pre-thrombotic state. Once the patient appeared placental abruption, eclampsia, intrauterine infection etc., coagulation-promoting substances (thrombin) are released into the maternal blood, then the fibrinolytic pathway is triggered. Due to clotting-fibrinolytic imbalance, the fibrinolytic system is over-activated subsequently causing in coagulation dysfunction and massive blood loss of life-threating syndrome [3]. Importantly, real-time monitoring of coagulation is requisite for PPH patients to keep successful delivery. Obviously, early diagnosis and intervention for PPH bleeding could save thousands of women's lives each year. Thromboelasmogram (TEG) can manifest the interactions among platelets, coagulation cascades, and fibrinogen [4]. However, the conventional (regular) coagulation test does not reveal some interference factors, such as the influence of stable fibrin clot formation. In the human body, the involvements of coagulation and anti-coagulation form a dynamic counterbalance. When PPH occurs, insufficient thrombin production and fibrinolytic hyperactivity appear consecutively, thereafter fibrinogen supplementation and anti-fibrinolytic can effectively prevent and treat PPH. In our case, the coagulation condition of the patient did not significantly revert after supplying fibrinogen anti-fibrinolytic therapy. In addition, thrombin production time (R time) was significantly prolonged. The pathological factors are the venture of the prolonged R value at TEG analysis containing lack of coagulation factors, decreased number and function of platelet fibrinogen, acidosis, hypothermia, hypocalcemia, liver and kidney function insufficiency, and anti-coagulant use [5, 6]. In fact, PPH was caused by lack of thrombin synthesis or fibrinogen and platelet loss in this case, the results illustrated that the R time of heparinase-modified TEG (hmTEG) returned to normal after protamine neutralizing. It is worth noting that the patient might produce endogenous heparin without any heparin appliance, and subsequently could lead to inhibiting of thrombin production.
2. Case Manifestation
The puerpera fully understood and signed a written informed consent to publish the case report. A 26-year-old puerpera with 39 weeks of menolipsis was hospitalized on November 14, 2018, and lack amniotic fluid of unknown reasons. The examinations of color Doppler ultrasound indicated that all tests were normal except the presence of only 64 mm amniotic fluid index, which might have been ceased by the malfunction or dysfunction of the placenta.
3. Labor and Delivery Presentation
On November 15, an intravenous drip of oxytocin was administered to the puerpera for odinapoeia until 5:15 pm. At 8:50 pm, she had a fever up to 39°C associated with chills and shivering. Blood tests showed the increases in the leukocyte count, neutrophil ratio, and C-reactive protein level. The fetal membrane was ruptured, the amniotic fluid was degree 1 turbid, fetal tachycardia occurred and irregular contractions of uterine were observed, implying intrauterine infection. At 9:50 pm, a cesarean section was performed to terminate the pregnancy, and the amniotic fluid of the puerpera was determined to be 3 degrees turbid. The puerpera gave birth to a baby boy at 10:11 pm.
4. Postpartum
The patient began severe vomiting at 10:55 pm, November 15, 2018. Her blood pressure dropped down to 82/45 mmHg, her heart rate raised up 131/min, and many places of capillary hemorrhage were observed in the omentum majus. Her nasal cavity spontaneously began to bleed, lasting for 7 minutes. And unexpectedly the patient’s blood did not manifest coagulation, resulting in the suspicion of disseminated intravascular coagulation (DIC). During hemorrhage of the greater omentum suture, the patient had obvious infiltration of the abdominal wall muscles and subcutaneous fat, and her DIC score reached 7 points. Based on the dominant DIC International Standard of Thrombus and Hemostasis Committee (ISTH Standard) assessment, revealing that she was in a state of severe hypo-coagulation or coagulopathy.
5. TEG Diagnosis and Intervention
A critical condition of PPH patient with DIC needed to be urgently treated. Therefore, TEG was critically employed to measure the coagulation state after sampling. Surprisingly, the TEG assay showed that the patient’s R value was extended and the line was sustained over 30 min. At 11:18 pm, based on the laboratory results, the patient was given 10 U of cryoprecipitate and 4 U of RBC, and adjunctive use of anti-fibrinolytic and anti-infective drugs, unfortunately, the patient's condition did not see improvement and continued to bleeding (Table 1).
Combined with the prolong and expansion of activated partial thromboplastin time (APTT) extension, thrombin time (TT), and R value, the hmTEG was also performed to measure the patient’s sample. The results showed that the coagulation state had significantly improved after the neutralization of heparinase, and the hmTEG results (R 8.5 min, angle 22.9º, MA 34.7 mm) were verified. These results indicated that the patient had severe heparinization (Figure 1), could be due to the self-production of heparin substance which was not derived from any heparin-related medication. Definitely, no heparin administration upon closure was performed and this state was reconfirmed by the surgical team. Immediately, the patient's coagulation function was significantly improved (R 12.5 min, angle 20.3°, MA 30.1 mm) after 40 mg of protamine intervention, indicating that the heparin was offset (Table 1 and Figure 1). However, the coagulation results still revealed a lack of fibrinogen and platelets. Platelets were not available, and 10 U of cryoprecipitate and 4 U of RBCs slightly promoted the coagulation. At 00:54 am on November 16, the patient’s condition became worsen again, as evidenced by 200 mL of blood exuding from the wound without clotting. Unexpectedly, the results showed that the R value was prolonged, the line was sustained to 30 min, and the antagonism of heparinase had improved (R 8 min, angle 23.6°, MA 34 mm). These results showed the abnormality of the coagulation again, and endogenous heparinization counteraction was deteriorated. After urgent injection with 40 mg protamine again, the patient’s coagulation function was significantly improved (R 8.6 min, angle 21.6°, MA 30 mm). After 15 U of cryoprecipitate, 2 doses of curative platelets and 4 g of fibrin preparation were transfused. The patient’s coagulation function gradually returned to normal at 02:43 am. The patient was sent to the ICU for further medicare and treatment at 03:50 am on November 16.
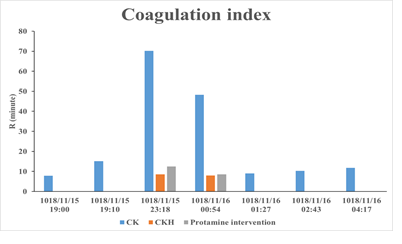
Figure 1: Coagulation profile in PPH with DIC patients using TEG measurement. Common test for thrombologram with kaolin as the activator (CK TEG). Heparinase modified TEG comparative detection (CKH TEG).
Table 1: History of medical examinations from the puerpera during the critical period.
Normal reference values of the coagulation index: APTT: 26.0-37.0S, TT: 12.0-17.0S, PT: 9.0-13.0S, FIB: 2.0-4.0 g/L, FDP: 0-5 μg/mL. Normal thromboelastic reference values are plotted as R: 5-10 min, MA: 50-70 mm, Angle: 53°-72°. CRP 0-10 mg/L; WBC (4-10) ×109/L; HGB 120-160 g/L; PCT 0-0.1 μg/L; AT 81.5-121.3%; TM 3.8-13.3 TU/mL; tPAI-C<10.5 ng/mL.
6. Laboratory Tests
In the meantime, we also examined two indicators: thrombo-regulatory protein (TM 3.8-13.3 TU/mL) and tissue plasminogen activator inhibitor 1 complex (tPAI-C<10.5 ng/mL) of vascular endothelial cell injury. At 09:10 pm on November 15, 2018, TM was 11.8 TU/mL and tPAI-C was 14.7 ng/mL, respectively; and subsequently TM was 14.9 TU/mL and tPAI-C was 27.5 ng/mL at 11:18 pm. TM was 23.6 TU/mL and tPAI-C was 32.3 ng/mL at 00:54 am, November 16, 2018. AT (normal range 81.5–121.3%) level was 22.56% at 11:18 pm, November 15, 2018. At 01:27 am, November 16, AT level was 39.21%, but the level was elevated to 58.23% at 04:41 am, November 16 (Table 1). During the treatments, the patient presented the symptoms of double lung respiratory sounds and wet rales. Bedside chest X-ray revealed double lung patch shadows and pleural effusion, and the hemogram of the patient exhibited persistent symptoms of infection. Blood was cultured on November 15, and the results depicted that the patient had an Escherichia infection. After anti-infection treatment, the patient became gradually stable and was discharged from the hospital on November 28, 2018.
7. Discussion
After supplying with fibrinogen, plasma and anti-fibrinolytic drugs, the coagulation disorder of the patient was not relapsed and thrombin production was still inadequate. Additionally, the bleeding was caused by the release of endogenous heparin rather than exogenous heparin use (the surgical team reconfirmed that heparin was not used for sealing the catheter). After protamine intervention, the coagulation disorder was instantly improved and the emergency rescue time was reduced to 5 hours. This case showed that the vital factor of blood loss is insufficient thrombin production caused by endogenous heparin production, rather than fibrinolytic hyperactivity or simple coagulation factor deficiency. As we known, heparin is a natural anti-coagulant substance in animals and human beings. Generally, there are two main sources of endogenous heparin from human tissues. One is the release of heparin by mast cells, lung, heart, liver, and muscle tissues [7]. The other is the endothelial glycocalyx layer, which is the key component of glycosaminoglycan (GAG) in vascular endothelial tissue. Heparin sulfate (HS) is the most abundant in GAG (about 50-90%). Under normal circumstances, heparin would not be released by human body, once trauma, infection, and poisoning occur, mast cells will release heparin as well as vascular endothelial injury will also cause glycocalyx shedding, resulting in endogenous heparinization [8]. Under physiological conditions, heparin can bind with anti-thrombin (also known as AT) to catalyze the inactivation of clotting factors (IIa, Xa, IXa, Xia, XIIa, etc.), that is the main mechanism of heparin anti-coagulation. The absence of heparin, clotting factors are very slowly inactivated by AT. Once heparin bound to AT, which a chronic thrombin inhibitor became a rapid inhibitor, and the inactivation rate of clotting factor can be increased 1000-2000 times. Notably, some of accident or diseases might be found the induced endogenous heparin production: (A) Traumatic coagulopathy: the mechanism of acute traumatic coagulopathy has been demonstrated such as activation of protein C, destruction of endothelial glycocalyx, consumption of fibrinogen, and platelet dysfunction [9, 10]. These multifactorial processes lead to the decreased blood clot intensity and hyper-fibrinolysis [11, 12], and then vascular endothelial injury is caused by ischemia-reperfusion deficiency results in auto-heparinization. (B) Sepsis: coagulation cascade in sepsis is mediated by tissue factor expression and cytokine release from the monocytes and macrophages. Cytokines such as TNF-α, IL-1, and IL-6 are up-regulated to inhibit the self-anticoagulation and fibrinolysis and subsequently caused endothelial damage. TNF-α and IL-1β can activate metalloproteinases and heparinase under inflammatory conditions, which directly promote the cleavage of endothelial glycocalyx and cause the release of endogenous heparin [13]. (C) Liver transplantation after acute liver failure: acute liver failure can cause severe systemic inflammation, and the endothelial glycocalyx degradation products increase in systemic circulation; the liver had rich blood transport and high content of GAG, which was aggravated by ischemia-reperfusion after transplantation. The severe reduction of liver function may greatly reduce the ability to eliminate GAG in the circulation, and the body is prone to endogenous heparinization [14]. (D) The shedding of endothelial glycocalyx layer in atherosclerosis can lead to endothelial cell exposure, promote monocyte adhesion and foam cell infiltration, increase oxidative stress response, and enhance plaque formation. When liver function is abnormal, the produced GAG cannot be degraded and heparinized substance residues can be detected. (E) Hyperglycemia leads to improved endothelial permeability, impaired nitric oxide synthase function, weakened vascular wall protection, and endothelial glycocalyx is easy to fall off. Hyperglycemia primes the formation of reactive oxygen species (ROS), which directly damages endothelial glycocalyx. (F) Under physiological conditions, the factors of platelets release heparinase are mainly expressed in platelets and placenta [7]. When damage occurs, the activated platelets release heparinase that causes the increases of heparin neutralization via heparinase. Subsequently, the incidence of platelet decrease is due to a large volume of blood loss; the neutralization of heparin was decreased with the decline level of heparinase. According to vascular endothelial injury, HS release cannot be fully degraded by heparinase, this may be one of the causes of high endogenous heparin. Because ischemia-reperfusion and infection (E.coli was found in blood cultures) can cause significant damage to vascular endothelial cells, TM, and TPAI-C levels (indicators of vascular endothelial damage) of patient are gradually elevated. The manifestations of decreased platelets associated with reduced release of heparinase in this patient might be the reason of endogenous heparin observed. Heparinase, an endo-β-D-glucuronidase abundant in platelets, is an enzyme that cleaves the side chains of HS in the cell surface and extracellular matrix [7]. In studies of HS oligosaccharides, heparinase has been shown to cleave them along the HS polysaccharide chain in a definite sulphating pattern. A study reported that heparinase could degrade HS on cell surface and extracellular matrix; additionally, it can degrade heparin and low molecular weight heparin. Compared the sensitivity of TEG with different heparin detection methods found that TEG detection was more sensitive than different heparin detection in most conventional coagulation tests except anti-FXa detection [15]. The calculated parameters for the difference between the standard (CK-TEG) and the heparinase-coated cup (CK-hmTEG) greatly increased the sensitivity of TEG assays due to the effects of these anticoagulants, even more than against FXa. Conventionally, clotting ends with the formation of a fibrin clot reflects the clotting level at a certain time point. TEG, a dynamic and comprehensive coagulation assessment, provides the sensitive approach to be qualitative and quantitative the differences in platelets, coagulation cascade proteins and fibrinolysis. Moreover, hmTEG can be used to inactivate heparin and other glycoaminoglycans (i.e. HS), the evaluated result can be better than using heparin in patients Accordingly, the sensitivity and rationality of hmTEG for the detection of endogenous heparin could also be shed light on this case. Based on the rescued experience of this case, we propose that infection and thrombocytopenia caused ischemia-reperfusion and blood loss would be the major for the increase of endogenous heparin-like substances in the patients. Currently, our clinical case reports found that the reduced coagulation latent caused by hyper-endogenous heparinization was concomitant with the binding of anti-thrombin and the triggering of fibrinolysis in massive wasp envenomation [16]. Mostly, endogenous heparinization seldom occurred in PPH patients for decades, nevertheless, PPH patients with endogenous heparinization has recently been reported [17]. Both of cases (including this study) have demonstrated the significance of hyper-heparinization appeared in PPH. Simple and accurate identification of endogenous heparinization can greatly shorten the rescue time of the emergency state after targeted therapy, suggesting that précising validation and reasonable intervention of endogenous heparinization are of great importance for the management of PPH coagulopathy.
8. Conclusions
The appliance of TEG and hmTEG on the coagulation assessment can specifically identify the heparinization of patients and assist resuscitation and transfusion. Timely use of protamine has a significant effect on reversing the occurrence and progress of coagulation disorder in the puerpera, which could be a momentous treatment for the PPH patients in the future. Infection, ischemia-reperfusion and low platelets level might be considered into the risk of high endogenous heparinization. The identification of endogenous heparinization in PPH of the puerpera is summarized in Figure 2. Therefore, precise identification and appropriate intervention of high endogenous heparinization should be taken into cautiousness in the management of PPH patients.
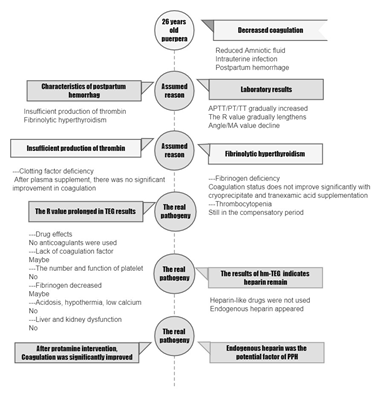
Figure 2: The identification of endogenous heparinization in postpartum hemorrhage of the puerpera.
Acknowledgements
The authors sincerely thank for his help in preparations of manuscript. Special thanks to John Koester for his English editing.
Authors’ Contributions
Tian Y and He F participated in research design, data analysis, and manuscript preparation. He F, Ji Cf, and Tian Y carried out laboratory tests and collected experimental data. Lai D and Weng CF designed the study and critically reviewed the manuscript. All authors reviewed and approved the final version of manuscript.
Funding
Youth research project of Fujian provincial commission of health and family planning:2018-2-70.
Availability of Data and Materials
Not applicable.
Declarations
Ethics Approval and Consent to Participate
This report received Institutional Review Board (IRB) review and approval. The IRB approved number is ChiCTR2100052226.
Consent for Publication
The patient signed a written consent allowing the case to be published.
Competing Interests
The authors declare that they have no competing interests.
References
- Munoz M, Stensballe J, Ducloy-Bouthors A, et al. Patient blood management in obstetrics: prevention and treatment of postpartum haemorrhage. A NATA consensus statement. Blood Transfus 17 (2019): 112-136.
- Burd JE, Quist-Nelson JA, Edwards SE, et al. Blood type and postpartum hemorrhage by mode of delivery: A retrospective cohort study. Eur J Obstet Gynecol Reprod Biol 256 (2021): 348-353.
- Gillissen A, van den Akker T, Caram-Deelder C, et al. Coagulation parameters during the course of severe postpartum hemorrhage: a nationwide retrospective cohort study. Blood Adv 2 (2018): 2433-2442.
- MacDonald SG, Luddington RJ. Critical factors contributing to the thromboelastography trace. Semin Thromb Hemost 36 (2010): 712-22.
- Schmidt AE, Israel AK, Refaai MA. The Utility of Thromboelastography to Guide Blood Product Transfusion. Am J Clin Pathol 152 (2019): 407-422.
- Mukhopadhyay T, Subramanian A, Pati HP, et al. Characterization of analytical errors in thromboelastography interpretation. Pract Lab Med 23 (2021): e00196.
- Nasser NJ, Fox J, Agbarya A. Potential Mechanisms of Cancer-Related Hypercoagulability. Cancers (Basel) 12 (2020): 566. doi: 10.3390/cancers12030566..
- Wang Y, Sha H, Zhou L, et al. The Mast Cell Is an Early Activator of Lipopolysaccharide-Induced Neuroinflammation and Blood-Brain Barrier Dysfunction in the Hippocampus. Mediators Inflamm 2020 (2020): 8098439.
- Dobson GP, Morris JL, Davenport LM, et al. Traumatic-Induced Coagulopathy as a Systems Failure: A New Window into Hemostasis. Semin Thromb Hemost 46 (2020): 199-214.
- Savioli G, et al. Trauma Coagulopathy and Its Outcomes. Medicina (Kaunas) 56 (20204): 205.
- Moore HB, Gando S, Iba T, et al. Defining trauma-induced coagulopathy with respect to future implications for patient management: Communication from the SSC of the ISTH. J Thromb Haemost 18 (2020): 740-747.
- Uchimido R, Schmidt EP, Shapiro NI. The glycocalyx: a novel diagnostic and therapeutic target in sepsis. Crit Care 23 (2019): 16.
- Senzolo M, Agarwal S, Zappoli P, et al. Heparin-like effect contributes to the coagulopathy in patients with acute liver failure undergoing liver transplantation. Liver Int 29 (2009): 754-759.
- Jedlicka J, Becker BF, Chappell D. Endothelial Glycocalyx. Crit Care Clin 36 (2020): 217-232.
- Coppell JA, Thalheimer U, Zambruni A, et al. The effects of unfractionated heparin, low molecular weight heparin and danaparoid on the thromboelastogram (TEG): an in-vitro comparison of standard and heparinase-modified TEGs with conventional coagulation assays. Blood Coagul Fibrinolysis 17 (2006): 97-104.
- Lai D, Tian Y, Zhang J, et al. Hyperendogenous Heparinization Suggests a Guideline for the Management of Massive Wasp Stings in Two Victims. Wilderness Environ Med 32 (2021): 344-350.
- Wang S, Qi C, Liu Z, et al. Endogenous Heparin-Like Substances May Cause Coagulopathy in a Patient with Severe Postpartum Hemorrhage. Transfus Med Hemother 47 (2020): 337-343.


 Impact Factor: * 5.3
Impact Factor: * 5.3 Acceptance Rate: 75.63%
Acceptance Rate: 75.63%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 
