The Impact of Chronic Electronic Cigarette Use on Alveolar Macrophage Lipid Content: Case Report
Molly Ruttenberg2, David Armstrong1, Diane Mellinger1, James Carroll1, and Alix Ashare1,2*
1Section of Pulmonary and Critical Care Medicine, Dartmouth Hitchcock Medical Center, Lebanon, NH, USA
2Department of Microbiology and Immunology, Geisel School of Medicine at Dartmouth, Hanover, NH, USA
*Corresponding Author: Alix Ashare, Department of Microbiology and Immunology, Geisel School of Medicine at Dartmouth, Hanover, NH, USA.
Received: 15 September 2022; Accepted: 27 September 2022; Published: 14 October 2022
Article Information
Citation: Molly Ruttenberg, David Armstrong, Diane Mellinger, James Carroll, and Alix Ashare. The Impact of Chronic Electronic Cigarette Use on Alveolar Macrophage Lipid Content: Case Report. Archives of Clinical and Medical Case Reports 6 (2022): 689-692.
View / Download Pdf Share at FacebookAbstract
Background: While acute respiratory distress following electronic cigarette (e-cig) use has been described, the effects of chronic e-cig use on lung health are currently unknown. Acute e-cigarette/vaping product useassociated lung injury (EVALI) has been highlighted recently in numerous cases across the United States. Numerous EVALI case reports highlight alterations in alveolar macrophages, justifying investigation of this key immune sentinel of the lung in habitual e-cig users.
Case Presentation: After informed consent, we performed a bronchoscopy on a 25 year asymptomatic woman who reported daily e-cig use. To evaluate for evidence of abnormal lipid homeostasis, we performed histologic and Oil Red O stain evaluation of alveolar macrophages obtained from bronchoalveolar lavage fluid. Our analyses demonstrate a prevalence of cells with high lipid accumulation in multiple, discrete cytoplasmic foci. We found a high lipid laden macrophage index within alveolar macrophages isolated from a chronic e-cig user. At the ultrastructural level, we found membrane-bound compartments filled with material of various densities segregated along curved phase separation lines reminiscent of suspensions of immiscible fluids.
Conclusions: We found a unique ultrastructural pattern in alveolar macrophages isolated from a chronic e-cig user that is unlike any other previously reported in aspiration syndromes and may represent a defining diagnostic feature of chronic e-cig use.
Keywords
<p>Alveolar Macrophages; Case Report; Cigarettes; Electronic; Lipid</p>
Article Details
List of Abbreviations:
E-cig- Electronic Cigarette; EVALI- e-Cigarette/Vaping Product Use-Associated Lung Injury; BAL- Bronchoalveolar Lavage; ORO- Oil Red O; TEM- Transmission Electron Microscopy; LLMI- Lipid Laden Macrophage Index; EIB- E-cig-Associated Inclusion Body
1. Background
Since entering the market in the United States in 2007, electronic cigarettes (e-cigs) have emerged as a popular recreational tool among adults and adolescents [1,2]. The starting liquid used in e-cig devices typically contains nicotine or THC as well as flavorings and sugar alcohols. In addition, polyethylene glycol, propylene glycol, glycerol, vitamin E acetate, and medium chain triglycerides are often added to increase the viscosity [3]. The rise of e-cigs has come at the helm of a shift from the popularity of typical tobacco products. Most e-cig companies market themselves as smoking cessation products. Since e-cigs are tobacco and smoke-free they are thought of as healthy, cheaper, sustainable alternative to smoking. While there is evidence to support the use of e-cigs as alternatives to smoking, their use is not without risks [4]. Recently, reports of an acute respiratory syndrome related to e-cig use have emerged. In 2019 and 2020, over 2000 cases of e-cigarette or vaping product use associated lung injury (EVALI) were reported in the U.S resulting in 57 confirmed deaths [5]. Despite the development of EVALI, the e-cig epidemic continues with 10 million adults and 5.2 million young people in the U.S. reporting current e-cig usage, including 27.5% of high school students and 10.5% of middle school students [2,6,7]. While there have been studies aimed at understanding EVALI, the continued chronic use of e-cigs in the U.S. indicates a need for studies investigating more long-term effects. EVALI case reports suggest alterations in the innate immune system including abnormalities in lung macrophages [5,8-10]. Alveolar macrophages play a major role in lipid homeostasis in the lung, which is essential for adequate gas exchange, maintenance of alveolar epithelial integrity, and maintenance of innate immune function. Pulmonary surfactant, a complex mixture of proteins and lipids, is a component of the airway surface liquid [11]. By reducing surface tension in the alveoli, surfactant maintains ventilation-perfusion matching. Impaired metabolism of surfactant by lung macrophages leads to accumulation of intracellular lipids as well as deposition of excess lipids in the alveolar space [12]. EVALI case reports show increased lipid content in innate immune cells of the lung suggesting a potential disruption of alveolar lipid homeostasis [13,14]. Here, we describe a novel macrophage phenotype observed in lung macrophages isolated from bronchoalveolar lavage (BAL) fluid from a chronic e-cig user.
2. Case Presentation
This study was approved by the Dartmouth-Hitchcock Institutional Review Board (#22781). The subject is a 25 year old chronic e-cig user who reports use of a 2 to 4 mL of e-cig liquid daily for a period of greater than two years. She is a former smoker with less than a 10 pack-year history of cigarette use and no cigarette use in over 3 years. She has used both nicotine-containing and THC-containing products. However, she denies use of any THC containing products during the 12 months prior to her bronchoscopy procedure. The nicotine-containing products she uses do not contain vitamin E acetate. She takes no chronic daily medications and denies any respiratory symptoms. She has no family history of lung disease. Her lung examination was normal. Following informed consent, she underwent flexible bronchoscopy in 2019 as part of a separate research study (Dartmouth Hitchcock IRB study #22781) as previously described [15]. Bronchoalveolar lavage (BAL) fluid was obtained from tertiary airways via instillation of 20mL of sterile saline followed by 10mL of air and repeated for a total of 5 times per airway. Alveolar macrophages were isolated as previously described [15,16]. Briefly, BAL fluid was mixed with magnetic beads bound to CD15, designed to bind neutrophils, and run over a magnetic column for neutrophil depletion [15]. The pellet was washed twice and resuspended in RPMI 1640. Cells were stained with Oil Red O (ORO) to assess lipid accumulation [17]. Cellular ultrastructure was examined by transmission electron microscopy (TEM). A 25 year old female subject who reported no cigarette or e-cig use throughout her lifetime was used as a control. There were no adverse events. To evaluate the lipid content of alveolar macrophages isolated from a chronic e-cig user, we performed histologic evaluation using oil red O staining (ORO). A heterogeneous distribution of ORO stain was seen across the alveolar macrophage population (Figure 1A). Some cells showed high accumulation of ORO staining droplets (arrow) while other cells showed little to no staining (arrowhead). Cells show ORO positive cytoplasmic droplets of varying size and varying subcellular localization (Figure 1B). To further quantify the lipid content within alveolar macrophages, we calculated a lipid-laden macrophage index (LLMI) from as previously described (17). Alveolar macrophages with no visible lipid cytoplasmic staining were scored “0”; macrophages with <50% of the cytoplasm opacified by lipid were scored “1+”; macrophages with >50% of the cytoplasm opacified by lipid were scored “2+”. The LLMI was calculated as follows: LLMI=((%1+ LMs) x 1) + ((% 2+ LMs) x 2). We found that alveolar macrophages isolated from a chronic e-cig user had a LLMI of 172.5, which is significantly increased compared to the LLMI of 2.3 in a healthy control. To gain further insight into the nature of these lipid accumulations, alveolar macrophage ultrastructure was examined by TEM (Figure 2). Alveolar macrophages from both a healthy non-smoking subject (Panel A) and a chronic e-cig user (Panels B-F) were approximately 25-30 mm in diameter. In the alveolar macrophages from an e-cig user, ultrastructural analysis demonstrates heterogenous lipid material accumulation in membrane bound intracellular compartments. We refer to this structure as an e-cig-associated inclusion body (EIB). The abundance of EIBs is heterogeneous across the alveolar macrophage population and range in size from 500 nm to nearly 10 mm in diameter. EIBs were not observed in alveolar macrophages of the non-e-cig users.
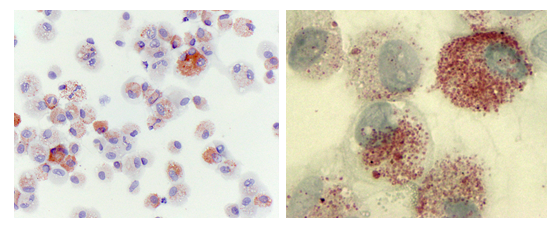
Figure 1: Lung Macrophages from e-Cig Users Stain Positive for Oil Red O.
Primary human alveolar macrophages were prepared via cytospin and stained for lipid content with Oil Red O (ORO). Heterogeneity of ORO staining across the cell population (Panel A) (Magnification 400 x). Higher magnification of alveolar macrophages (1000 x, Panel B) further illustrates both the ORO staining heterogeneity between cells and the size of the cytoplasmic organelles staining ORO positive.
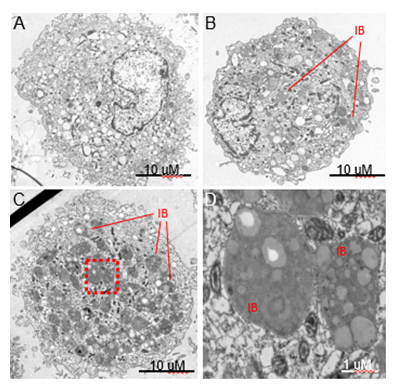
Figure 2: Human Alveolar Macrophages from Asymptomatic e-Cig Users Contain Novel Inclusion Bodies. Transmission electron micrographs of primary alvevolar macrophages collected by bronchoalveolar lavage from a healthy control subject (A) and a frequent e-cig user (B-D). Common features in alveolar macrophages from both subjects were numerous mitochondria, translucent vacuoles and cells approximately 25-30 mM in diameter.
- A) Representative image of an alveolar macrophage from a control subject.
- B) E-cig alveolar macrophages demonstrating a novel ultrastructural feature we refer to as an E-cigarette-associated inclusion body (EIB).
- C) E-cig user alveolar macrophages with a high abundance of inclusion bodies (red box outlines the region selected for higher magnification imaging in D).
- D) Higher magnification of the EIB ultrastructure reveals multiple regions of varying electron density within the organelle.
3. Discussion and Conclusions
The long-term effects of habitual e-cig use on pulmonary immune cells are unknown. Alveolar macrophages are among the first cells in the lung to respond to inhaled foreign substances, particles or pathogens. To explore possible phenotype alterations to alveolar macrophages in response to e-cig use, we examined the cytology and ultrastructure of alveolar macrophages from BAL fluid from an e-cig user compared to a control subject. We have identified a novel cytosolic accumulation of heterogeneous electron density which we refer to as an EIB, e-cig-associated inclusion body. Alveolar macrophages are the primary phagocytes of the innate immune system. Alveolar macrophages also play a major role in lipid homeostasis in the lung, which is essential for adequate gas exchange, maintenance of alveolar epithelial integrity, and maintenance of innate immune function. Abnormal lipid accumulation with macrophages has been shown to impair phagocytsosis and other functions [18]. A murine study of chronic e-cig exposure found aberrant lipid accumulation within alveolar macrophages leading to increased susceptibility to viral infection [19]. Taken together, these studies suggest that abnormal lipid homeostasis in alveolar macrophages in response to chronic e-cig use may have long term effects on the innate immune response in the lung. Historically, cytology-based studies utilizing Oil Red O staining have been used to demonstrate the presence of lipid-laden macrophages in the lung [5,8,17,20]. There are limited ultrastructural studies showing lipid-like cytosolic inclusions in alveolar macrophages from BAL fluid. M. tuberculosis infection studies [21,22], mineral oil-exposure related [23,24] or petroleum aspiration [25] reports come closest to describing similar cytosolic organelles; nonetheless these structures are clearly not the heterogeneous structures we have observed in e-cig users. To our knowledge, this is the first report describing alveolar macrophage ultrastructure associated with habitual e-cig product use. Most notably, we observed a novel pattern of cytosolic accumulation within membrane bound compartments with heterogenous electron density. This pattern does not resemble any pattern published in the prior literature, including EVALI and aspiration pneumonia. One hypothesis of the likely composition of EIBs is that they are the vaporized derivatives of lipids in the e-cig liquid. Future studies should investigate the content of lipids withing alveolar macrophages isolated from chronic e-cig users to better understand the lipid pathways that are disrupted and gain a greater understanding of the potential downstream clinical implications of continued e-cig use.
Declarations
Ethics Approval and Consent to Participate
The study was reviewed and approved by the Dartmouth-Hitchcock Institutional Review Board (#22781). Informed consent was obtained.
Competing Interests
The authors declare that they have no competing interests.
Funding
R01HL122372 (funding for bronchoscopies, cell isolation, and microscopy studies) and K24HL150453 (funding for salary for AA and DM) to AA.
Author Contributions
MR was a major contributor to the writing of this manuscript; DA conceived of the idea for this case report; DM isolated and processed cells for analysis; JC performed the bronchoscopies and contributed to the writing of this manuscript; AA conceived of the idea, designed the protocols, and was a major contributor to the writing of this manuscript. All authors have read and approved this manuscript.
Acknowledgments
We appreciate the help of Dr. Radu Stan in obtaining electron microscopy images.
References
- King BA, Jones CM, Baldwin GT, et al. The EVALI and Youth Vaping Epidemics - Implications for Public Health. The New England journal of medicine 382 (2020): 689-691.
- Hartnett KP, Kite-Powell A, Patel MT, et al. Syndromic Surveillance for E-Cigarette, or Vaping, Product Use-Associated Lung Injury. The New England journal of medicine 382 (2020): 766-772.
- Gutsche J, Pasternak R, Campbell D, et al. A 19-Year-Old Man With Vaping-Associated Lung Injury. Air Med J 39 (2020): 6-8.
- Farsalinos KE, Polosa R. Safety evaluation and risk assessment of electronic cigarettes as tobacco cigarette substitutes: a systematic review. Ther Adv Drug Saf 5 (2014): 67-86.
- Layden JE, Ghinai I, Pray I, et al. Pulmonary Illness Related to E-Cigarette Use in Illinois and Wisconsin - Final Report. The New England journal of medicine 382 (2020): 903-916.
- Gentzke AS, Creamer M, Cullen KA, et al. Vital Signs: Tobacco Product Use Among Middle and High School Students - United States, 2011-2018. MMWR Morb Mortal Wkly Rep 68 (2019): 157-164.
- Mirbolouk M, Charkhchi P, Kianoush S, et al. Prevalence and Distribution of E-Cigarette Use Among U.S. Adults: Behavioral Risk Factor Surveillance System, 2016. Ann Intern Med 169 (2018): 429-438.
- Guerrini V, Panettieri RA, Jr., Gennaro ML. Lipid-laden macrophages as biomarkers of vaping-associated lung injury. The lancet Respiratory medicine 8 (2020): e6.
- Butt YM, Smith ML, Tazelaar HD, et al. Pathology of Vaping-Associated Lung Injury. N Engl J Med 381 (2019): 1780-1781.
- Maddock SD, Cirulis MM, Callahan SJ, et al. Pulmonary Lipid-Laden Macrophages and Vaping. N Engl J Med 381 (2019): 1488-1489.
- Han S, Mallampalli RK. The Role of Surfactant in Lung Disease and Host Defense against Pulmonary Infections. Ann Am Thorac Soc 12 (2015): 765-774.
- Remmerie A, Scott CL. Macrophages and lipid metabolism. Cellular immunology 330 (2018): 27-42.
- Marsden L, Michalicek ZD, Christensen ED. More on the Pathology of Vaping-Associated Lung Injury. N Engl J Med 382 (2020): 387-388.
- Davidson KR, Fox DL. More on the Pathology of Vaping-Associated Lung Injury. N Engl J Med 382 (2020): 388.
- Bessich JL, Nymon AB, Moulton LA, et al. Low levels of insulin-like growth factor-1 contribute to alveolar macrophage dysfunction in cystic fibrosis. J Immunol 191 (2013): 378-385.
- Chen Y, Armstrong DA, Salas LA, et al. Genome-wide DNA methylation profiling shows a distinct epigenetic signature associated with lung macrophages in cystic fibrosis. Clin Epigenetics 10 (2018): 152.
- Colombo JL, Hallberg TK. Recurrent aspiration in children: lipid-laden alveolar macrophage quantitation. Pediatr Pulmonol 3 (1987): 86-89.
- Schrijvers DM, De Meyer GR, Kockx MM, et al. Phagocytosis of apoptotic cells by macrophages is impaired in atherosclerosis. Arteriosclerosis, thrombosis, and vascular biology 25 (2005): 1256-1261.
- Madison MC, Landers CT, Gu BH, et al. Electronic cigarettes disrupt lung lipid homeostasis and innate immunity independent of nicotine. The Journal of clinical investigation 129 (2019): 4290-4304.
- Ocampo-Gonzalez FA, Park JW. Cytologic features of vaping-induced lung injury: A case report. Diagn Cytopathol 48 (2020): 174-176.
- Armstrong JA, Hart PD. Response of cultured macrophages to Mycobacterium tuberculosis, with observations on fusion of lysosomes with phagosomes. J Exp Med 134 (1971): 713-740.
- Borelli V, Vita F, Soranzo MR, et al. Ultrastructure of the interaction between mycobacterium tuberculosis- H37Rv-containing phagosomes and the lysosomal compartment in human alveolar macrophages. Exp Mol Pathol 73 (2002): 128-134.
- Ohwada A, Yoshioka Y, Shimanuki Y, et al. Exogenous lipoid pneumonia following ingestion of liquid paraffin. Intern Med 41 (2002): 483-486.
- Osman GA, Ricci A, Terzo F, et al. Exogenous lipoid pneumonia induced by nasal decongestant. Clin Respir J 12 (2018): 524-531.
- Burkhardt O, Merker HJ, Shakibaei M, et al. Electron microscopic findings in BAL of a fire-eater after petroleum aspiration. Chest 124 (2003): 398-400.


 Impact Factor: * 5.3
Impact Factor: * 5.3 Acceptance Rate: 75.63%
Acceptance Rate: 75.63%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 
