First Radiobiological Characterization of Skin and Bone Cells from A Patient Suffering from the PI3KCA-Related Overgrowth Spectrum (PROS) Syndrome
Jean-Thomas Bachelet#, Adeline Granzotto#, Mélanie L. Ferlazzo, Laurène Sonzogni, Elise Berthel, Clément Devic and Nicolas Foray
#Authors contributed equally
Institut National des Sciences et de la Recherche Médicale, UA8 Unit, Radiations: Defense, Health and Environment, Centre Léon-Bérard, Lyon, France
*Corresponding Author: Dr. Nicolas Foray, Institut National des Sciences et de la Recherche Médicale, UA8 Unit, Radiations: Defense, Health and Environment, Centre Léon-Bérard, Lyon, France
Received: 11 August 2020; Accepted: 18 September 2020; Published: 06 November 2020
Article Information
Citation:
Jean-Thomas Bachelet, Adeline Granzotto, Mélanie Ferlazzo, Laurène Sonzogni, Elise Berthel, Clément Devic and Nicolas Foray. First Radiobiological Characterization of Skin and Bone Cells from A Patient Suffering from the PI3KCA-Related Overgrowth Spectrum (PROS) Syndrome. Archives of Clinical and Medical Case Reports 4 (2020): 1052-1066.
View / Download Pdf Share at FacebookAbstract
The phosphatidylinositol 3-kinase catalytic subunit (PI3KCA) is an oncogene involved in the control of cellular proliferation. Some somatic mosaic heterozygous mutations of PI3KCA are associated with overgrowth malformations in skin, vasculature, bones, fat or brain tissues, gathered under the common term of “PI3KCA-related overgrowth spectrum” (PROS) syndromes. Since PROS patients may be exposed to ionizing radiation through anti-tumor radiotherapy and radiodiagnosis, the evaluation of the radiation-induced risk potentially linked to PROS syndrome is needed. However, no radiobiological characterization of this syndrome was available yet. Primary fibroblast and osteoblast cell lines derived from a PROS patient were exposed to radiation in realistic conditions. The PROS patient cells appeared to be associated with a moderate but significant radiosensitivity, a delayed radiation-induced nucleoshuttling of the ATM kinase, and an impairment of DNA double-strand breaks repair and signaling. Such phenotype may be partially corrected by using bisphosphonates combined with statins, which renders cells more radioresistant. Our data suggest that the PI3KCA protein may contribute to the individual Archives of Clinical and Medical Case Reports 1053 radiation response, as an ATM substrate. Furthermore, our findings suggest that exposure to radiation of PROS patients should be therefore justified carefully.
Keywords
<p>PROS syndrome; Radiation; Radiosensitivity; ATM; DNA double-strand breaks; PI3KCA</p>
Article Details
1. Introduction
The phosphatidylinositol 3-kinase (PI3K) is composed of a 85 kDa regulatory subunit and a 110 kDa catalytic subunit. Among the numerous variants of these subunits, the p110α one, also called PI3KCA, is found mutated in a number of cancers, more particularly in breast tumors [1-3]. However, in addition to its function as oncogene, the protein PI3KCA was also implied in the control of cellular proliferation since some somatic mosaic mutations of PI3KCA are associated with overgrowth syndromes. This is notably the case of the heterogeneous segmental overgrowth phenotypes like fibroadipose hyperplasia or overgrowth, hemihyperplasia multiple lipomatosis, congenital lipomatous overgrowth, vascular malformations, epidermal nevi, scoliosis/skeletal and spinal (CLOVE) syndrome, macrodactyly, fibroadipose infiltrating lipomatosis, and the related megalencephaly syndromes, megalencephaly-capillary malformation and dysplastic megalencephaly [4-8]. All these syndromes are associated with overgrowth malformations in skin, vasculature, bones, fat or brain tissues due to somatic mosaic heterozygous mutations leading to over-activity of the PI3K kinase. In a recent workshop, scientists and clinicians proposed to gather all these syndromes under the general term “the PI3KCA-related overgrowth spectrum (PROS) syndrome” [4-8].
PROS patients are frequently exposed to ionizing radiation (IR) for imaging. Furthermore, since some cases of cancers have been reported in the PROS patients [9, 10], radiotherapy can be also evoked in the treatment of the disease. Surprisingly, while the radiobiological characterization of the PROS syndromes is of clinical interest and the PI3K kinase is known to be involved in the response to genotoxic stress [11], the major risks related to IR, namely, radiosensitivity (toxicity in healthy tissues) and radiosusceptibility (IR-induced cancers) have not been addressed for PROS patients yet. In the last decade, there have been considerable advances in the radiation biology research field with regard to the individual response to radiation, and more particularly in the repair and signaling of DNA double-strand breaks (DSB), the most severe radiation-induced DNA damage [12]. Upstream the earliest molecular events that follow an exposure to IR, the activation of the ATM protein kinase was shown to be a crucial step [13-17]. More recently, from a collection of human fibroblasts representing a very large spectrum of radiation response, the radiation-induced nucleo-shuttling of ATM (RIANS) was found to be one of the most reliable predictor of radiosensitivity [12, 18-21]. Interestingly, ATM also belongs to the PI3K family and ATM and PI3K have been shown to interact and activate in response to genotoxic stress [11, 22]. Here, in order to ask whether the PROS syndrome is associated with radiosensitivity and radiosusceptibility, the functionality of the ATM-dependent pathways activated by IR were investigated, for the first time to our knowledge, in both fibroblasts and osteoblasts derived from one PROS patient.
2. Subjects and Methods
2.1 Case report
One case of PROS patient (patient 03F/03O) was analysed (Figure S1). This is an 18-year-old woman presenting since childhood a polymalformative syndrome with multiple predominant adipovascular tumors in the abdomen and left hemi-face initially diagnosed as neurofibromatosis type I because of the association corpo-facial deformities + "café au lait" spots. The tumors present in childhood correspond to adipovascular masses pushing the noble organs and invading the soft tissues. Bone deformations and tumor compression were observed. A normal pressure hydrocephalus without neurological and cognitive impact was confirmed by an imaging review. The patient presented no pain, but showed mechanical limitations due to the volume of lesions. At the clinical level, the patient presented a major facial deformity to type of hemi-facial hypertrophy affecting the three floors of the face with hypoesthesia and hemi-facial paralysis. There is also a large cervical cutaneous hamartoma homo-lateral hypertrophy. The patient also presented with multiple extracorporeal and intraperitoneal compressional thoraco-abdominal tumors. An exhaustive imaging review revealed a normal pressure hydrocephalus. The diagnosis of Proteus syndrome was then evoked in front of evocative facial deformities. A skin lesion biopsy suggestive of epidermal haemoma found to hold somatic mutations post-zygotic of the PI3KCA gene resulting in an array of congenital mosaic pathology. The patient was then integrated into the diagnostic framework of the PROS and the diagnosis of Proteus syndrome (AKT1 mutation) was then rejected.
Another patient (patient 07F/07O) who did not suffer from PROS served as a control. He was a 22-year-old man who was coming for the avulsion of the four wisdom teeth under general anesthesia. His consent was obtained for a bone biopsy next to the alveolar bone that was completed by a skin biopsy. Skin and bone samples were taken during surgery with the informed consent of each patient and in the regulatory frame of the COPERNIC cell biobank (EROS subcollection), that gathers a number of cases of different radiosensitivity. This collection was approved by a national ethical committee, declared under the number DC2011-1437 by the French Ministry of Research and was already described elsewhere [20].
2.2 Cell lines
All the experiments were performed with untransformed fibroblast and osteoblasts cells in plateau phase of growth to overcome both cell cycle and immortalization effects. The establishment of 03F/03O and 07F/07O cells as cell lines were performed according to standard procedures whose details have been described elsewhere [20]. The reference code for fibroblast and osteoblast cell lines derived from both patients were EROS 03F and EROS 03O for the PROS patient and EROS 07F and EROS 07O for the control patient. The major radiobiological features of the radioresistant control 1BR3 and the hyper-radiosensitive ATM-mutated AT4BI and LIG4-mutated 180BR cell lines used in this study for intercomparisons were published elsewhere [13].
2.3 Irradiations
All the irradiations were performed on a clinical irradiator at anti-cancer Centre Léon-Bérard, (Lyon, France) at a dose of 2 Gy at a dose-rate 6 Gy.min-1 as published previously [23].
2.4 ZOPRA treatment
The combination of 1 µM pravastatin (a statin drug; Sigma-Aldrich France, Saint-Quentin-Fallavier, France) and 1 µM zoledronate (a biphosphonate agent; Sigma-Aldrich, France), the ZOPRA cocktail, was added in cell culture as previously published [23, 24]. The culture medium was renewed immediately before irradiation.
2.5 Cell survival
The intrinsic radiosensitivity was quantified by applyingthe standard clonogenic assay technique described elsewhere that consists in plating cells 24 h after irradiation [25]. The survival data were fitted to the linear-quadratic (LQ) model that described cell survival S as a function of dose D as follows :
S(D) = exp(-αD-βD2) (1)
in which α and β are the two adjustable LQ parameters. The surviving fraction at 2 Gy (SF2) was determined to reflect intrinsic radiosensitivity after a standard session of radiotherapy [18].
2.6 Immunofluorescence
In order to assess the subcellular localization of PI3KCA protein and to quantify the nuclear gH2AX, pATM and MRE11 foci formed by in irradiated cells, standard immunofluorescence protocol described elsewhere [26, 27] was applied to the cells described above. Anti-gH2AXser139 antibody (#05636; Upstate Biotechnology-Euromedex, Mundolsheim, France) was used at 1:800. Anti-pATMser1981 (#2888; Abcam, Cambridge, UK), -PI3KCA (#40776; Abcam), and -MRE11 (#56211; Abcys, Paris, France) were used at 1:100. Slides were mounted in 4',6' Diamidino-2-Phényl-indole (DAPI)-stained Vectashield (Abcys) and examined with Olympus fluorescence microscope.
2.7 Statistical analysis
The kinetics of nuclear foci formed by the proteins described above were analyzed according to previous studies [19]. Statistical analysis was performed by using Kaleidagraph v4 (Synergy Software, Reading, PA, USA).
3. Results and Analysis
3.1 Localization and expression of PI3KCA in PROS fibroblasts
Quiescent radioresistant 1BR3 fibroblasts showed cytoplasmic PI3KCA staining but the immunofluorescence signal appeared to be weak. By contrast, the PROS 03F fibroblasts elicited both cytoplasmic and perinuclear staining at higher intensity than the 1BR3 controls (Figure S2). These observations did not change after irradiation (data not shown).
3.2 The PROS fibroblasts show a moderate but significant cellular radiosensitivity
The clonogenic survival assay was applied to the radioresistant control 1BR3, the hyper-radiosensitive ATM-mutated AT4BI and the PROS 03F fibroblasts. The resulting LQ parameters were: α = 0.14 ± 0.03 Gy-1 and β = 0.001 ± 0.0005 Gy-2 with an SF2 of 64 ± 3%, and α = 2.0 ± 0.1 Gy-1 and β = 0 ± 0 Gy-2 with an SF2 of 2 ± 1 %, and α = 0.76 ± 0.09 Gy-1 and β = 0.001 ± 0.0005 Gy-2 with an SF2 of 35.3 ± 2 %, respectively, supporting that the PROS fibroblasts tested here showed a moderate but significant cellular radiosensitivity (Figure 1A). Radiation-induced micronuclei were found to be correlated with cellular radiosensitivity [28]. The PROS fibroblasts did not show any significant number of spontaneous micronuclei. The number of micronuclei assessed 24 h post-irradiation was found significantly higher than that of the radioresistant 1BR3 controls (11 ± 2 vs. 1 ± 1; p<0.001), but lower than that of the hyper-radiosensitive AT4BI cells (45.3 ± 4), supporting again a moderate radiosensitive phenotype (Figure 1B).
3.3 The PROS fibroblasts show an impaired DSB repair
Micronuclei are generally due to unrepaired DSB. The gH2AX foci formation is considered as the earliest recognition step of DSB managed by the non-homologous end-joining (NHEJ), the major DSB repair pathway in humans [29-32]. The number of spontaneous gH2AX foci in the radioresistant 1BR3 controls was not significantly different from those assessed in the PROS fibroblasts (data not shown ). In the radioresistant 1BR3 controls, the number of gH2AX foci scored 10 min after 2 Gy was 78 ± 3 per cell. This number was significantly lower in the PROS fibroblasts (30 ± 1 foci per cell on average, respectively; p<0.001), suggesting a slower DSB recognition by NHEJ via the H2AX phosphorylation (Figure 1C). By contrast, after 24 h post-irradiation, there was no significant difference between the number of gH2AX foci scored in PROS fibroblasts and that scored in radioresistant 1BR3 controls (p>0.8) (Figure 1C). In addition, the number of gH2AX foci remaining at 24 h after 2 Gy observed in the PROS cells was found significantly lower than that of the hyper-radiosensitive ATM and LIG4-mutated fibroblasts (p<0.001) [12] (Figure 1C). The SF2 and gH2AX data obtained from the PROS fibroblasts were found in quantitative agreement with the correlation obtained from 40 untransformed human fibroblasts differing by their radiosensitivity [12] (Figure 1D).
3.4 The radiation-induced ATM nucleo-shuttling is delayed in the PROS fibroblasts
The gH2AX foci generally result from a phosphorylation by the ATM protein kinase that also forms nuclear foci when auto-phosphorylated and the number of pATM foci reflects the rate of the RIANS [12, 18-20]. An exposure to 2 Gy X-rays resulted in an early formation of nuclear pATM foci in radioresistant controls. Conversely, the number of pATM after 10 min post-irradiation was found significantly lower in the PROS fibroblasts (12 ± 2 pATM foci per cell) than in the radioresistant 1BR3 control (44 ± 8 pATM foci per cell; p<0.05) (Figure 2A). Furthermore, the kinetics of appearance/disappearance of pATM foci were found very different with a maximum reached between 10 min and 1 h post-irradiation in the PROS fibroblasts. Such findings suggest that PROS cells are associated with a delayed RIANS.
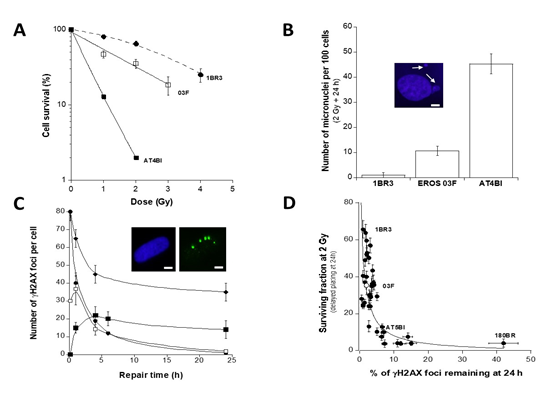
Figure 1: Cellular and molecular radiosensitivity of PROS fibroblasts. A. Clonogenic cell survival curves of irradiated fibroblast cell lines obtained after 24 h delayed plating: 1BR3 fibroblast cell line (?) is a radioresistant control; AT4BI (?) is a hyper-radiosensitive ATM-mutated cell line, and 03F (?) are PROS fibroblasts. Data plots represent the mean of triplicate experiments ± standard error of the mean (SEM). Survival data were fitted to the LQ model [18]. B. Number of micronuclei per 100 cells assessed 24 h after 2 Gy X-rays. Data plots represent the mean of triplicate experiments ± SEM. Insert. Representative example of micronucleus observed with the DAPI counterstaining. The arrow indicates the micronucleus. The white bar corresponds to 5 µm. C. IR-induced γH2AX foci kinetics assessed by immunofluorescence. Data were expressed as a number of γH2AX foci per cell. 180BR (♦) is a hyper-radiosensitive LIG4-mutated cell line. Data plots represent the mean of triplicate experiments ± SEM. Data were fitted to the Bodgi’s formula [19]. Insert. Representative examples of γH2AX foci after 2 Gy followed by 24 h in MAS fibroblasts with the DAPI counterstaining. The white bar corresponds to 5 µm. D. Correlation between SF2 and γH2AX foci remaining after 2 Gy followed by 24 h. Survival data shown in panel A were plotted against the corresponding γH2AX foci data shown in panel C. Cellular radiosensitivity (SF2) was found to be inversely proportional to the corresponding percentage of γH2AX foci remaining at 24 h post-irradiation. The best data fit was obtained with: (y = 100 / (x + 1)) - 1; r = 0.76 (solid line).
3.5 The PROS fibroblasts show abnormally high MRE11 activity after irradiation
The MRE11 nuclease activity was shown to be implied in a recombination-like DSB repair pathway that is responsible for DSB misrepair and genomic instability [12]. MRE11 foci appeared from 1 to 4 h after 2Gy and reached their maximal yield at 4 h in the radioresistant 1BR3 controls (Figure 2B). By contrast, the PROS fibroblasts showed an earlier occurrence of radiation-induced MRE11 foci (reached in the first hour post-irradiation) with a higher number of foci (13 ± 4 MRE11 foci in 03F fibroblasts vs. 3 ± 1 MRE11 foci in the radioresistant 1BR3 controls at 10 min post-irradiation (p<0.001) (Figure 2B).
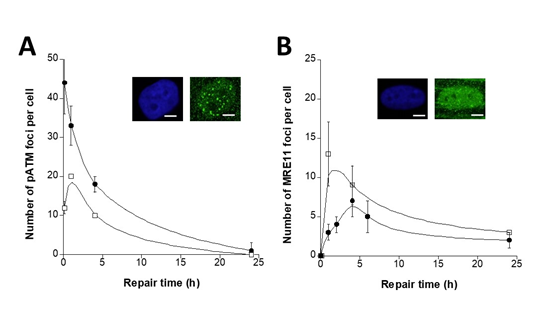
Figure 2: pATM and MRE11 foci in PROS fibroblasts in response to IR. A. Kinetics of IR-induced pATM foci assessed by immunofluorescence in the 03F PROS (?) and the radioresistant 1BR3 control (?) fibroblast cell lines. Data were expressed as a number of pATM foci per cell. Data plots represent the mean of triplicate experiments ± SEM. Insert. Representative examples of pATM foci after 2 Gy followed by 10 min for repair in PROS fibroblasts. The white bar corresponds to 5 µm. B. Kinetics of IR-induced MRE11 foci assessed by immunofluorescence in the same fibroblast cell lines. Data were expressed as a number of MRE11 foci per cell. Data plots represent the mean of triplicate experiments ± SEM. All the data were fitted to the Bodgi’s formula [19]. Insert. Representative examples of MRE11 foci after 2 Gy followed by 1 h for repair in PROS fibroblasts. The white bar corresponds to 5 µm.
3.6 Statins+bisphosphonates treatment results in the radioprotection of the PROS fibroblasts
The ZOPRA treatment was shown to accelerate the RIANS [23, 24]. We therefore investigated the effect of ZOPRA treatment to the previously defined molecular endpoints. In agreement with published data, the ZOPRA treatment did not affect the number of micronuclei, the kinetics of gH2AX and pATM foci in radioresistant 1BR3 control cells [23, 24]. Conversely, in the PROS cells treated with ZOPRA, the number of micronuclei assessed 24 h post-irradiation was reduced significantly (5.7 ± 1.2 vs. 11 ± 2 micronuclei per 100 cells treated and untreated, respectively) (Figure 3A). With regard to the gH2AX foci scored 24 h post-irradiation, the PROS 03F data were found similar to that of the radioresistant controls (1.84 ± 1 vs. 1 ± 1 gH2AX foci per cells treated and untreated, respectively) (Figure 3B). In addition, the ZOPRA treatment significantly accelerated the RIANS in the PROS cells (38 ± 2 pATM foci after 2 Gy for the 1BR3 controls versus 37 ± 2 for the ZOPRA-treated 03F fibroblasts, respectively; p>0.8) (Figure 3C).
3.7 The PROS osteoblasts and fibroblasts show similar radiobiological features
We applied the same protocols to the PROS 03O osteoblasts derived from the same PROS patient who provided 03F fibroblasts. However, since there was no osteoblast counterpart of the radioresistant 1BR3 control fibroblasts, we used the 07O osteoblasts derived from the patient 2 as PROS-negative control. The numbers of residual micronuclei in PROS 03O osteoblasts were found significantly higher than those of their fibroblasts counterparts (p<0.05) and their 07O controls (p<0.01) (Figure S3A). The number of the early gH2AX foci (Figure S3B) and the the pATM foci (Figure S3C) assessed in PROS 03O osteoblasts were significantly found lower than those of their fibroblasts counterparts (p<0.001 for both gH2AX and pATM) and their 07O controls (p<0.001 for gH2AX and p<0.01 for pATM), respectively. The number of MRE11 foci assessed in PROS 03O osteoblasts was found to be similar to that assessed in their fibroblasts counterparts but much higher than that assessed in their controls (Figure S3D).
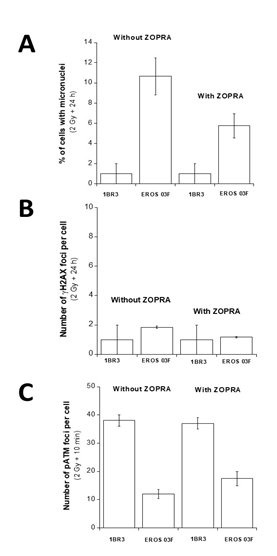
Figure 3: Effect of ZOPRA treatment in PROS fibroblasts in response to IR. The indicated fibroblast cell lines were treated or not with ZOPRA and irradiated at 2 Gy. The corresponding number of micronuclei per 100 cells assessed after 2 Gy + 24 h (A), the number of γH2AX foci remaining after 2 Gy + 24 h (B), the number of pATM foci remaining after 2 Gy + 10 min (C), were represented by the mean of triplicate experiments ± SEM.
4. Discussion
4.1 A moderate radiosensitivity, a novel clinical feature associated with PROS syndrome?
The phosphatidylinositol 3-kinase (PI3K)/Akt pathway is a key cascade downstream of several protein kinases like DNA-PK and ATM, that is involved in the DNA damage signalling and the cell cycle arrest. Hyperactivation of the PI3K/Akt pathway, notably via the phosphorylation of AKT1 in S473 is correlated with cellular proliferation, transformation and tumor development [11, 33] That somatic mosaic mutations of the PI3KCA subunit lead to overgrowth syndromes like PROS confirms the importance of the PI3K/Akt pathway in the control of cell growth and genomic instability. Besides, it was recently shown that PI3K inhibitor treatment may reverse some PROS [34]. However, in this study, while radiosusceptibility (radiation-induced cancers) may be explained by the hyperactivation of the PI3K protein, radiosensitivity appears to be a new clinical feature of PROS that is not necessarily explained by known PI3K functions, and, overall, not necessarily explained by exacerbated PI3K activity. Besides, it was shown recently that restraining Akt phosphorylation attenuates DSB repair and leads to radiosensitization [35]. Altogether, according to our algorithms of the radiosensitivity prediction from the RIANS model [20, 21], our data suggest that the exposure to IR of the PROS 03F/03O patient may be responsible of post-radiotherapy grades 2-4 tissue reactions, which may correspond to severe tissue injuries. However, further investigations on more PROS patients are needed to consolidate our hypotheses.
4.2 A novel mechanistic model for the PROS syndrome
How to explain the moderate but significant radiosensitivity observed in the cells from the PROS patient tested? Interestingly, such moderate radiosensitivity is very similar to that encountered in some syndromes like tuberous sclerosis, Huntington’s disease, Xeroderma Pigmentosum. It is also noteworthy that ZOPRA treatments were shown to decrease the radiosensitivity phenotype in cells derived from these syndromes, providing new insights for the use of statins and/or bisphosphonates to improve the patients outcome [23, 27, 36]. To date, the RIANS model permits to propose a relevant molecular explanation to the moderate radiosensitivity and the efficiency of the ZOPRA treatment without contradicting the “historical” models for specific clinical feature like overgrowth and tumor susceptibility [20]. Briefly, our recent findings suggest that IR induce the monomerization of cytoplasmic ATM dimers which facilitates the diffusion of ATM monomers in the nucleus [19, 20]. Once in the nucleus, the ATM monomers trigger phosphorylation of H2AX protein, which 1) initiates the recognition of DSB managed by NHEJ, the major DSB repair pathway in humans; 2) inhibits the activity of MRE11 nuclease that may be at the origin of genomic instability and cancer proneness (Figure 4). If the RIANS is delayed, the recognition of DSB by NHEJ is less efficient and more DSB are managed by the MRE11-dependent repair pathway [21]. As a result, cells are more radiosensitive and more susceptible to transformation. Conversely, any acceleration of the RIANS, like that observed with ZOPRA-treated cells, can render cells more radioresistant and less transformable (Figure 4). Interestingly, while the mutations of PI3KCA likely lead to an overactive PI3KCA function, immunofluorescence PI3KCA signals appeared to be more intense in PROS than in control cells. Since interaction between PI3K and ATM has been demonstrated [22], more frequent interactions between radiation-induced ATM monomers and more abundant PI3KCA may occur in cytoplasm and lead to a perinuclear accumulation of the ATM-PI3KCA complexes. Such accumulation of the multiprotein complexes close to the nuclear membrane may prevent the diffusion of ATM monomers in nucleus. Hence, a delayed RIANS may appear as an important molecular feature of PROS cells and the RIANS model may provide an explanation of radiosensitivity due to the PI3KCA protein “substrate” rather than the PI3KCA protein “function”. Obviously, the relevance of the RIANS model for PROS must be verified by the demonstration that the PI3KCA mutations leading to PROS are associated by a deregulation of the protein resulting in a higher PI3KCA expression in cytoplasm in PROS cells than in controls.
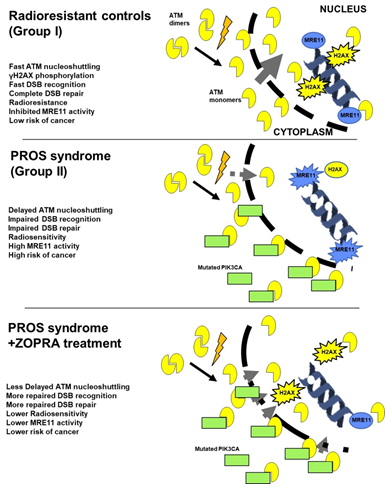
Figure 4: PROS syndrome and the RIANS model. Schematic illustration of the RIANS model for the (Group I) radioresistant cells, the (Group II) PROS and the ZOPRA-treated cells. The IR-induced ATM monomers can diffuse in nucleus in which they trigger DSB repair pathway by phosphorylating H2AX (activation of NHEJ) and MRE11 (inhibition of the recombination-like process). In PROS cells, the PI3KCA mutations favour the formation of ATM-PI3KCA complex in cytoplasm, which sequestrates the ATM monomers. As a result, a significant RIANS occurs. It is alleviated by the ZOPRA treatment.
5. Conclusions
The radiobiological characterization of skin and bone cells derived from a PROS patient suggest that the PROS syndrome may belong to the group of syndromes associated with moderate radiosensitivity and delayed RIANS. Furthermore, the application of statin and/or bisphosphonate treatment to the PROS cells appears to complement this phenotype. However, further investigations on more clinical cases are needed to verify whether the RIANS model is relevant for any PROS patients.
Acknowledgements
TB was awarded by the Association des Gueules Cassées.
Disclosure Statement
The authors have no conflicts of interest to declare.
Funding Sources
This work was supported by the Commissariat General à l’Investissement (CGI) (INDIRA project), the Institut National du Cancer (INCA) (PROUST project), the Centre National d’Etudes Spatiales (CNES) (BERNADOTTE Project).
Ethics Statement
Cell sampling, conservation and use were approved by a national ethical committee, declared under the number DC2011-1437 by the French Ministry of Research.
Informed Consent
Skin and Bone sampling was performed in agreement with French laws and notably with the informed consent of the patients.
References
- Vanhaesebroeck B, Whitehead MA, Pineiro R. Molecules in medicine mini-review: isoforms of PI3K in biology and disease. J Mol Med (Berl) 94 (2016): 5-11.
- Yuan TL, Cantley LC. PI3K pathway alterations in cancer: variations on a theme. Oncogene 27 (2008): 5497-510.
- Yang SX, Polley E, Lipkowitz S. New insights on PI3K/AKT pathway alterations and clinical outcomes in breast cancer. Cancer Treat Rev 45 (2016): 87-96.
- Keppler-Noreuil KM, Rios JJ, Parker VE, et al. PIK3CA-related overgrowth spectrum (PROS): diagnostic and testing eligibility criteria, differential diagnosis, and evaluation. Am J Med Genet A 167A (2015): 287-295.
- Brunner HG, van Driel MA. From syndrome families to functional genomics. Nat Rev Genet 5 (2004): 545-551.
- Cohen M, Neri G, Weksberg R. Overgrowth syndromes. New York: Oxford University Press (2002).
- Martinez-Lopez A, Blasco-Morente G, Perez-Lopez I, et al. CLOVES syndrome: review of a PIK3CA-related overgrowth spectrum (PROS). Clin Genet 91 (2017): 14-21.
- Venot Q, Canaud G. PIK3CA-related overgrowth syndrome (PROS)]. Nephrol Ther 13 Suppl 1 (2017): S155-S156.
- Peterman CM, Fevurly RD, Alomari AI, et al. Sonographic screening for Wilms tumor in children with CLOVES syndrome. Pediatr Blood Cancer 64 (2017).
- Madsen RR, Vanhaesebroeck B, Semple RK. Cancer-Associated PIK3CA Mutations in Overgrowth Disorders. Trends Mol Med 24 (2018): 856-870.
- Toulamy M, Rodemann HP. Phosphatidylinositol 3-kinase/Akt signaling as a key mediator of tumor cell responsiveness to radiation. Seminar in Cancer Biology 35 (2015): 180-190.
- Joubert A, Zimmerman KM, Bencokova Z. et al. DNA double-strand break repair defects in syndromes associated with acute radiation response: at least two different assays to predict intrinsic radiosensitivity? International journal of radiation biology 84 (2008): 1-19.
- Foray N, Priestley A, Alsbeih G, et al. Hypersensitivity of ataxia telangiectasia fibroblasts to ionizing radiation is associated with a repair deficiency of DNA double-strand breaks. International journal of radiation biology 72 (1997): 271-283.
- Taylor AM, Byrd PJ, McConville CM, et al. Genetic and cellular features of ataxia telangiectasia. International journal of radiation biology 65 (1994): 65-70.
- Taylor AM, Harnden DG, Arlett CF, et al. Ataxia telangiectasia: a human mutation with abnormal radiation sensitivity. Nature 258 (1975): 427-429.
- Savitsky K, Bar-Shira A, Gilad S, et al. A single ataxia telangiectasia gene with a product similar to PI-3 kinase. Science 268 (1995): 1749-1753.
- Savitsky K, Sfez S, Tagle DA, et al., The complete sequence of the coding region of the ATM gene reveals similarity to cell cycle regulators in different species. Human molecular genetics 4 (1995): 2025-2032.
- Bodgi L, Foray N, The nucleo-shuttling of the ATM protein as a basis for a novel theory of radiation response: resolution of the linear-quadratic model. International journal of radiation biology 92 (2016): 117-131.
- Bodgi L, Granzotto A, Devic C, et al. A single formula to describe radiation-induced protein relocalization: towards a mathematical definition of individual radiosensitivity. Journal of theoretical biology 333 (2013): 135-145.
- Granzotto A, Benadjaoud MA, Vogin G, et al. Influence of Nucleoshuttling of the ATM Protein in the Healthy Tissues Response to Radiation Therapy: Toward a Molecular Classification of Human Radiosensitivity. International journal of radiation oncology, biology, physics 94 (2016): 450-460.
- Berthel E, Foray N, Ferlazzo ML. The Nucleoshuttling of the ATM Protein: A Unified Model to Describe the Individual Response to High- and Low-Dose of Radiation? Cancers 11 (2019).
- Irarrazabal CE, Burg MB, Ward SG, et al. Phosphatidylinositol 3-kinase mediates activation of ATM by high NaCl and by ionizing radiation: Role in osmoprotective transcriptional regulation. Proceedings of the National Academy of Sciences of the United States of America 103 (2006): 8882-8887.
- Ferlazzo ML, Sonzogni L, Granzotto A, et al. Mutations of the Huntington's Disease Protein Impact on the ATM-Dependent Signaling and Repair Pathways of the Radiation-Induced DNA Double-Strand Breaks: Corrective Effect of Statins and Bisphosphonates. Molecular neurobiology 49 (2014): 1200-1211.
- Varela I, Pereira S, Ugalde AP, et al. Combined treatment with statins and aminobisphosphonates extends longevity in a mouse model of human premature aging. Nature medicine 14 (2008): 767-772.
- Badie C, Iliakis G, Foray N, et al. Induction and rejoining of DNA double-strand breaks and interphase chromosome breaks after exposure to X rays in one normal and two hypersensitive human fibroblast cell lines. Radiation research 144 (1995): 26-35.
- Foray N, Marot D, Gabriel A, et al. A subset of ATM- and ATR-dependent phosphorylation events requires the BRCA1 protein. The EMBO journal 22 (2003): 2860-2871.
- Ferlazzo M, Berthel E, Granzotto A, et al. Some mutations in the xeroderma pigmentosum D gene may lead to moderate but significant radiosensitivity associated with a delayed radiation-induced ATM nuclear localization. International journal of radiation biology (2019): 1-17.
- Foray N, Bourguignon M, Hamada N, Individual response to ionizing radiation. Mutation Research Review 770 (2016): 369-86.
- Rothkamm K, Lobrich M, Evidence for a lack of DNA double-strand break repair in human cells exposed to very low x-ray doses. Proceedings of the National Academy of Sciences of the United States of America 100 (2003): 5057-62.
- Burma S, Chen BP, Murphy M, et al. ATM phosphorylates histone H2AX in response to DNA double-strand breaks. The Journal of biological chemistry 276 (2001): 42462-42467.
- Rogakou EP, Pilch DR, Orr AH, et al. DNA double-stranded breaks induce histone H2AX phosphorylation on serine 139. The Journal of biological chemistry 273 (1998): 5858-5868.
- Bakkenist CJ, Kastan MB. DNA damage activates ATM through intermolecular autophosphorylation and dimer dissociation. Nature 421 (2003): 499-506.
- Yudushkin I, Getting the Akt Together: Guiding Intracellular Akt Activity by PI3K. Biomolecules 9 (2019).
- Crunkhorn S, Genetic disorders: PI3K inhibitor reverses overgrowth syndrome. Nat Rev Drug Discov 17 (2018): 545.
- Szymonowicz K, Oeck S, Krysztofiak A, et al. Restraining Akt1 Phosphorylation Attenuates the Repair of Radiation-Induced DNA Double-Strand Breaks and Reduces the Survival of Irradiated Cancer Cells. International journal of molecular sciences 19 (2018).
- Ferlazzo ML, Bach-Tobdji MKE, Djerad A, et al. Radiobiological characterization of tuberous sclerosis: A delay in the nucleo-shuttling of ATM may be responsible for radiosensitivity. Molecular neurobiology 55 (2017).


 Impact Factor: * 5.3
Impact Factor: * 5.3 Acceptance Rate: 75.63%
Acceptance Rate: 75.63%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 
