Neonate with Severe Tricuspid Regurgitation and Flail Anterior Leaflet of the Tricuspid Valve
Matthew F Pizzuto*, Chris S Iskander, J. Ryan Shea
Department of Pediatrics, University of North Carolina at Chapel Hill, North Carolina Children’s Hospital
*Corresponding Author: Matthew F Pizzuto, Campus Box 7221, 417 MacNider Hall, Chapel Hill, North Carolina.
Received: 30 August 2023; Accepted: 13 September 2023; Published: 23 October 2023
Article Information
Citation: Matthew F Pizzuto, Chris S Iskander, J. Ryan Shea. Neonate with Severe Tricuspid Regurgitation and Flail Anterior Leaflet of the Tricuspid Valve. Archives of Clinical and Medical Case Reports. 7 (2023): 374-376.
View / Download Pdf Share at FacebookAbstract
Flail anterior tricuspid valve leaflet is a rare source of primary tricuspid regurgitation. It can be challenging to manage medically in neonates and often requires surgical repair. This case describes the use of medical management of a patient with flail tricuspid valve leaflet with severe tricuspid regurgitation to avoid neonatal cardiac surgery
Keywords
<p>Flail tricuspid valve; Tricuspid regurgitation; Neonate; Congenital Heart Disease</p>
Article Details
1. Introduction
Primary tricuspid regurgitation occurs due to a structural abnormality of the tricuspid valve. This abnormality causes inappropriate coaptation of one or multiple leaflets of the valve relative to the other(s). Flail anterior tricuspid valve leaflet is a rare source of primary tricuspid regurgitation typically caused by damage to the papillary muscle.
There are multiple potential causes of papillary muscle injury in the neonate including prenatal and perinatal factors, genetic factors, and concomitant congenital heart disease lesions [1].
2. Case Presentation
A 2.5kg male infant was born at 36 weeks gestation to 38-year-old G6P4 mother whose pregnancy was complicated by gestational diabetes, pre-eclampsia, bipolar disorder, chronic kidney disease, and maternal drug use. Prenatal ultrasounds were unremarkable. APGARs were 8 and 9 at 1 and 5 minutes, respectively. The patient became cyanotic within the first hour of life requiring tracheal intubation. Pulse oximetry saturations remained approximately 70% despite 100% FiO2. A murmur was noted and given the concern for congenital heart disease, prostaglandin E1 (PGE) was started. Echocardiography revealed severe tricuspid valve regurgitation, prompting transfer to a tertiary care center.
Repeat echocardiography on arrival was notable for severe tricuspid regurgitation with prolapse of the anterior leaflet of the tricuspid valve (Figure 1). The septal leaflet was minimally tethered. Right ventricular size and function were normal for age. Apical displacement of the tricuspid valve was only 5.9 mm/m2 (An index of ≥8mm/m2 is supportive of the diagnosis of Ebstein’s anomaly) [2].
There was a small patent ductus arteriosus (PDA) with left to right shunting and a moderate patent foramen ovale with right to left shunting. Initially on day of life 1 there was functional pulmonary atresia presumably due to elevated pulmonary vascular resistance (PVR) as well as left to right ductal flow.
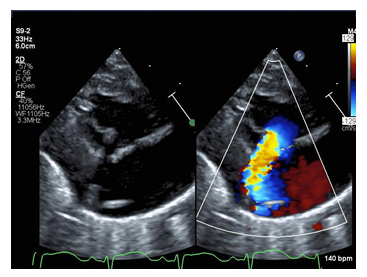
Figure 1: Echocardiogram still image of the parasternal long axis view with color compare of the patient demonstrating flail tricuspid valve leaflet (arrow) and corresponding severe tricuspid regurgitation jet with color.
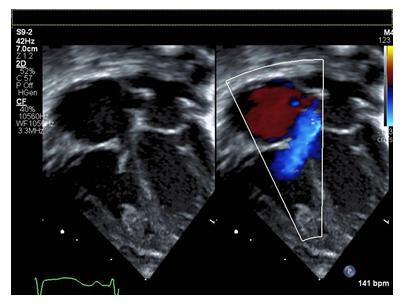
Figure 2: Apical four chamber view of flail tricuspid valve with color compare in systole demonstrating failure of coaptation and wide based regurgitant jet.
Considering the increased PVR and functional pulmonary atresia, inhaled nitric oxide (iNO) was started, mechanical ventilation was titrated, and PGE was continued. This led to a clinical improvement in oxygen saturations to 87%. The management strategy was to give time to allow for the PVR to decrease as normally occurs in all newborns, leading to adequate antegrade flow across the pulmonary valve. At day of life 4, the patient was initially trialed off PGE. He developed prohibitive hypoxemia requiring reinstitution of PGE and the decision was made to give more time for the PVR to decrease further. His second trial off PGE was successful and by 13 days of life the patient was weaned off iNO, PGE, and the ventilator. The decision was made to delay surgical intervention given continued clinical improvement. At 4 weeks of age, he was discharged home in good condition, feeding by mouth, and gaining weight.
He initially did well but at 23 months of age he developed symptoms of fatigue and periorbital edema. He then underwent surgical repair of his abnormal tricuspid valve. Intraoperative inspection showed a thickened anterior leaflet with prolapse. The subvalvar apparatus consisted of a minor chordal attachment but no obvious papillary muscle present. His surgery consisted of a repair of tricuspid valve with a modified annuloplasty, PDA ligation, and closure of patent foramen ovale. There were no complications. Echocardiography showed that his pre-operative tricuspid regurgitation was now mild and without significant tricuspid stenosis; there was normal biventricular systolic function and mild-moderate right ventricular dilation. He was discharged home on post-operative day six.
3. Discussion
Flail anterior tricuspid valve leaflet is a rare, but life-threatening diagnosis made based on imaging in the context of a clinical picture consisting of murmur, cyanosis, and respiratory distress failing to improve despite optimized ventilation and oxygenation. Though typically provoked by a ruptured papillary muscle, this pathology should remain on the differential diagnosis even with intact papillary muscles.
Medical management of a neonate with flail tricuspid valve is challenging. Optimizing multiple sources of pulmonary blood flow is key. Maneuvers to achieve this goal include intubation with supplemental oxygen and ventilator titration to minimize PVR, iNO, milrinone, and a tincture of time. PGE is also useful to keep the PDA open for additional pulmonary blood flow and act as an additional pulmonary vasodilator. Many of the management strategies are physiologically similar to that of neonatal Ebstein’s anomaly [1, 3].
It should be noted, however, that in certain scenarios, PGE could be detrimental and may prevent antegrade flow across the pulmonary valve causing functional pulmonary atresia [4]. When to start and stop PGE can be a difficult decision for the clinician at the bedside and must be individualized to the patient.
4. Conclusions
Understanding this rare anatomy and complex physiology of flail tricuspid valve as well as ways to optimize pulmonary blood flow were key to successful medical management of this case. Importantly, this led to avoidance of surgical intervention in the neonatal period.
Acknowledgements
The authors acknowledge the support from the Department of Pediatrics at University of North Carolina at Chapel Hill.
Financial Support
This research received no specific grant from any funding agency, commercial or not-for-profit sectors.
Conflict of Interest
None.
References
- Loftus PD, Arrington CB, Kaza AK. Neonatal flail tricuspid valve: diagnosis and management. Ann Thorac Surg. 2014;98(3):1098-1101.
- Attenhofer Jost CH, Connolly HM, Dearani JA, Edwards WD, Danielson GK. Ebstein's anomaly. Circulation. 2007;115(2):277-285.
- Baek JS, Yu JJ, Im YM, Yun TJ. Outcomes of neonatal Ebstein's anomaly without right ventricular forward flow [published correction appears in J Thorac Cardiovasc Surg. 2016 Dec;152(6):1644]. J Thorac Cardiovasc Surg. 2016;152(2):516-521.
- Wald RM, Adatia I, Van Arsdell GS, Hornberger LK. Relation of limiting ductal patency to survival in neonatal Ebstein's anomaly. Am J Cardiol. 2005;96(6):851-856.


 Impact Factor: * 5.3
Impact Factor: * 5.3 Acceptance Rate: 75.63%
Acceptance Rate: 75.63%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 
