The Physiological and Perceptual Stress Response During Data Collection in Altitude: A Single-Case Report of A Healthy Researcher
Livia Freitag1*, Ron Clijsen1,2,3,4, Erich Hohenauer1,2,4,5
1Rehabilitation Research Laboratory (2rLab), Rehabilitation and Exercise Science Group, Department of Business Economics, Health and Social Care, University of Applied Sciences and Arts of Southern Switzerland, Landquart / Manno, Switzerland
2International University of Applied Sciences THIM, Landquart, Switzerland
3Department of Health, Bern University of Applied Sciences, Berne, Switzerland
4Department of Movement and Sport Sciences, Vrije Universiteit Brussel, Brussels, Belgium
5Department of Neurosciences and Movement Sciences, University of Fribourg, Fribourg, Switzerland
*Corresponding Author: Livia Freitag, Weststrasse 8, 7302 Landquart, Switzerland
Received: 20 October 2021; Accepted: 03 November 2021; Published: 16 November 2021
Article Information
Citation:
Livia Freitag, Ron Clijsen, Erich Hohenauer. The Physiological and Perceptual Stress Response During Data Collection in Altitude: A Single-Case Report of A Healthy Researcher. Archives of Clinical and Medical Case Reports 5 (2021): 821-831.
View / Download Pdf Share at FacebookAbstract
Environmental conditions such as altitude exposure can lead to diverse physiological adjustments and stimulate a kind of stress response which can be measured with validated biomarkers. Stress negatively affects the cognitive performance and potential increases source of error. The aim of the following case study is to evaluate the physiological stress response of a healthy researcher during the conduct of an extraordinary study in altitude. The study was performed at an altitude of nearly 3000 meters above sea level in a mountain laboratory. Data were compared to conditions in a professional research laboratory and office at 550 meters above sea level. Parameters were mitochondrial activity, salivary cortisol, stress level and body battery collected by a smartwatch and scores of the daily stress inventory questionnaire. Almost all results showed a similar pattern: Outcomes were slightly impaired during laboratory conditions (LAB), compared to a normal office day in research (NOR). The highest impact on all measured values were observed on the first day of altitude exposure (M1), followed by a recovery until the last mountain day (M4). On the last day (POST), data returned to the level of NOR condition. The results of this case report show, that researching in altitude exposure can lead to an increased stress response. Particularly during data collection in such extraordinary study conditions, great attention must be given to prevent errors. Previously performed acclimatization procedures on the researcher might influence the results.
Keywords
<p>Mitochondrial activity; Salivary cortisol; Altitude exposure; Cognitive performance; Hypoxia</p>
Article Details
1. Introduction
Physiological adjustments, such as increased heart rate, cardiac output, decrease in hemoglobin oxygen saturation and peripheral resistance can be observed in altitude exposure [1, 2]. Terrestrial altitude exposure stimulates a stress response in the human body. Validated biomarkers, which are affected as a result of this stress responses are cortisol levels, mitochondrial function, peroxisome proliferator-activated receptor-gamma coactivator (PGC-1α) and the bioenergetic health index (BHI) [3-5]. The release of cortisol is considered to be a reliable measure of hypothalamus-pituitary-adrenal axis and has been shown to be related to the experience or anticipation of stress [6, 7]. PGC-1α has been demonstrated to play a central role in mitochondrial biogenesis, providing energy for most cellular functions [8, 9]. The BHI represents a dynamically sensitive indicator to oxidative stress in human monocytes and is calculated based on basal respiratory, proton leak, maximal oxygen consumption, ATP production, reserve respiratory capacity and non-mitochondrial respiration [10]. Acute stress on healthy humans can cause a multitude of unfavorable health consequences both on physical and mental functioning [11].
Currently, research focuses on the impact of hypoxia on participants during altitude exposure but not on the examiner. The environmental conditions as well as the work-related stress itself can affect the researcher and probably influence the measurements and results. To our knowledge, no study has focused on different stress biomarkers from a healthy researcher yet, during study execution in altitude. Therefore, the aim of our case report is to evaluate the stress response of a healthy researcher, while conducting a large-scale study under extraordinary conditions, using objective and subjective assessments in altitude in comparison to normal laboratory conditions.
2. Materials and Methods
2.1 Study design
Data collection took place within one month: at a normal office day in research (NOR) which acted as the control condition, at a day in the laboratory where usual research measurements were conducted (LAB), at four consecutive days at the mountain laboratory (M1 until M4) and a three-day post-altitude follow-up in normal research office working conditions (POST) as seen in Figure 1.
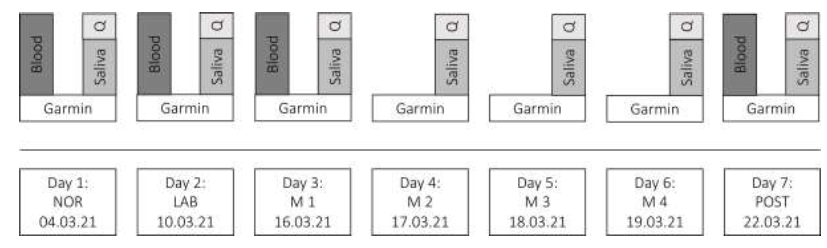
Figure 1: Study plan.
Abbreviations: Q, Questionnaires; NOR, normal office day; LAB, laboratory measurements; M1, mountain measurements day 1; M2, mountain measurements day 2; M3, mountain measurements day 3; M4, mountain measurements day 4; POST, normal office day after mountain exposure.
All samplings at 550 m above sea level (NOR, LAB, POST) corresponded to a professional research laboratory respectively in the nearby office (Landquart, CH-7302, Switzerland). The laboratory has the following dimensions: 7m x 14m x 2.5m and the environmental conditions can be controlled.
Data from the mountain measuring days (M1, M2, M3 and M4) were collected at an altitude of 2972 m above sea level in the mountain laboratory, where the hypobaric hypoxia measurement took place (Diavolezza, CH-7504 Pontresina, Switzerland). The mountain laboratory room had a size of about 4m x 5m x 2.3m. The transport, as well as the complete assembly of all devices, were carried out independently by the research team. To ensure a balance of strong temperature fluctuations, the room was heated in the mornings and the windows were temporarily darkened to minimize daily solar radiation. On each mountain day were five measurements from the large-scale study performed with an average duration of 1h 32min (total measurement time/day: 7h 42min).
2.2 Subject description
The subject of this case report is a healthy 37-years-old male research collaborator. The subject is a non-smoker and does not use any medication. The validated Copenhagen Psychosocial Questionnaire (COPSOQ) showed a low mental stress at baseline [12]. No surgical, psychosocial, or depressive disorder nor relevant medical history existed neither for the subject nor in the family history. No excessive physical activity nor caffeine consumption were permitted. The subject was asked to maintain his usual daily activities and habits during the investigation period.
2.3 Instrumentation
For the containment of the COVID-19 pandemic, all applicable federal regulations were considered.
2.3.1 Mitochondrial activity: For determination of the total number of mitochondrial cells, BHI and PGC-1α, a venous blood sample (±6 mL) was taken from the median cubital vein. This was performed by a professionally trained medical person (LF) at the same time of each measuring day. The analysis of the mitochondrial activity is based on the integrity of the mitochondrial membrane. After fluorescent coloration of the mitochondrial cells, cytometric determination was conducted.
2.3.2 Saliva cortisol: The saliva cortisol levels were assessed, as suggested, always at the evening of the corresponding measurement day before going to bed, ideally from 8:25 PM to 12:00 PM [13]. Saliva collection was performed at the earliest 30 minutes after ingestion of solid and liquid food. Previously, the oral cavity was rinsed with tap water for about one minute. Through a straw, ±3 mL saliva was gathered into two tubes. Then, the closed saliva tubes were stored in the refrigerator until dispatch to the external laboratory (Biovis Diagnostik MVZ GmbH, Limburg an der Lahn, Germany).
2.3.3 Smartwatch tracking: Heart rate, breathing and sleep were recorded during the whole day and the following night by a smartwatch (VENU, Garmin Ltd., Olathe, Kansas, USA). For the outcomes of interest (body battery and validated stress level) the manufacturers’ specific algorithms were used [14, 15].
2.3.4 Questionnaire: In the evening of each measurement day, the subject was also asked to complete the German version of the Daily Stress Inventory (DSI). The DSI includes an assessment of 58 potentially stressful everyday events of the past 24 h plus a well-being questionnaire [16]. The questionnaire was demonstrated to be reliable and valid to assess to individual impact of relatively minor stressful events [17].
2.4 Data reporting
Outcomes are presented as raw (absolute) data except the data of body battery and stress level from the smartwatch tracking. Results were described descriptively.
3. Results
3.1 Mitochondrial activity
The values of all blood mitochondrial samples are presented in Figure 2. The baseline values in NOR showed the highest total number of intact mitochondrial cells (97%), with only 3% being damaged. During LAB, the total number of intact mitochondrial cells decreased slightly to 95%. The lowest value of the total number of intact mitochondrial cells was observed in M1, where only 73% of the mitochondrial cells was intact. Of the remaining 27% damaged mitochondrial cells, 95% are irreversibly damaged and 5% are reversible. In other words, 22% of the intact mitochondrial cells were destroyed during six days (from LAB to M1). However, in the POST condition, mitochondrial cells recovered nearly to baseline values (96%).
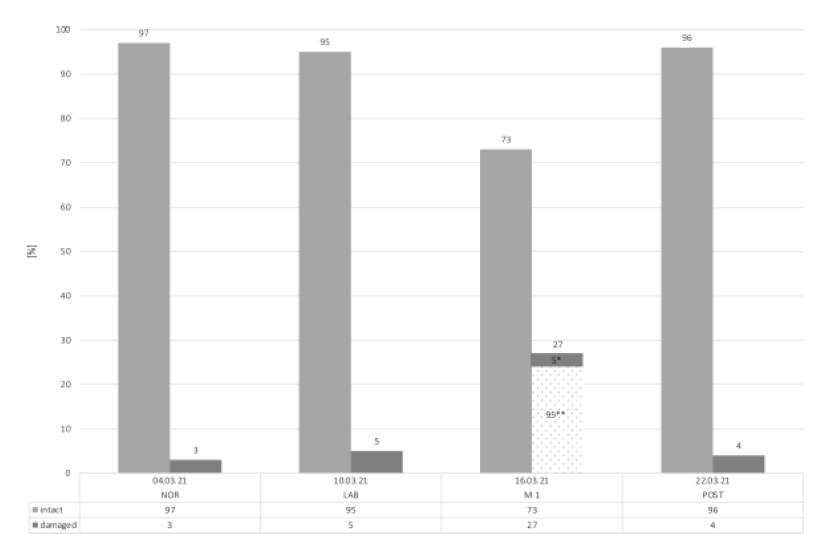
Figure 2: Number of total mitochondrial cells.
Abbreviations: *, reversible damaged; **, irreversible damaged; NOR, normal office day; LAB, laboratory measurements; M1, mountain measurements day 1; POST, normal office day after mountain exposure.
The BHI in NOR was 1.88 and slightly decreased in the LAB condition to 1.85. On M1, the lowest BHI was assessed with a value of 1.58. (as seen in Table 1). The values from the basal respiration, the maximal oxygen consumption, the reserve respiratory capacity, and the non-mitochondrial respiration were increased during LAB compared to NOR. The lowest value was observed on M1. The ATP production showed a similar pattern like the BHI: from NOR (90.4%) to LAB (86.98%) to M1 (86.26%) the value decreased continuously. In contrast, the lowest value of the proton leak is seen in NOR (9.6%) and increased during LAB (13.02%). The highest value is observed on M1 (13.74%). For the POST-condition, no BHI levels could be investigated because the laboratory was not able to isolate enough stimulable cells from the venous blood sample. PGC-1α in NOR condition were observed as “expressed” and in M1 and LAB as “not expressed” (Table 1). Indicating, that the regulation of mitochondrial biogenesis was decreased during altitude and laboratory measurements.
|
NOR 04.03.21 |
LAB 10.03.21 |
M 1 16.03.21 |
||
|
BHI |
index |
1.88 |
1.85 |
1.58 |
|
Basal respiration |
pmol O2/min |
29.78 |
33.89 |
25.51 |
|
Proton leak |
% |
9.6 |
13.02 |
13.74 |
|
Max. oxygen consumption |
% |
374.5 |
457.81 |
332.71 |
|
ATP production |
% |
90.4 |
86.98 |
86.26 |
|
Reserve respiratory capacity |
% |
274.5 |
357.81 |
232.71 |
|
Non-mito. respiration |
pmol O2/min |
10.03 |
11.57 |
9.83 |
|
PGC-1α |
expressed |
not expressed |
not expressed |
Abbreviations: NOR, normal office day; LAB, laboratory measurements; M1, mountain measurements day 1; BHI, bioenergetic health index; max., maximal; ATP, adenosine triphosphate production; Non-mito., non-mitochondrial
Table 1: Bioenergetic health index and PGC-1α.
3.2 Saliva cortisol
All results from the saliva cortisol samples are depicted in Figure 3. A strong increase can be seen from NOR (0.24 µg/l) to M1 (0.52 µg/l), which also reflects the highest values throughout the measurements. In M3, the saliva cortisol levels decreased to 0.42 µg/l from M1 and M2. Contrary to our expectations, the LAB-day showed lower cortisol values (0.12 µg/l) compared to NOR. In the POST condition, saliva cortisol decreased to 0.10 µg/l.
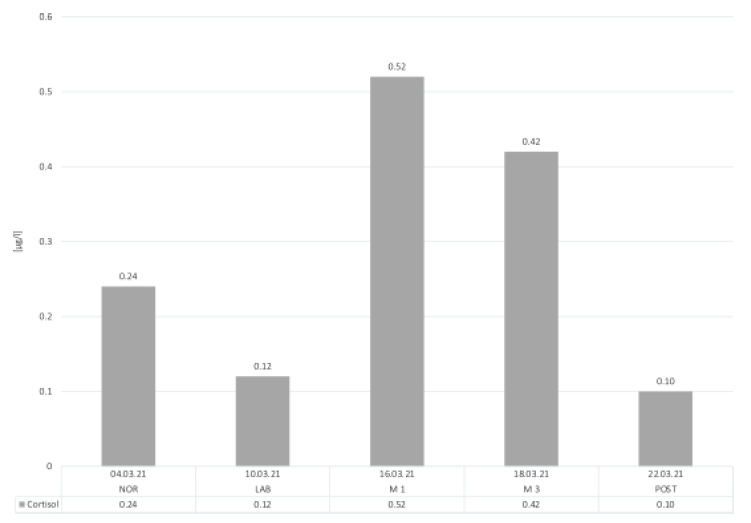
Figure 3: Salivary cortisol.
Abbreviations: NOR, normal office day; LAB, laboratory measurements; M1, mountain measurements day 1; M3, mountain measurements day 3; POST, normal office day after mountain exposure.
3.3 Smartwatch tracking
All outcomes of the Garmin smartwatch tracking are displayed in Figure 4. The stress level value during the LAB condition (stress level: 28) was decreased compared to NOR (stress level: 20). The highest level of stress was observed again during M1 (stress level: 44) and decreased through the altitude exposure (M4, stress level: 25). Again, during the POST condition, the values decreased to a stress level of 16.
The results of the body battery demonstrated an inverse-pattern compared to the stress level assessment. During NOR, the body battery was recorded a value of 60/100, whilst the lowest value was observed during M2 (body battery: 12/100), after the most demanding measurement day. However, after M2, the subject’ body battery started to increase (M3, M4, body battery: 35/100, 48/100) and reached the highest values during POST (body battery: 72/100).
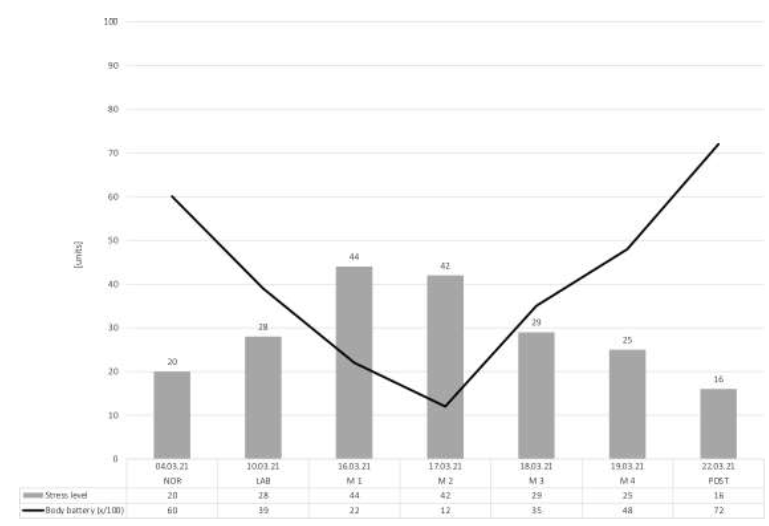
Figure 4: Stress level and body battery.
Abbreviations: NOR, normal office day; LAB, laboratory measurements; M1, mountain measurements day 1; M2, mountain measurements day 2; M3, mountain measurements day 3; M4, mountain measurements day 4; POST, normal office day after mountain exposure.
3.4 Questionnaire
The highest result of the DSI, indicating most daily stress inventory, was identified in the LAB condition with a score of 31 points (Figure 5). A slight increase in daily stress inventory can be seen during M1 (28 points) and M3 (16 points) compared to NOR (9 points). POST-day demonstrated the lowest stress score of 7 points, indicating that the DSI was even lower than baseline.
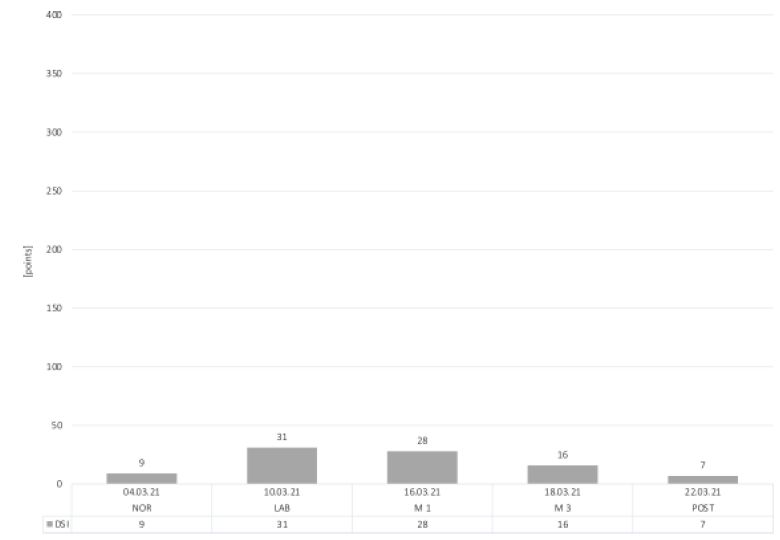
Figure 5: Daily Stress Inventory.
Abbreviations: DSI, Daily Stress Inventory; NOR, normal office day; LAB, laboratory measurements; M1, mountain measurements day 1; M3, mountain measurements day 3; POST, normal office day after mountain exposure.
4. Discussion
The aim of this case study was to assess the physiological stress response from a healthy researcher during measurements in altitude. In general, the results of the current case report show that, research under altitude exposure can lead to an increased stress response compared to normal laboratory conditions (LAB). The POST-measurements demonstrated that the subject recovered from the objective and subjective stress response. The total number of intact mitochondrial cells at M1 dropped below the value in NOR, which indicates, that the environment and stress due to the research on the mountain might have a strong impact on mitochondrial functioning, compared to all other conditions. A low number of mitochondrial cells is an important indicator for depressive disorders and lack of concentration but also represents the possible attendance of diseases like chronic fatigue syndrome, burnout, neurodegenerative-, cardiovascular diseases and metabolic syndrome [8, 18]. During all conditions, BHI values were below the normal range (normal value >2.00). This reduced index indicates, that the subject started already at the beginning of the examination with an insufficient capacity to generate enough ATP [19]. NOR measurement of PGC-1α showed regular values and decreased in M1 as well as during LAB. In case of a decreased number of mitochondrial cells, PGC-1α is an key regulator for induction of the mitochondrial biogenesis [20, 21]. Unfortunately, due to missing data of the PGC-1α in the POST condition, the effect of altitude exposure on the mitochondrial biogenesis remains unclear.
The negative effect from stress on different body functions has been frequently studied [22-26]. An above-average release of hormones such as cortisol are negatively associated with cognitive performance [6, 27]. Therefore, the impact from stress on researcher should be paid sufficient attention. Perhaps, sources of error could increase due to the combination of stress and environmental conditions. The observed mitochondrial function of this case report demonstrates the changes of these parameters in altitude exposure. Future studies should focus on the impact of an acclimatization period on the physiological and perceptual response of the researcher.
5. Conclusion
The results of our case report show, that physiological and subjective responses during measurements under hypoxia provoke stress and can potentially affect the cognitive performance of a researcher. The return to baseline stress values suggests a full recovery of the subject after altitude exposure.
Acknowledgement
The authors thank the Thim van der Laan foundation for financial support.
Conflict of Interest
The authors declare no conflict of interest.
References
- Vogel JA, Hartley LH, Cruz JC, et al. Cardiac output during exercise in sea-level residents at sea level and high altitude. J Appl Physiol 36 (1974): 169-172.
- Klausen K. Cardiac output in man in rest and work during and after acclimatization to 3,800 m. J Appl Physiol 21 (1966): 609-616.
- Picard M, McEwen BS. Psychological Stress and Mitochondria: A Systematic Review. Psychosom Med 80 (2018): 141-153.
- Chacko BK, Kramer PA, Ravi S, et al. The Bioenergetic Health Index: a new concept in mitochondrial translational research. Clin Sci (Lond) 127 (2014): 367-373.
- Chu B, Marwaha K, Sanvictores T, et al. Physiology, Stress Reaction. In: StatPearls. Treasure Island (FL): StatPearls Publishing. Copyright © 2021, StatPearls Publishing LLC (2021).
- Hammerfald K, Eberle C, Grau M, et al. Persistent effects of cognitive-behavioral stress management on cortisol responses to acute stress in healthy subjects-a randomized controlled trial. Psychoneuroendocrinology 31 (2006): 333-339.
- Hellhammer DH, Wust S, Kudielka BM. Salivary cortisol as a biomarker in stress research. Psychoneuroendocrinology 34 (2009): 163-171.
- Picard M, Prather AA, Puterman E, et al. A Mitochondrial Health Index Sensitive to Mood and Caregiving Stress. Biol Psychiatry 84 (2018): 9-17.
- Austin S, St-Pierre J. PGC1α and mitochondrial metabolism--emerging concepts and relevance in ageing and neurodegenerative disorders. J Cell Sci 125 (2012): 4963-4971.
- Chacko BK, Zhi D, Darley-Usmar VM, et al. The Bioenergetic Health Index is a sensitive measure of oxidative stress in human monocytes. Redox Biology 8 (2016): 43-50.
- Thoits PA. Stress and Health: Major Findings and Policy Implications. Journal of Health and Social Behavior 51 (2010): S41-S53.
- Nübling M, Hasselhorn H. The Copenhagen Psychosocial Questionnaire in Germany: From the validation of the instrument to the formation of a job-specific database of psychosocial factors at work. Scandinavian journal of public health 38 (2010): 120-124.
- Raff H, Phillips JM. Bedtime Salivary Cortisol and Cortisone by LC-MS/MS in Healthy Adult Subjects: Evaluation of Sampling Time. J Endocr Soc 3 (2019): 1631-1640.
- Witt D, Kellogg R, Snyder M, Dunn J. Windows Into Human Health Through Wearables Data Analytics. Curr Opin Biomed Eng 9 (2019): 28-46.
- Wagner M, Engel F, Klier K, et al. Zur Reliabilität von Wearable Devices am Beispiel einer Premium Multisport-Smartwatch. German Journal of Exercise and Sport Research 51 (2021): 49-62.
- Brantley PJ, Waggoner CD, Jones GN, et al. A Daily Stress Inventory: development, reliability, and validity. J Behav Med 10 (1987): 61-74.
- Traue HC, Hrabal V, Kosarz P. Alltags Belastungs Fragebogen (ABF): Zur inneren Konsistenz, Validierung und Stressdiagnostik mit dem deutschsprachigen Daily Stress Inventory. Verhaltenstherapie und Verhaltensmedizin 21 (2000): 15-38.
- Picard M, Wallace DC, Burelle Y. The rise of mitochondria in medicine. Mitochondrion 30 (2016): 105-116.
- Brand MD, Nicholls DG. Assessing mitochondrial dysfunction in cells. Biochem J 435 (2011): 297-312.
- Rius-Pérez S, Torres-Cuevas I, Millán I, et al. PGC-1α, Inflammation, and Oxidative Stress: An Integrative View in Metabolism. Oxid Med Cell Longev (2020): 1452696.
- Benton CR, Wright DC, Bonen A. PGC-1alpha-mediated regulation of gene expression and metabolism: implications for nutrition and exercise prescriptions. Appl Physiol Nutr Metab 33 (2008): 843-862.
- Yaribeygi H, Panahi Y, Sahraei H, et al. The impact of stress on body function: A review. EXCLI J 16 (2017): 1057-1072.
- Marin MF, Lord C, Andrews J, et al. Chronic stress, cognitive functioning and mental health. Neurobiol Learn Mem 96 (2011): 583-595.
- Sun MK, Alkon DL. Stress: perspectives on its impact on cognition and pharmacological treatment. Behav Pharmacol 25 (2014): 410-424.
- Quaedflieg C, Schwabe L. Memory dynamics under stress. Memory 26 (2018): 364-376.
- McEwen BS, Sapolsky RM. Stress and cognitive function. Curr Opin Neurobiol 5 (1995): 205-216.
- Law R, Clow A. Stress, the cortisol awakening response and cognitive function. Int Rev Neurobiol 150 (2020): 187-217.


 Impact Factor: * 5.3
Impact Factor: * 5.3 Acceptance Rate: 75.63%
Acceptance Rate: 75.63%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 
