A Tale of Two Hearts: Cardiac Manifestations of Coronavirus-2019. A Case Series and Literature Review
Rohit Aloor1*, Shravana Aryal1, Debajit Roy1, Beth Allen2, Louis Saade1, 3, Christopher James Haas1, 3
1MedStar Health, Internal Medicine Residency Program, Maryland, USA
2Department of Pathology, MedStar Health, Maryland, USA
3Assistant Professor of Medicine, Georgetown University Medical Center, DC, USA
*Corresponding Author: Rohit Aloor, MedStar Health, Internal Medicine Residency Program, Maryland, USA
Received: 28 December 2020; Accepted: 19 January 2021; Published: 04 May 2021
Article Information
Citation: Rohit Aloor, Shravana Aryal, Debajit Roy, Beth Allen, Louis Saade, Christopher James Haas. A Tale of Two Hearts: Cardiac Manifestations of Coronavirus-2019. A Case Series and Literature Review. Archives of Clinical and Medical Case Reports 5 (2021): 373-387.
View / Download Pdf Share at FacebookAbstract
Coronavirus disease 2019 (COVID-19) originally emerged as respiratory disease from a novel coronavirus, SARS-CoV-2. While initial cases revealed a spectrum of respiratory-associated clinical presentations ranging from mild shortness of breath with associated cough to severe respiratory distress, extrapulmonary symptoms, with or without concomitant respiratory disease, have been increasingly reported. Cardiovascular complications are noted in a subset of patients with presentations ranging from non-ST elevation myocardial infarction to fulminant myopericarditis. While cardiac myocytes express the SARS-CoV-2 receptor, Angiotensin converting enzyme 2 (ACE2), the mechanism of cardiac effects remains unknown, further underscoring the complex nature of COVID-19. Here we report two cases of COVID-19 in which cardiac manifestations – pericarditis with associated pericardial effusion and tamponade physiology and fulminant myocarditis – were the predominant manifestation of COVID-19. We hypothesize that many of COVID-19 pulmonary and extra-pulmonary manifestations manifest not only from direct SARS-CoV-2 toxicity in select tissues, but also its interaction with a complex interplay of host factors including genetic and epigenetic modifications of crucial genes – Angiotensin Converting Enzyme 2, Angiotensin Converting Enzyme 1, and Transmembrane protease serine-2 – and their respective affiliation with multiple signaling cascades with resultant dysregulated proinflammatory effects.
Keywords
<p>COVID-19; Cardiac Manifestations</p>
Article Details
1. Introduction
Towards the end of 2019, a surge of unexplained cases of pneumonia were confirmed in Wuhan, China [1]. A novel coronavirus, Severe Acute Respiratory Syndrome (SARS) Coronavirus-2 (SARS-CoV-2) was found to be responsible for the outbreak. The coronavirus family is diverse and causes disease ranging from the common cold to more severe pathologies, including Severe Acute Respiratory Syndrome (SARS) and Middle East Respiratory Syndrome (MERS), with the latter two having zoonotic tropism and reservoir. Since the incident cases, SARS-CoV-2 has spread rapidly and has been epidemiologically termed a pandemic. While initial research efforts were directed towards understanding the epidemiological spread of the virus, these efforts have shifted to understanding not only the pathobiology and pathophysiology, but also the therapeutic management of the infection.
Much like the spectrum of diseases of other members of the coronavirus family, SARS-CoV-2 infection has a spectrum of clinical presentations ranging from asymptomatic carriers, to mild shortness of breath, to severe critical outcomes, including refractory hypoxia, acute respiratory distress syndrome, and cytokine storm with multiorgan failure. While respiratory symptoms have been a prominent clinical finding in SARS-CoV-2, the spectrum of respiratory presentations has been further underscored not only by a variety of non-classical, non-respiratory presentations including diarrhea, hepatitis, venous thromboembolism, headache, anosmia, ageusia, and cerebrovascular accident, but also myocardial infarction, myopericarditis, and pericardial effusion that may occur with or without concomitant respiratory symptomatology. While underlying cardiovascular disease has been associated with increased morbidity and mortality, SARS-CoV-2 has not only been shown to exacerbate underlying cardiovascular disease with resultant myocardial infarction, but also result in primary cardiac involvement (myocarditis, pericarditis, pericardial effusion), even in the absence of respiratory symptomatology [2]. To that end, we present two cases of SARS-CoV-2 infection, one complicated by pericarditis with associated pericardial effusion with tamponade physiology, and another complicated by severe myopericarditis with a dramatically elevated troponin.
2. Case Presentations
2.1 Case 1
An 82-year-old female with a history of hypertension, chronic pulmonary obstructive disease without home oxygen requirements, paroxysmal atrial fibrillation (not on anticoagulation due to recurrent falls), and Sick Sinus Syndrome status post dual chamber pacemaker placement due to symptomatic pauses (negative cardiac catherization for obstruction), initially presented with a five day history of productive cough, fever, chills, and intermittent diarrhea. Additional history was notable for a family history of hypertension and a personal history that was negative for tobacco, alcohol, or illicit drug use. On admission, hemodynamics were stable with a preserved heart rate (60s), blood pressure (110s/60s), and oxygen saturation on room air (95%). Initial physical examination revealed a well-developed, well-nourished but tired appearing elderly woman with dry oral mucosa. Initial cardiac examination was unremarkable with appreciated S1 and S2 heart sounds, preserved pulses, and no evidence of pulsus paradoxus. Pulmonary examination was unremarkable. Laboratory diagnostics were notable for a negative troponin but an elevated pro-BNP (12,115 pg/mL, elevated from 1,767 pg/mL three months prior). Complete blood count demonstrated leukopenia (2.8 WBC/μl) with no left shift, but an absolute neutrophil count of 1.8/μl and an absolute lymphocyte count of 0.6/μl, as well as a chronic, normocytic anemia. Metabolic panel was notable for a hypotonic, hypovolemic, hyponatremia (122 mEq/L) but was otherwise unremarkable. SARS-CoV-2 testing was noted to be positive. Adjunctive markers of SARS-CoV-2 infection, CRP (25.5 mg/L), ESR (20 mm/hr), LDH (286 U/L), and Ferritin (732.7 ng/mL) were all noted be elevated.
Diagnostics included an electrocardiogram (EKG) showing an atrial paced rhythm with prolonged PR interval, and inverted T waves in leads II, III, avF, V3, V4, V5, and V6 with low voltage in the limb but not precordial leads, and no evidence of electrical alternans (Figure 1a). Chest x-ray revealed cardiomegaly with bibasilar atelectasis (Figure 2). Transthoracic echocardiogram revealed an ejection fraction of 50-55%, mildly dilated left atrium, abnormal hypokinetic apex, and small pericardial effusion globally located around the entire heart with no tamponade physiology (Figure 3a). Given her SARS-CoV-2 positivity and lack of clinical evidence for hydroxychloroquine/azithromycin, she was managed conservatively. In the setting of her elevated proBNP and EKG changes, she underwent repeat Echocardiography, which demonstrated an increased size of the pericardial effusion with evidence of early diastolic collapse of the right ventricle, suggestive of tamponade physiology (Figure 3b-c). In this setting, coupled with the development of ongoing symptomatic palpitations, tachycardia, chest tightness, increased PVC burden (Figure 1b), and ongoing shortness of breath she was recommended for pericardiocentesis. Pericardiocentesis was performed with removal of 400cc of straw colored, clear fluid and a pericardial drain was placed. She was placed on prophylactic amiodarone. Pericardial fluid analysis demonstrated a transudative, culture negative fluid (Table 1) with reactive mesothelial cells, macrophages, and rare degenerated inflammatory cells on a background of fibrinous material, with no evidence of malignant cells (Figure 4). Repeat echocardiograms showed interval improvement in the pericardial fluid collection with resolution of diastolic collapse. The patient improved symptomatically and was subsequently discharged home.
2.2 Case 2
A 26-year-old man with past medical history of mild intermittent asthma and daily tobacco and marijuana use presented with progressive chest pain of seven days duration that acutely worsened one day prior to admission. He described a constant, non-radiating, heavy pressure, 5/10 in intensity that was aggravated by ambulation and supine positioning. His symptoms were not pleuritic in nature and were not alleviated with home albuterol. During this time, he also noted dyspnea upon exertion, orthopnea, subjective chills, nausea, generalized malaise, and occasional dizziness. His family history was notable for a mother with coronary disease requiring percutaneous intervention in her 50s. On presentation he was afebrile, with a preserved heart rate (60-80s) and blood pressure (120s/80s), with preserved saturations on room air. Physical exam demonstrated a well-developed male in no acute distress. Cardiac auscultation demonstrated a regular rate and rhythm but an associated friction rub. Laboratory diagnostics demonstrated an unremarkable Complete blood count but a metabolic panel that was remarkable for an elevated AST (120 U/L), but normal ALT (33 U/L), lactate of 3.0 mmol/L, and a troponin on presentation was elevated to 21.2 ng/mL. Though SARS-CoV-2 PCR was negative, there was clinical suspicion for an atypical presentation for which SARS-CoV-2 inflammatory were collected – CRP (9.84 mg/L), Ferritin (114 ng/mL), Interleukin-6 (<5), ESR (5 mm/hr), CK (844 U/L), and LDH (404 U/L). Chest radiography remained unremarkable (Figure 5).
Electrocardiogram revealed subtle Q waves in V2 with mild ST depressions in inferior leads II, III, avF, and V5 (Figure 6a) with repeat EKG showing a marked ST abnormality (Figure 6b), suggestive of inferolateral subendocardial injury versus reciprocal changes to acute infarct with ST depressions in inferior leads. During his hospital course his ALT, Lactate, and Troponin continued to rise to 723 U/L, greater than 15 mmol/L, and 83.5 ng/mL respectively. Additional imaging included a negative Magnetic resonance imaging/angiography of chest and abdomen to rule out a dissection and a transthoracic echo that demonstrated a preserved ejection fraction (60%), no regional wall motion abnormalities, and no evidence of pericardial effusion. Heparin drip was deferred due to concern for myopericarditis and the theoretical risk of bleeding and he was initiated on low dose Lisinopril, high dose Aspirin, ibuprofen, and colchicine. He subsequently underwent cardiac catheterization that demonstrated clean coronaries with a LVEDP of 22mmHg (Figure 7). Troponin continued to rise, peaking at >200 ng/mL. On the following day the patient suffered a cardiac arrest with rhythm analysis revealing multiple episodes of pulseless ventricular tachycardia, ventricular fibrillation, and pulseless electrical activity, with subsequent ROSC. He was started on levophed, epinephrine, and vasopressin for blood pressure support and lidocaine for arrhythmia management. Repeat bedside echocardiogram demonstrated a newly reduced EF to <10% and the patient was subsequently initiated on VA-ECMO. Unfortunately, the patient suffered multiple subsequent PEA arrests and passed. Full viral panel was negative for parainfluenza (1, 2, 3, and 4), B pertussis, B parapertussis, C pneumoniae, Adenovirus DNA, RSV PCR, Rhinovirus/enterovirus RNA, Coronavirus OC43/HKU1/229E/NL63, Influenza A and B, Human metapneumovirus, and M pneumoniae DNA. Repeat SARS-CoV2 was negative with Human RNAse P positive by PCR.
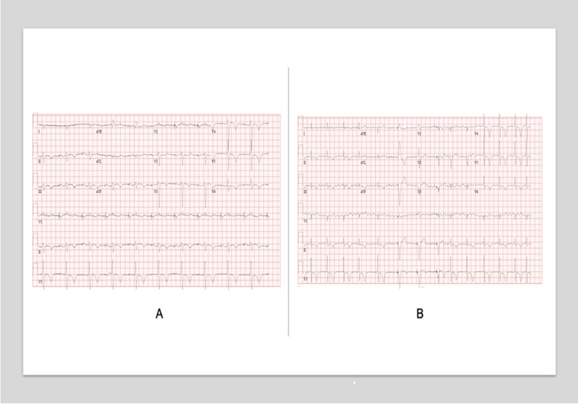
Figure 1: Serial Electrocardiograms in a patient with Pericarditis complicated by pericardial effusion and tamponade physiology. A, Initial EKG on presentation demonstrating an atrial-paced rhythm with noted left axis deviation, delayed R-wave progression, and diffuse T-wave inversions. B, EKG during the hospital course with noted increased ectopy.
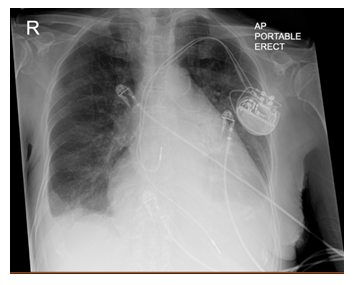
Figure 2: Chest Radiograph. Chest radiograph demonstrates a Portable AP film with evidence of cardiomegaly, left atrial enlargement, bibasilar atelectasis, and a small right-sided pleural effusion. Dual chamber pacemaker is noted in the left chest wall with leads in the right atrium and right ventricle.
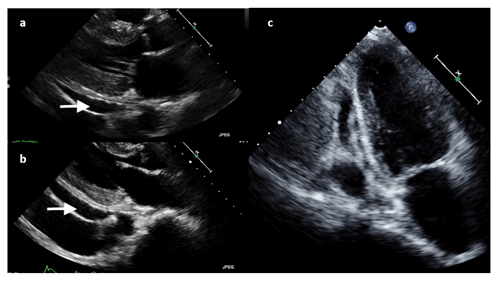
Figure 3: Transthoracic Echocardiography. A, Echocardiogram revealed an ejection fraction of 50-55%, mildly dilated left atrium, abnormal hypokinetic apex, and small pericardial effusion globally located around the entire heart with no tamponade physiology. B, C Echocardiogram demonstrated an increased size of the pericardial effusion with evidence of early diastolic collapse of the right ventricle, suggestive of tamponade physiology in (b) parasternal long-axis and (c) apical four-chamber views.
|
Test |
Patient |
|
Color |
Straw colored |
|
pH |
> 9.00 |
|
WBC with differential |
103 (1 Neutrophil, 16 Lymphocytes, 83 Mononuclear) |
|
RBC |
1000 |
|
Glucose |
> 40 |
|
Protein |
3.9 (Albumin 2.2) |
|
LDH |
< 200 |
|
Specific gravity |
1.025 |
Table 1: Pericardial fluid Analysis.
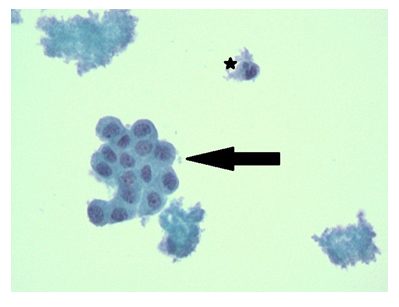
Figure 4: Papanicolaou Staining of Pericardial Fluid. Papanicolaou staining demonstrates clumped acellular fibrin with admixed red blood cells and fragments of reactive mesothelial cells (black arrow) with rare neutrophil/ inflammatory cells (asterixis). 400x Magnification.
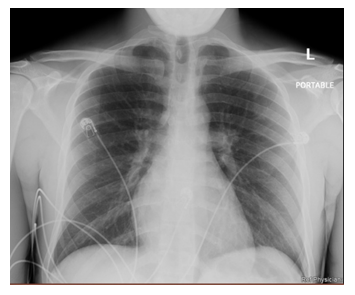
Figure 5: Chest Radiograph. Unremarkable chest radiography with no evidence of acute intrathoracic pathology.
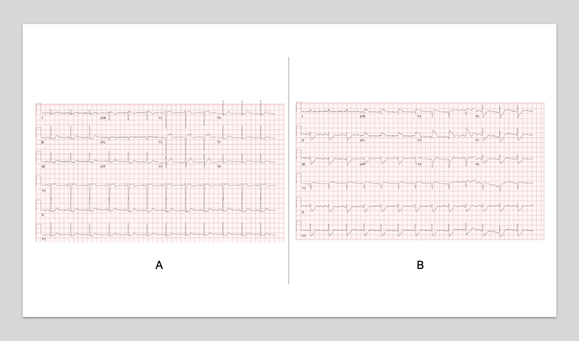
Figure 6: Serial Electrocardiograms. A, Electrocardiogram revealed subtle Q waves in V2 with mild ST depressions in inferior leads II, III, avF, and V5. B, Repeat electrocardiogram demonstrates marked ST abnormality, suggestive of inferolateral subendocardial injury versus reciprocal changes to acute infarct with ST depressions in inferior leads.
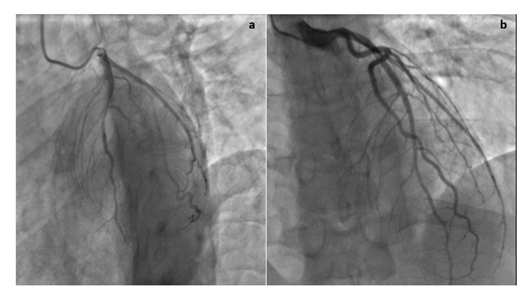
Figure 7: Cardiac Catheterization. Cardiac catheterization demonstrates no evidence of coronary artery disease in the (a) Left anterior oblique and (b) Right anterior oblique views.
3. Discussion
In this case series, we present two patients with laboratory confirmed or highly presumed SARS-CoV-2 infection who presented with cardiac complications. In the original SARS epidemic, SARS-CoV-1 infection was associated with transient cardiac arrythmia, cardiac arrest, and subclinical systolic and diastolic dysfunction [3], whereas acute myocarditis and acute onset systolic heart failure were reported with MERV-CoV [4]. Indeed, SARS-CoV-2, along with SARS-CoV-1 and MERV-CoV, are three beta-coronavirus that cause severe infections in the human species, with the remainder causing the common cold. To mediate downstream effects these zoonotic, positive-sense, enveloped RNA viruses must bind to cell surface receptors and undergo receptor-mediated endocytosis. In the case of SARS-CoV-1 and SARS-CoV-2, molecular sequencing has demonstrated over 75% sequence homology, with nearly identical sequences in the highly conserved viral Spike (S)-glycoprotein which plays a critical role in binding to the ACE2 receptor [5]. The S-protein, however, requires priming by transmembrane protease serine-2 (TMPRSS2), a protease exploited by multiple pathogenic viruses, including not only SARS-CoV-1 and SARS-CoV-2, but also Influenza, which serendipitously also utilizes ACE2 as its functional receptor and is capable of multiple cardiac sequalae – acute myocardial infarction, heart failure exacerbation, and TMPRSS2 subsequently cleaves the S-protein into two subunits, S1 and S2, with the S1 carboxy-terminal domain functioning as the receptor binding domain, forming an S1-trimeric head with an S2 stalk. Single nucleotide polymorphisms in the underlying genetic sequence have resulted in amino acid substitutions at critical sites and thus different levels of affinity amongst different strains of each individual virus with presumed dramatic effects on viral transmissibility and fitness 6]. Intriguingly, even in the absence of ACE2 or TRMPSS2, SARS-CoV-1 and SARS-CoV-2 may still enter cells, presumably due to the presence of an alternative receptor or protease-mediated cleavage by cathepsins [7, 8]. Indeed, prior studies have demonstrated that both ACE2 and TMPRSS2 (albeit at significantly lower levels) is expressed on both endothelial surfaces as well as cardiac tissues [9-11], suggesting that direct cardiac myocyte toxicity is plausible.
Intriguingly, the expression of ACE2 has been shown to regulated not only by underlying single nucleotide polymorphisms in coding and non-coding regions of the ACE2 gene itself [12, 13], but also by underlying epigenetic (e.g. DNA hypomethylation/hypermethylation and histone acetylation/deacetylation) variability [14-16]. As an example of this epigenetic variability and its association with disease states, systemic lupus erythematosus, hypertension, as well as patient-dependent and environmental factors, including highly methylated diets, lack of physical activity, and psychosocial stressors have been shown to be associated with epigenetic variability of the ACE2 promoter [15, 17]. Similar reports demonstrated upregulated ACE2 transcription and translation in human cardiac myocytes in the context of idiopathic and ischemic cardiomyopathy [18]. Furthermore, polymorphisms of additional genes, including the insertion/ deletion polymorphism of Angiotensin Converting Enzyme 1 (ACE1), which has been associated not only with an increased risk of hypertension, heart failure, and acute coronary syndrome [19], but also with sepsis and ARDS, including during the SARS epidemic [20-24] has been associated with modulation of ACE2 levels [25]. Similarly, though TMPRSS2 is expressed at significantly lower levels in cardiac myocytes and endothelial cells compared to respiratory mucosa [11], single nucleotide polymorphisms were shown to be associated with an increased risk of infection, specifically during the H1N1 pandemic (which also uses the ACE2 receptor) [26]. Intriguingly, epidemiologic studies have demonstrated a possible male predominance of SARS-CoV-2 as well as influenza [27], with reports noting that the TMPRSS2 promoter contains an Androgen-receptor element that facilitates transcription, suggesting that increased interaction between androgen receptor and host androgens, could subsequently co-localize to the TMPRSS2 promoter region and increase surface levels of TMPRSS2 with resultant increased predisposition to infection [11, 27]. Despite cardiac expression of ACE2 and to a lesser extent, TMPRSS2, multiple prior studies with SARS demonstrated no evidence of SARS-CoV-1 expression in cardiac myocytes or human heart tissues [28, 29]. In contrast, at least one study demonstrated the presence of SARS-CoV-1 RNA on autopsy in a minority of patients with severe SARS, a finding that was associated with increased macrophage infiltration, associated myocardial damage, and reduced levels of ACE2 protein [30], a known sequela of SARS-CoV-1 infection.
In this limited case series, we present two patients with confirmed or high-suspicion for SARS-CoV-2 infection, whose clinical course was notable by mild pericarditis complicated a progressive pericardial effusion progressing to tamponade physiology and a case of severe fulminant myopericarditis complicated by cardiac failure necessitating initiation of VA-ECMO. Indeed, multiple recent studies have noted a spectrum of cardiac injury as a consequence of SARS-CoV-2 infection [31-34], however the exact mechanism remains to be determined. Though the human heart and more specifically, cardiac myocytes, express ACE2, similar to SARS-CoV-1, pathological data seemed to suggest that even in cases of myocarditis, there appeared to minimal evidence of direct myocardial damage, with scant, scattered mononuclear infiltrate [35]. While there was no evidence of direct SARS-CoV-2 infiltration in cardiac myocytes, [34] there was evidence of direct pericyte infection and resultant capillary endothelial cell and microvascular dysfunction [36]. In contrast, at least two reports have demonstrated that in cases of SARS-CoV-2 induced fulminant, severe myocarditis, there appears to be marked lymphocytic inflammatory infiltrates with associated interstitial edema and only limited focal necrosis, with a persistent inability to detect viral sequences [33].
Thus, while the presence of ACE2 within the heart would seem to suggest a potential for direct SARS-CoV-2 mediated damage to cardiac myocytes, pathological specimens have failed to demonstrate the presence of viral sequences, suggesting an alternative etiology for SARS-CoV-2 induced myocyte damage such as systemic inflammation/cytokine release syndrome. This scenario is characterized by elevated levels of inflammatory markers (e.g. ferritin, erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), D-Dimer, LDH, Interleukin-6 (IL-6), and Interleukin-1 (IL1)), as mediated by SARS-CoV-2 induced impaired immune responsiveness, imbalance of the renin-angiotensin aldosterone system, and thrombotic microangiopathy [37]. Prior reports have noted that SARS-CoV-2 induced several inflammatory cytokines (IL-1, IL-6, IFN-y, MCP-1) [38]. In addition, SARS-CoV-2 mediated downregulation of the ACE2 receptor and the subsequent activation of the Renin-Angiotensin-Aldosterone system. In normal physiologic conditions, ACE2 functions to cleave Angiotensin I and Angiotensin II, not only preventing Angiotensin I and Angiotensin II mediated effects, but also generating Ang1-9 and Ang1-7, respectively, peptide fragments that have a variety of effects that are antagonistic to that of angiotensin II [39]. Decreased levels of Ang1-7 have been shown to be associated with increased TNF-alpha production resulting in a pro-inflammatory state [40]. Furthermore, the increased renin-angiotensin-aldosterone signaling as a result of ACE2 downregulation results in increased vascular permeability and cardiac remodeling/fibrosis [40]. Taken together these studies suggest that cardiac myocytes and the resultant cardiac dysfunction may merely be innocent bystanders in a developing hyperinflammatory state, analogous to the effect of SARS-CoV-2 and its effect on the pulmonary vasculature. While more readily apparent in critically ill patients with resultant organ failure, there may exist a spectrum of systemic inflammatory states ranging from mild to severe with effects on multiple organ systems including the gastrointestinal tract, manifesting as elevated liver enzymes, to the cardiovascular system, manifesting as myocarditis, pericarditis with associated pericardial effusion, or myocardial infarction, based on as of yet undetermined genetic susceptibilities.
The patients presented in this mini-series demonstrated divergent pathologies - pericarditis complicated by a transudative pericardial effusion with progression to tamponade physiology necessitating pericardiocentesis (Case 1) and fulminant myocarditis with markedly elevated troponins, clean coronary angiography, and progression to overt cardiac failure necessitating VA-ECMO (Case 2) - highlighting the spectrum of clinical presentations of cardiac involvement as well as the varied ages of affected individuals. Surprisingly in this mini-series, a relatively young, otherwise healthy male had the more severe outcome, and ultimately succumbed to COVID-19. Like many cases of SARS-CoV-2, our patients demonstrated an antecedent history of mild upper respiratory symptoms with progression to associated shortness of breath and overt cardiac involvement. Both cases had relatively clear chest radiography, often seen in COVID-19 infections, as well as a progressive worsening of symptoms prior to admission that cascaded into their respective pathologies. Our cases serve to highlight the spectrum of severity of SARS-CoV-2 infection from a cardiac perspective.
4. Conclusion
SARS-CoV-2 has been shown to be a formidable virus that can lead to a spectrum of clinical presentations ranging from asymptomatic infection to hypoxemic respiratory failure and acute respiratory distress syndrome. In a significant number of cases, multi-organ dysfunction, likely due to a combination of direct viral cytopathological effects due to direct SARS-CoV-2 infection and/ or due to an indirect elaborated viral-induced abrogation of innate and adaptive immune responses with a subsequent elaborated inflammatory response, results in significant morbidity and mortality. While pulmonary manifestations of COVID-19 are the dominant clinical finding, emerging data has found concomitant cardiac pathology that significantly contributes to COVID-19 morbidity and mortality, ranging from pericarditis, pericardial effusion, and overt fulminant myopericarditis. While therapeutic strategies targeting ACE2 and TMPRSS2 could prevent viral cell binding, the temporal relationship between therapeutic administration and the timing of infection, as well as the roles these proteins play in normal cell and cardiovascular and cardiopulmonary physiology are important considerations. Alternatively, therapies targeting the viral S-protein may be more prudent, but in the case of severe infection with cardiac involvement or multiple organ dysfunction syndrome, therapies targeting the underlying dysregulation of the innate and adaptive immune systems as well as the associated cytokine storm may be necessary.
References
- Lu H, Stratton C W, Tang Y-W. Outbreak of pneumonia of unknown etiology in Wuhan, China: The mystery and the miracle. Journal of Medical Virology 92 (2020): 401-402.
- Bansal M. Cardiovascular disease and COVID-19. Diabetes & Metabolic Syndrome 14 (2020): 247-250.
- Yu C, Wong R S, Wu E B, et al. Cardiovascular complications of severe acute respiratory syndrome. Postgraduate Medical Journal 82 (2006): 140-144.
- Alhogbani T. Acute myocarditis associated with novel Middle East respiratory syndrome coronavirus. Annals of Saudi Medicine 36 (2016): 78-80.
- Nadeem M S, Zamzami M A, Choudhry H, et al. Origin, Potential Therapeutic Targets and Treatment for Coronavirus Disease (COVID-19). Pathogens 9 (2020): 307.
- Cui J, Li F, Shi Z-L. Origin and evolution of pathogenic coronaviruses. Nature Reviews Microbiology, 17 (2019): 181-192.
- Huang I-C, Bosch B J, Li F, et al. SARS coronavirus, but not human coronavirus NL63, utilizes cathepsin L to infect ACE2-expressing cells. The Journal of Biological Chemistry 281 (2006): 3198-3203.
- Li F. Receptor recognition and cross-species infections of SARS coronavirus. Antiviral Research, 100 (2013): 246-254.
- Hamming I, Timens W, Bulthuis M L C, et al. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. The Journal of Pathology 203 (2004): 631-637.
- Harmer D, Gilbert M, Borman R, et al. Quantitative mRNA expression profiling of ACE 2, a novel homologue of angiotensin converting enzyme. FEBS Letters 532 (2002): 107-110.
- Stopsack K H, Mucci L A, Antonarakis E S, et al. TMPRSS2 and COVID-19: Serendipity or Opportunity for Intervention?. Cancer Discovery (2020).
- Chiu R W K, Tang N L S, Hui D S C, et al. ACE2 gene polymorphisms do not affect outcome of severe acute respiratory syndrome. Clinical Chemistry 50 (2004): 1683-1686.
- Itoyama S, Keicho N, Hijikata M, et al. Identification of an alternative 5’-untranslated exon and new polymorphisms of angiotensin-converting enzyme 2 gene: Lack of association with SARS in the Vietnamese population. American Journal of Medical Genetics. Part A 136 (2005): 52-57.
- Chai P, Yu J, Ge S, et al. Genetic alteration, RNA expression, and DNA methylation profiling of coronavirus disease 2019 (COVID-19) receptor ACE2 in malignancies: A pan-cancer analysis. Journal of Hematology & Oncology 13 (2020): 43.
- Sawalha A H, Zhao M, Coit P, et al. Epigenetic dysregulation of ACE2 and interferon-regulated genes might suggest increased COVID-19 susceptibility and severity in lupus patients. Clinical Immunology (Orlando, Fla.) 215 (2020): 108410.
- Tikoo K, Patel G, Kumar S, et al. Tissue specific up regulation of ACE2 in rabbit model of atherosclerosis by atorvastatin: Role of epigenetic histone modifications. Biochemical Pharmacology 93 (2015): 343-351.
- Holmes L, Lim A, Comeaux C R, et al. DNA Methylation of Candidate Genes (ACE II, IFN-γ, AGTR 1, CKG, ADD1, SCNN1B and TLR2) in Essential Hypertension: A Systematic Review and Quantitative Evidence Synthesis. International Journal of Environmental Research and Public Health 16 (2019).
- Goulter A B, Goddard M J, Allen J C, et al. ACE2 gene expression is up-regulated in the human failing heart. BMC Medicine 2 (2004): 19.
- Di Pasquale P, Cannizzaro S, Paterna S. Does angiotensin-converting enzyme gene polymorphism affect blood pressure? Findings after 6 years of follow-up in healthy subjects. European Journal of Heart Failure, 6 (2004): 11-16.
- Adamzik M, Frey U, Sixt S, et al. ACE I/D but not AGT (-6)A/G polymorphism is a risk factor for mortality in ARDS. The European Respiratory Journal 29 (2007): 482-488.
- Cardinal-Fernández P, Ferruelo A, El-Assar M, et al. Genetic predisposition to acute respiratory distress syndrome in patients with severe sepsis. Shock (Augusta, Ga.) 39 (2013): 255-260.
- Itoyama S, Keicho N, Quy T, et al. ACE1 polymorphism and progression of SARS. Biochemical and Biophysical Research Communications 323 (2004): 1124-1129.
- Jerng J-S, Yu C-J, Wang H-C, et al. Polymorphism of the angiotensin-converting enzyme gene affects the outcome of acute respiratory distress syndrome. Critical Care Medicine 34 (2006): 1001-1006.
- Marshall R P, Webb S, Bellingan G J, et al. Angiotensin converting enzyme insertion/deletion polymorphism is associated with susceptibility and outcome in acute respiratory distress syndrome. American Journal of Respiratory and Critical Care Medicine 166 (2002): 646-650.
- Delanghe, J R, Speeckaert M M, De Buyzere M L. The host’s angiotensin-converting enzyme polymorphism may explain epidemiological findings in COVID-19 infections. Clinica Chimica Acta; International Journal of Clinical Chemistry 505 (2020): 192-193.
- Cheng Z, Zhou J, To K K-W, et al.. Identification of TMPRSS2 as a Susceptibility Gene for Severe 2009 Pandemic A(H1N1) Influenza and A(H7N9) Influenza. The Journal of Infectious Diseases 212 (2015): 1214-1221.
- Wambier C G, Goren A. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection is likely to be androgen mediated. Journal of the American Academy of Dermatology (2020).
- Ding Y, He L, Zhang Q, et al. Organ distribution of severe acute respiratory syndrome (SARS) associated coronavirus (SARS-CoV) in SARS patients: Implications for pathogenesis and virus transmission pathways. The Journal of Pathology 203 (2004): 622-630.
- Gu J, Gong E, Zhang B, et al. Multiple organ infection and the pathogenesis of SARS. The Journal of Experimental Medicine 202 (2005): 415-424.
- Oudit G Y, Kassiri Z, Jiang C, et al. SARS-coronavirus modulation of myocardial ACE2 expression and inflammation in patients with SARS. European Journal of Clinical Investigation 39 (2009): 618-625.
- Hu H, Ma F, Wei X, et al. Coronavirus fulminant myocarditis saved with glucocorticoid and human immunoglobulin. European Heart Journal (2020).
- Inciardi R M, Lupi L, Zaccone G, et al. Cardiac Involvement in a Patient With Coronavirus Disease 2019 (COVID-19). JAMA Cardiology (2020).
- Sala S, Peretto G, Gramegna M, et al. Acute myocarditis presenting as a reverse Tako-Tsubo syndrome in a patient with SARS-CoV-2 respiratory infection. European Heart Journal (2020).
- Yao X H, Li T Y, He Z C, et al. [A pathological report of three COVID-19 cases by minimally invasive autopsies]. Zhonghua Bing Li Xue Za Zhi = Chinese Journal of Pathology 49 (2020): E009.
- Xu Z, Shi L, Wang Y, et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. The Lancet. Respiratory Medicine 8 (2020): 420-422.
- Chen L, Li X, Chen M, et al. The ACE2 expression in human heart indicates new potential mechanism of heart injury among patients infected with SARS-CoV-2. Cardiovascular Research 116 (2020): 1097-1100.
- Henry B M, Vikse J, Benoit S, et al. Hyperinflammation and derangement of renin-angiotensin-aldosterone system in COVID-19: A novel hypothesis for clinically suspected hypercoagulopathy and microvascular immunothrombosis. Clinica Chimica Acta; International Journal of Clinical Chemistry 507 (2020): 167–173.
- Chen C, Zhou Y, Wang D W. SARS-CoV-2: A potential novel etiology of fulminant myocarditis. Herz, 45 (2020): 230-232.
- Gheblawi M, Wang K, Viveiros A, et al. Angiotensin-Converting Enzyme 2: SARS-CoV-2 Receptor and Regulator of the Renin-Angiotensin System: Celebrating the 20th Anniversary of the Discovery of ACE2. Circulation Research 126 (2020): 1456-1474.
- Maisch, B. Cardio-Immunology of Myocarditis: Focus on Immune Mechanisms and Treatment Options. Frontiers in Cardiovascular Medicine 6 (2019): 48.
- Davis J L, Moutinho V, Panageas K S, et al. A peripheral blood biomarker estimates probability of survival: The neutrophil–lymphocyte ratio in noncancer patients. Biomarkers in Medicine 10 (2016): 953-957.


 Impact Factor: * 5.3
Impact Factor: * 5.3 Acceptance Rate: 75.63%
Acceptance Rate: 75.63%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 
