An Unusual Skin Adverse Event after Switching from Intravenous to Subcutaneous VEDOLIZUMAB Therapy in Patients with Ulcerative Colitis
Anne-Sophie Peaucelle1, Claire Gay2, Marine-Alexia Lefevre1, Mathilde Barrau1, Gilles Boschetti2, Stéphane Nancey2 and Xavier Roblin1*
1Department of Gastroenterology, CHU Saint Etienne, Saint Etienne, France
2Department of Gastroenterology, CH Lyon Sud, Hospices Civils de Lyon, Lyon, France and Inserm U1111, Lyon
*Corresponding Author: Xavier Roblin, Department of Gastroenterology, CHU Saint Etienne, Saint Etienne, France.
Received: 11 December 2022; Accepted: 25 January 2023; Published: 10 February 2023
Article Information
Citation: Anne-Sophie Peaucelle, Claire Gay, Marine-Alexia Lefevre, Mathilde Barrau, Gilles Boschetti, Stéphane Nancey and Xavier Roblin. An Unusual Skin Adverse Event after Switching from Intravenous to Subcutaneous VEDOLIZUMAB Therapy in Patients with Ulcerative Colitis. Archives of Clinical and Medical Case Reports 7 (2023): 77-79.
View / Download Pdf Share at FacebookAbstract
Here, we report for the first time 2 cases of patients with ulcerative colitis (UC) (55 and 35 years old women) in clinical remission under maintenance regimen under vedolizumab infusions 300 mg every 8 weeks. After having switched the patients to subcutaneous (sc) vedolizumab 108 mg every two weeks, both patients experienced urticaria like skin lesions located on the site of drug injection with favorable outcomes. Both were then back switched to infusion of vedolizumab and surprisingly developed again at the same previous spot similar and reversible urticaria like skin lesions.
Keywords
Adverse Effect; Subcutaneous; Ulcerative Colitis; Vedolizumab
Adverse Effect articles; Subcutaneous articles; Ulcerative Colitis articles; Vedolizumab articles
Adverse Effect articles Adverse Effect Research articles Adverse Effect review articles Adverse Effect PubMed articles Adverse Effect PubMed Central articles Adverse Effect 2023 articles Adverse Effect 2024 articles Adverse Effect Scopus articles Adverse Effect impact factor journals Adverse Effect Scopus journals Adverse Effect PubMed journals Adverse Effect medical journals Adverse Effect free journals Adverse Effect best journals Adverse Effect top journals Adverse Effect free medical journals Adverse Effect famous journals Adverse Effect Google Scholar indexed journals Ultrasound articles Ultrasound Research articles Ultrasound review articles Ultrasound PubMed articles Ultrasound PubMed Central articles Ultrasound 2023 articles Ultrasound 2024 articles Ultrasound Scopus articles Ultrasound impact factor journals Ultrasound Scopus journals Ultrasound PubMed journals Ultrasound medical journals Ultrasound free journals Ultrasound best journals Ultrasound top journals Ultrasound free medical journals Ultrasound famous journals Ultrasound Google Scholar indexed journals Subcutaneous articles Subcutaneous Research articles Subcutaneous review articles Subcutaneous PubMed articles Subcutaneous PubMed Central articles Subcutaneous 2023 articles Subcutaneous 2024 articles Subcutaneous Scopus articles Subcutaneous impact factor journals Subcutaneous Scopus journals Subcutaneous PubMed journals Subcutaneous medical journals Subcutaneous free journals Subcutaneous best journals Subcutaneous top journals Subcutaneous free medical journals Subcutaneous famous journals Subcutaneous Google Scholar indexed journals Ulcerative Colitis articles Ulcerative Colitis Research articles Ulcerative Colitis review articles Ulcerative Colitis PubMed articles Ulcerative Colitis PubMed Central articles Ulcerative Colitis 2023 articles Ulcerative Colitis 2024 articles Ulcerative Colitis Scopus articles Ulcerative Colitis impact factor journals Ulcerative Colitis Scopus journals Ulcerative Colitis PubMed journals Ulcerative Colitis medical journals Ulcerative Colitis free journals Ulcerative Colitis best journals Ulcerative Colitis top journals Ulcerative Colitis free medical journals Ulcerative Colitis famous journals Ulcerative Colitis Google Scholar indexed journals Nasal Oxygen articles Nasal Oxygen Research articles Nasal Oxygen review articles Nasal Oxygen PubMed articles Nasal Oxygen PubMed Central articles Nasal Oxygen 2023 articles Nasal Oxygen 2024 articles Nasal Oxygen Scopus articles Nasal Oxygen impact factor journals Nasal Oxygen Scopus journals Nasal Oxygen PubMed journals Nasal Oxygen medical journals Nasal Oxygen free journals Nasal Oxygen best journals Nasal Oxygen top journals Nasal Oxygen free medical journals Nasal Oxygen famous journals Nasal Oxygen Google Scholar indexed journals treatment articles treatment Research articles treatment review articles treatment PubMed articles treatment PubMed Central articles treatment 2023 articles treatment 2024 articles treatment Scopus articles treatment impact factor journals treatment Scopus journals treatment PubMed journals treatment medical journals treatment free journals treatment best journals treatment top journals treatment free medical journals treatment famous journals treatment Google Scholar indexed journals CT articles CT Research articles CT review articles CT PubMed articles CT PubMed Central articles CT 2023 articles CT 2024 articles CT Scopus articles CT impact factor journals CT Scopus journals CT PubMed journals CT medical journals CT free journals CT best journals CT top journals CT free medical journals CT famous journals CT Google Scholar indexed journals surgery articles surgery Research articles surgery review articles surgery PubMed articles surgery PubMed Central articles surgery 2023 articles surgery 2024 articles surgery Scopus articles surgery impact factor journals surgery Scopus journals surgery PubMed journals surgery medical journals surgery free journals surgery best journals surgery top journals surgery free medical journals surgery famous journals surgery Google Scholar indexed journals Laryngectomy articles Laryngectomy Research articles Laryngectomy review articles Laryngectomy PubMed articles Laryngectomy PubMed Central articles Laryngectomy 2023 articles Laryngectomy 2024 articles Laryngectomy Scopus articles Laryngectomy impact factor journals Laryngectomy Scopus journals Laryngectomy PubMed journals Laryngectomy medical journals Laryngectomy free journals Laryngectomy best journals Laryngectomy top journals Laryngectomy free medical journals Laryngectomy famous journals Laryngectomy Google Scholar indexed journals Vedolizumab articles Vedolizumab Research articles Vedolizumab review articles Vedolizumab PubMed articles Vedolizumab PubMed Central articles Vedolizumab 2023 articles Vedolizumab 2024 articles Vedolizumab Scopus articles Vedolizumab impact factor journals Vedolizumab Scopus journals Vedolizumab PubMed journals Vedolizumab medical journals Vedolizumab free journals Vedolizumab best journals Vedolizumab top journals Vedolizumab free medical journals Vedolizumab famous journals Vedolizumab Google Scholar indexed journals
Article Details
1. Introduction
Vedolizumab, a monoclonal antibody that inhibits the gut-selective α4β7 integrin, has been approved for many years now using an intravenous administration in UC cases, with a high safety and tolerance profile [1]. Recently, the VISIBLE 1 study [2] showed that sc vedolizumab was non inferior and represents an alternative in the maintenance treatment for patients with moderately to severely active UC. The French National Association for medications (ANSM) approved the use of the sc vedolizumab 108 mg every two weeks following an iv vedolizumab induction at weeks 0 and 2, or as a relay for patients in remission under maintenance treatment with iv vedolizumab 300 mg every 8 weeks. During the last past months, in our centers we started switching UC patients in clinical and endoscopic remission (Partial Mayo score 0, endoscopic Mayo score 0 and fecal calprotectin level < 150 ug/g) from iv maintenance vedolizumab therapy to sc treatment.
2. Case Report
2.1 Case 1
A 55 years old woman was diagnosed with UC in 1997. She had no medical history of urticaria. After experiencing a drug failure with two different tumor necrosis factor antagonists (anti- TNFs), a treatment with Vedolizumab was started in June 2020 according to the usual regime of induction (300 mg at W0, W2 and W6) and maintenance (300 mg every 8 weeks). In September 2021, the patient was in endoscopic and clinical remission under IV Vedolizumab therapy which was perfectly tolerated and wished switching to the sc vedolizumab for convenient reasons. Meeting the VISIBLE 1 criteria, the patient received the first sc injection of vedolizumab (dose 108 mg) in October 2021 by an IBD specialized nurse at home 8 weeks after the last drug infusion. Soon, she complained about occurrence of urticaria-like skin reactions (with erythema and pruritus) located on the puncture point on her abdominal wall. The lesion disappeared spontaneously in few days but relapsed at every SC vedolizumab injection. Given the skin-induced disturbances, it was proposed to backswitch to iv vedolizumab infusion. Therefore, 2 weeks after the last SC injection, she received an initial iv vedolizumab infusion of 300 mg in 30 minutes followed by one hour of follow-up. At this time, the vedolizumab trough levels was 29.7 ug/mL with no antibody detectable (using a drug-sensitive method) [3]. Forty-five minutes after the end of infusion, she experienced a relapse of urticaria-like skin lesion, on the right side of the umbilical region of her abdomen, surprisingly located on one of the sites she used previously for drug sc injection (Figure 1). The lesion was localized, raised, edematous, and itchy. No other sign of hypersensitivity was recorded, no other location of urticaria on the whole body, no hemodynamic failure, or associated bronchospasm. A treatment with iv anti-histaminic was proposed and despite both the pruritus as well as the skin edema disappeared, an erythematous skin eruption remained detectable for few days. The patient was offered a medical visit with a Dermatologist-Allergist one month later for further investigations. All the intradermal test using low dose of vedolizumab and patch test testing polysorbate 80 (a common excipient of both sc and iv forms) were negative. Given the efficacy of vedolizumab to control UC and the non-severe induced skin reaction it was decided to pursue the treatment under the same modalities, but to prevent a recurrence of skin reaction, an oral administration of anti-histaminic was proposed 2 days prior iv vedolizumab. Eight weeks later, as scheduled, vedolizumab infusion was again administered with a more slowly speed to improve tolerance. She experienced again similar skin urticaria lesions occurring 15 minutes before the end of the drug infusion, at the same spot (Figure 2) but of smaller size compared with that reported previously. The pruritus and skin edema entirely disappeared under topical steroid application but the erythema remained for next few days, exactly as during the first eruption. Since the tolerance of such adverse event was good, the treatment was pursued and the patient did not experience new skin reaction after the subsequent, with a previous therapy of anti-histaminics two days before and after the infusion, and application of dermocorticoids on the abdominal skin.
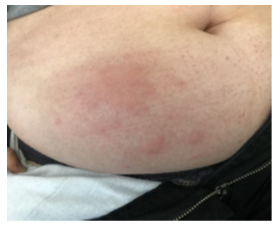
Figure 1: First urticarial skin reaction after reintroduction IV VEDOLIZUMAB, patient A.
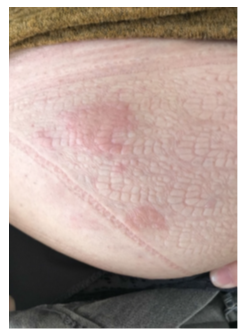
Figure 2: Second urticarial reaction, during the second infusion of VEDOLIZUMAB, patient A.
2.2 Case 2
The second case concerns a 35 years old woman, with a diagnosis of UC since 2000. The patient was firstly treated with golimumab and experienced a secondary loss of response and finally failed to the treatment despite dose intensification. She also failed to tofacitinib and finally started vedolizumab with regular infusions (300 mg every 8 weeks as maintenance regimen) in January 2021. Transition from intravenous to subcutaneous vedolizumab was proposed in September 2021. She rapidly experienced similar disturbing urticaria like skin reactions specifically located on the puncture sites and was finally backswitched to iv vedolizumab. Again, at the end of the vedolizumab infusion, she complained about urticaria skin lesions located on the abdomen, exactly in the spot of the former injection sites (Figure 3).
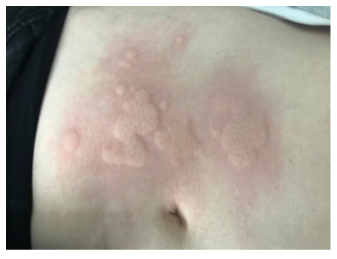
Figure 3: Urticarial reaction after reintroduction of IV VEDOLIZUMAB, patient B.
3. Discussion
In VISIBLE 1 Study, the incidence of injection-site reactions (ISR) (mainly rash, swelling, erythema, and pruritus) was reported more frequently in patients under subcutaneous vedolizumab (10.4%) when compared with intravenous vedolizumab (1.9%) or placebo (0%). These skin reactions were not associated with treatment limitations, most being mild, and do not result in drug discontinuation. Interestingly, these ISR trended down over time with the patient’s experience with sc injection. At our knowledge, these kind of -drug-reactivated urticarial skin reaction located on the puncture point after switching back from SC to IV vedolizumab has never been described so far. The physiopathological mechanism of this event is unknown. . Urticaria skin lesion represents a type 1 hypersensitivity reaction featured by antigen-specific IgE bound on the surface of mast cells and basophils. Interactions with antigens degranulates the cells leading to the release of various inflammatory mediators (with histamine, prostaglandin, leukotrienes) and results into fast vasodilatation, increased vascular permeability responsible for skin redness and edema. We practiced a cutaneous biopsy for the second patient, and the histological findings showed a significant lymphocytic infiltration, with an innate immune response (TNF and interleukine 1b) and an adaptative response TH2 (interleukines 4 and 13). The reasons why skin lesions were specifically located at the site of previous skin sc drug injection are of paramount interest but remain unclear. Even though the case of our two patients showed no sign of severity, it has been causing doubts about the safety of pursuing the treatment, especially for the case one, who preferred to stop entirely therapy. The prevalence of such urticaria-like skin adverse events occurring after transitioning from subcutaneous to intravenous vedolizumab should be considered in the future.
Funding
None.
Conflict of Interest
All authors have no personal conflicts of interest or financial relationships relevant to this publication.
Author Contributions
A.S.P., C.G., MB. : Clinical management of the patients in the gastroenterology units and paper writing; M.A.L: clinical consultation in allergology and histopathology; X.R., S.N. and G.B. : critical revision of the manuscript for important intellectual content.
Supplementary Data
Data statement of the two patients are available and the coordinator author can provide more data to the journal.
References
- Feagan BG, Rutgeerts P, Sands BE, et al. Vedolizumab as induction and maintenance therapy for ulcerative colitis. N Engl J Med 369 (2013): 699-710.
- Sandborn WJ, Baert F, Danese S, et al. Efficacy and Safety of Vedolizumab Subcutaneous Formulation in a Randomized Trial of Patients With Ulcerative Colitis. Gastroenterology 158 (2020): 562-572.e12.
- Wyant T, Yang L, Lirio RA, et al. Vedolizumab Immunogenicity with Long-Term or Interrupted Treatment of Patients with Inflammatory Bowel Disease. J Clin Pharmacol 61 (2021): 1174-1181.


 Impact Factor: * 5.3
Impact Factor: * 5.3 Acceptance Rate: 75.63%
Acceptance Rate: 75.63%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 
