Complete Response to Chemotherapy in A Patient with Unresectable Extrahepatic Cholangiocarcinoma
Ihsan Shaheen*, Miriam Lobo, Mahmoud Shahin, Jose Angel García, Atilio Navarro, Carlos Camps
Departamento de Oncología Médica, Consorcio Hospital General Universitario de Valencia, Valencia, Spain
*Corresponding Author: Dr. Ihsan Shaheen, Departamento de Oncología Médica, Consorcio Hospital General Universitario de Valencia, Valencia, Spain
Received: 01 September 2019; Accepted: 20 September 2019; Published: 28 November 2019
Article Information
Citation: Ihsan Shaheen, Miriam Lobo, Mahmoud Shahin, Jose Angel GarcÃa, Atilio Navarro, Carlos Camps. Complete Response to Chemotherapy in A Patient with Unresectable Extrahepatic Cholangiocarcinoma. Archives of Clinical and Medical Case Reports 3 (2019): 636-645.
View / Download Pdf Share at FacebookAbstract
Introduction: Biliary tract cancer (BTC) is an uncommon tumor with bad prognosis. There is no curative treatment for patients with unresectable disease at diagnosis. There is a limited experience with second-line chemotherapy in advanced BTC since clinical trials are difficult to perform due to the rarity and heterogeneity of these tumors. Recent molecular studies have increased our understanding of the pathogenetic mechanism that underly the development of cholangiocarcinoma. These will help us to determine the significance of molecular alterations that occur in this disease and will direct the development of targeted therapy.
Case Report: This case report describes a radiological complete response after three cycles of second-line chemotherapy with the combination of capecitabine and oxaliplatin in a patient with unresectable extrahepatic cholangiocarcinoma, after treatment with the combination of gemcitabine and cisplatin as first-line chemotherapy.
Conclusion: Our case suggests that selected patients may demonstrate very good responses to chemotherapy. There is an urgent need to identify different molecular subtypes that could direct management of these patients.
Keywords
<p>Cholangiocarcinoma; Targeted treatment; Cronic Inflamation</p>
Article Details
1. Introduction
BTC is a rare malignancy that carries bad prognosis. Complete surgical resection is the only curative treatment. However, even if curative intent surgery is applied to selected patients, 5-year survival rates still remain low (33.1% for bile duct cancer, 52.8% for ampullary cancer, and 41.6% for gallbladder cancer) [1]. BTC patients are often diagnosed with advanced stages and treated with systemic chemotherapy or palliative treatment settings rather than curative surgery. Gemcitabine has been the cornerstone of the systemic chemotherapy treatment of BTC. Moreover, recent advances in the development of chemotherapy regimens have gained additional survival benefits for patients with advanced BTC. Different combination chemotherapy regimens containing gemcitabine, antracyclines, platinum analogs, S1, etoposide, fluoropyrimidines and mitomycin C reported an ORR of 15–45% with median survival of 6–11 months and 1-year survival ranging from 20% to 40% [2, 3].
Despite the considerable progress that has been made towards molecular profiling of BTC, there remain considerable gaps in our understanding of carcinogenesis of these tumors. It is likely that the complex interactions between various signaling pathways hold the key to deepening our understanding of the basis of cancer heterogeneity and predicting susceptibility of individual tumors to targeted therapy.
2. Case Report
A 56-year-old male with a medical history of dyslipidemia and 10.8 pack-years smoker, presented in October 2016 with colicky right hypochondrium abdominal pain of one-month duration associated with obstructive jaundice. Diagnostic imaging consisted of abdominal ultrasound followed by magnetic resonance cholangiopancreatography (MRCP) demonstrating a 26 mm focal lesion isointense to the hepatic parenchyma in T2 sequence, trapping the cystic duct, the gallbladder infundibulum and the intrahepatic bile duct with retrograde secondary dilation. The lesion showed intense enhancement after intravenous contrast administration. Another 40 mm focal hepatic lesion slightly hyperintense in T2 sequence, adjacent to the gallbladder fundus was shown (Figure 1A-1C).
Endoscopic ultrasound was then performed in November 2016, describing a solid 18 x 25 mm hypoechoic lesion at the level of the common bile duct infiltrating the gallbladder, with probable vascular infiltration of the portal vein.
Fine needle aspiration cytology of the common bile duct tumor demonstrated groups of irregular and hyperchromatic nuclei compatible with adenocarcinoma (Figure 2 F). Further staging was performed to determine whether the patient was an appropriate candidate for surgery. Computerized Tomography (CT) scan confirmed severe dilation of the intrahepatic bile duct with stenosis in the common hepatic duct, with no distant lesions.
The patient underwent an exploratory laparotomy, showing the hepatic lesion adjacent to the gallbladder fundus, and another common bile duct tumor infiltrating the right portal branch and hepatic 4b and 5 segments, which was considered unresectable. A biopsy was taken with immunohistochemical (IHC) stains positive for cytokeratin CK7, MOC31, and negative for CK20, with a high mitotic index measured with Ki67, consistent with an extrahepatic cholangiocarcinoma (Figure 2A-2F). Microsatellite instability (MSI) was not detected. Finally, an endobiliary metalic stent was placed resulting in successful biliary decompression.
Based on these findings, he was diagnosed with unresectable extrahepatic cholangiocarcinoma and started first-line chemotherapy with the combination of gemcitabine and cisplatin. CT evaluation after 3 cycles of treatment revealed a partial response to treatment (PR), with the disappearance of the gallbladder fundus lesion and stability of the infundibulum lesion (Figure 1D, 1E).
The patient presented grade 4 afebrile neutropenia, grade 1 anemia and grade 3 renal failure as main treatment toxicities. For this reason, after 6 cycles of chemotherapy with maintained PR, we decided to continue with gemcitabine monotherapy. After 3 cycles of treatment in September 2017, the CT scan revealed disease progression, with an increase of the infundibulum tumor volume (Figure 1F).
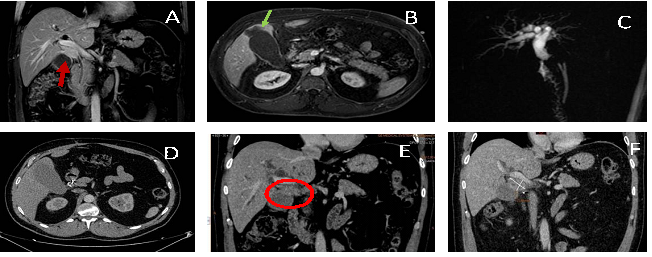
Figure 1: A. MRI Imaging showing focal hepatic lesion trapping the bile duct (red arrow). B. focal hepatic lesion adjacent to the gallblader fundus (green arrow). C. MRCP shows Bile duct dilation with stenosis of the common bile duct. D.E. CT scan shows disappearance of the gallbladder fundus lesion and stability of the infundibulum lesion after treatment (red circle). F. Disease progression, with an increase of the infundibulum tumor volume.
At this point, the patient had recovered from the previous treatment toxicities and maintained an excellent general status, so we started second-line chemotherapy treatment with capecitabine and oxaliplatin combination. A new CT scan was performed in December 2017 after receiving 3 cycles of treatment, which showed disappearance of the tumor lesion. The CR was also confirmed with Hepatic magnetic resonance imaging (MRI) (Figure 3). As an incidental finding, a mold of biliary mud with retrograde secondary dilation of the hepatic common duct was seen.
An Endoscopic retrograde cholangiopancreatography (ERCP) with direct cholangioscopy was then performed, confirming the absence of the tumor and the existence of the lithiasic mold which was partially removed. The biliary stent was changed and another ERCP was programed for complete stone removal. Given the accumulative hematological toxicity and the absence of disease in the imaging tests, we decided to stop chemotherapy treatment in February 2018. Another ERCP with direct cholangioscopy was done in January 2019, in which a fibrinoid ulcer was seen and biopsied with no signs suggestive of malignancy in the histopathological examination. At the present time, the patient remains without evidence of tumor recurrence, more than 30 months from the time of his initial diagnoses.
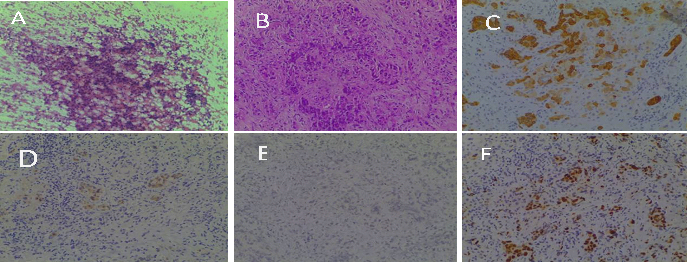
Figure 2: A. Citology: groups of irregular and hyperchromatic nuclei compatible with adenocarcinoma. A B C D E : H&E ( B): Stromal infiltration of poorly differenciated adenocarcinoma; Immunohistochemical stains positive for cytokeratin CK7, MOC31 (C, D), negative for CK20 (E), and a high mitotic index measured with ki67 (F), consistent with an extrahepatic colangiocarcinoma.
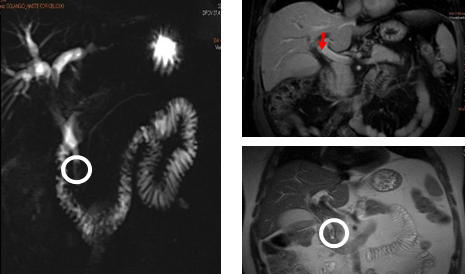
Figure 3: MRI imaging shows complete response to treatment (red arrow). Marked dilation of the bile duct by a biliary mud mold (white circle).
3. Discussion
BTC is an uncommon disease with bad prognosis. More than 75% of the patients are considered unresectable at the time of diagnosis and, even in the subset of patients with resectable disease, relapse rate remains high [4]. Chemotherapy has been commonly used to improve the outcome of these patients and to delay tumor progression. Few prospective trials have been performed in the first-line setting in advanced BTC. In the multicenter ABC-02 trial, cisplatin plus gemcitabine was associated with a significant overall survival (OS) advantage without the addition of substantial toxicity, as compared with gemcitabine alone (11.7 vs 8.1 months) [5]. A similar study was performed in a Japanese population, demonstrating a greater OS with the combination regimen (11.2 vs 7.7 months) [6].
However, gemcitabine plus cisplatin chemotherapy has not been directly compared in phase III trials with other gemcitabine-containing regimens (capecitabine, irinotecan or oxaliplatin), with the exception of gemcitabine plus S-1 in the Japanese phase III FUGA-BT trial. In a preliminary report of this trial presented at the 2018 American Society of Clinical Oncology (ASCO) Gastrointestinal Cancers Symposium, Gemcitabine plus S-1 was not inferior in terms of median OS, median progression-free survival (PFS) and overall response rate (ORR) [7]. Despite the outcome improvement with first-line chemotherapy, almost all of the patients will experience disease progression. Approximately a half of them maintain a good performance status and are willing to undergo further treatment. No standard salvage chemotherapy regimen has been identified in this setting. Limited experience with second-line chemotherapy in advanced BTC is reported in the literature since clinical trials are difficult to perform due to the rarity and heterogeneity of these tumors.
For patients progressing on gemcitabine plus cisplatin regimen, options for chemotherapy include a fluoropyrimide, alone or in combination with oxaliplatin. The addition of oxaliplatin to capecitabine in patients progressing after first-line treatment with gemcitabine-based chemotherapy yielded an ORR of 3–8.5% and a median PFS and median OS of 15–17 and 17–24.7 weeks respectively which, probably, may be in the range of the results observed with a single agent therapy [8, 9].
In other study, 56 patients diagnosed with BTC (36 cholangiocarcinoma and 20 gallbladder cancer) received the combination of capecitabine and oxaliplatin. In a preliminary report, two complete and seven partial responses were observed, and a great proportion of patients experienced prolonged periods of stable disease [10]. The unusual good response to chemotherapy of our case is worth reporting, especially because systemic chemotherapy is considered little effective in disseminated or unresectable cholangiocarcinoma and there is still no established protocol in the second-line setting. Previous studies have reported isolated cases of pathological complete response (pCR) to chemotherapy in advanced BTC. In a single center phase II study that evaluated the combination chemotherapy of (GEMOX) for advanced Gallbladder cancer patients, Sharma et al. reported one case of pCR [11]. Another case report also showed a pCR in a patient with BTC after five courses of the GEMOX regimen [12]. Walker et al. reported a case of pCR in a locally advanced common bile duct cancer with the gemcitabine-cisplatin combination regimen [13]. Other authors have also described cases of pCR in advanced disease with the combination chemotherapy of gemcitabine and S-1 [14, 15] and gemcitabine-cisplatin-S1 [16] (Table 1).
Table 1: Published case reports of Biliary Tract Cancer with Complete Response.
Recent molecular studies have increased our understanding of pathogenetic mechanism that underly the development of cholangiocarcinoma. These have helped us to determine the significance of molecular alterations that occur in this disease and will direct the development of targeted therapy. Different studies have revealed that BTC develops in the context of chronic inflammation and cholestasis [17]. In these studies cholangiocarcinogenesis is associated with proinflammatory cytokines such as interleukin-6 (IL-6) [18]. BTC cells synthesize and secrete IL-6, with subsequent auto-and paracrine stimulation of the IL-6 receptor. Negative feedback mechanisms regulating IL-6 signaling are frequently inactivated in these tumor cells. Activation of the IL-6 receptor results in activation of JAK/STAT3, MAPK, ERK1/2 and PI3K/Akt pathways and carcinogenesis [18].
Inducible nitric oxide synthase (iNOS) has also been implicated in cholangiocarcinogenesis [18]. iNOS over-expression could be induced in BTC cell lines by proinflammatory cytokines [19]. iNOS causes oxidative damage to DNA and limits the cellular ability to repair such damage. Once malignant transformation has occurred; cells gain the ability of uncontrolled proliferation, invasion across the basement membrane, and escape apoptotic pathways [20]. Among others, erb-2, cyclooxygenase-2 and epidermal growth factor receptors (EGFR) have been identified as key molecular contributors in cholangiocarcinogenesis [21].
Erlotinib is a tyrosine kinase inhibitor (TKI) that prevents activation of EGFR through reversible blockade of the receptor’s ATP binding site. It has been studied in combination to either gemcitabine and oxaliplatin (GEMOX) [21] or the VEGF inhibitor bevacizumab [22].The addition of erlotinib in these studies failed to prolong survival beyond that which would be expected from GEMOX or bevacizumab alone in patients with BTC. Cetuximab and panitumumab are monoclonal antibodies that selectively block the extracellular ligand-binding domain of EGFR Receptor. In combination with GEMOX, cetuximab failed to demonstrate a benefit of PFS or OS in the final analysis of the study [23].
Panitumumab, on the other hand, has consistently improved survival in patients with BTC. A single arm study of 35 patients with cholangiocarcinoma that received treatment with gemcitabine, irinotecan, and panitumumab had a median PFS and OS of 9.7 mo and 12.9 mo respectively [24]. The results of this trial, while promising, were demonstrated in relatively small patient population that lacked a control group for comparison. In addition, future studies should identify biomarkers to predict response to cetuximab and panitumab, such as EGFR, KRAS, and BRAF mutations.
Bevacizumab is a humanized monoclonal antibody that blocks VEGF receptor. This agent has been studied in combination to chemotherapy with promising results. A single arm phase II study of bevacizumab with GEMOX demonstrated good efficacy against BTC, with median PFS of 7 mo and OS of 12.7 mo [25]. These results, while encouraging, should be approached with precaution as the known efficacy of GEMOX and absence of an internal control group makes it difficult to estimate the true benefit conferred by bevacizumab. The MEK inhibitor Selumetinib is a newer targeted treatment that has demonstrated activity against BTC with a favorable toxicity profile [26]. Studies of melanoma and colorectal cancer have suggested that tumors with activating mutations of BRAF are sensitive to MEK inhibition [27]. This association has not yet been investigated in BTC.
Genetic heterogeneity of cholangiocarcinomas was detected in the whole genome and epigenomic analysis of 489 tumors, performed by the International Cancer Genome Consortium. In this analysis four distinct genetic clusters were identified, defined by mutation and copy number profiles, gene expression, and epigenetics [28]. Cluster 1 was enriched in TP53, ARID1A, BRCA1/2 mutations, and HER2 amplification. Cluster 2 was enriched with TP53 mutations. Both clusters occurred equally as extrahepatic and intrahepatic tumors and were liver fluke-positive or fluke-negative. Cluster 4 was enriched in BAP1 and IDH1/2 mutations as well as fibroblast growth factor receptor (FGFR) alterations and was predominantly intrahepatic and fluke-negative, as was cluster 3. In this study, approximately 60% of patients in cluster 4 were alive at 7 years, compared with 0% to 40% of patients in the other clusters (P < 0.0001).
The better prognosis for cluster 4 may be partly due to its enriched presence of FGFR2 fusion, as they have been associated with improved outcomes. FGFR fusions are driver events that result in ligand-independent activation of the FGFR pathway. BGJ398 is an orally, selective, ATP-competitive pan-FGFR inhibitor that showed activity in tumor models with FGFR alterations. In the phase II trial of BGJ398 in 61 heavily pretreated patients with FGFR alterations (79% had FGFR fusions), ORR was 14.8% (18.8% FGFR2 fusions only), disease control rate was 75.4% (83.3% FGFR2 fusions only), and estimated median PFS was 5.8 months (95% CI, 4.3 to 7.6 months) [29]. Other agents have shown activity against FGFR2 resistance mutations. In a phase I/II basket trial of TAS-120 that included 23 patients with FGFR2 fusion and other FGFR-altered cholangiocarcinomas, 4 of 9 patients achieved a partial response, and 8 patients had tumor regression. TAS-120 is currently being evaluated in a large basket trial with planned enrollment of over 800 patients [30].
Another new target is the isocitrate dehydrogenase-1 (IDH1) mutation, which occurs in about 20% of intrahepatic tumors. Ivosidenib (AG-120), is an oral, selective, reversible inhibitor of mutant IDH1 currently been evaluated in phase III trials of cholangiocarcinoma and acute myelogenous leukemia [31]. About 2.5% of cholangiocarcinomas have mismatch repair (MMR) deficiency, which makes them a target for programmed cell death protein 1 (PD-1) inhibitors. In a series of five uncontrolled, single-arm, multi-center clinical trials, pembrolizumab was assesed in MMR-deficient (dMMR)/MSI-high (MSI-H) advanced solid tumors (N = 149) [32]. Eleven of the 149 patients enrolled in these studies had BTCs. This small subset of BTCs showed an ORR of 27%, with a duration of response ranging from 11.6 to 19.6 months [26]. dMMR/MSI occurred across all BTC subtypes, most frequently in intrahepatic cholangiocarcinoma [33].
Finally, by integrating targeted therapy with the molecular profiles of tumor, we hope to accomplish the goal of precision treatment of patients with malignant diseases of the biliary tract.
4. Conclusion
In conclusion, we experienced a patient with unresectable BTC who was treated with capecitabine and oxaliplatin combination and achieved complete response after progression to first-line treatment with gemcitabine-cisplatin. Our case also suggests that selected patients may demonstrate robust or even complete responses to chemotherapy. There is a need to further characterize the molecular networks driving its progression and identify different molecular subtypes that could direct management of these patients.
References
- Miyakawa S, Ishihara S, Horiguchi A, et al. Biliary tract cancer treatment: 5,584 results from the Biliary Tract Cancer Statistics Registry from 1998 to 2004 in Japan. J Hepatobiliary Pancreat Surg 16 (2009): 1-7.
- Junji Furuse, Tadahiro Takada, Masaru Miyazaki, et al. Guidelines for chemotherapy of biliary tract and ampullary carcinomas. J Hepatobiliary Pancreat Surg 15 (2008): 55-62.
- Eckel F, Schmid RM. Chemotherapy in advanced biliary tract carci- noma: a pooled analysis of clinical trials. BR J Cancer 96 (2007): 896-902.
- De Groen PC, Gores JC, LaRusso NF, et al. Biliary tract cancers. N Engl J Med 341 (1999): 1368-1378.
- Juan Valle, Harpreet Wasan, Daniel H. Palmer, et al. Cisplatin plus Gemcitabine versus Gemcitabine for Biliary Tract Cancer. N Engl J Med 362 (2010): 1273-1281.
- Okusaka T, Nakachi K, Fukutomi A, et al. Gemcitabine alone or in combination with cisplatin in patients with biliary tract cancer: a comparative multicentre study in Japan. Br J Cancer 103 (2010): 469-474.
- Morizane C, Okusaka T, Mizusawa J, et al. Randomized phase III study of gemcitabine plus S-1 combination therapy versus gemcitabine plus cisplatin combination therapy in advanced biliary tract cancer: A Japan Clinical Oncology Group study (JCOG1113, FUGA-BT). J Clin Oncol 36 (2018): 4S.
- Mane J, Iruarrizaga E, Rubio I, et al. Second-line chemotherapy with capecitabine and oxaliplatin in patients with pancreatic or biliary tree adenocarcinoma. J Clin Oncol 29 (2011): 4s.
- Sancho A, Ferreiro J, Mane J, et al. Oxaliplatin and capecitabine after gemcitabine failure in patients with advanced pancreatic, biliary, and gallbladder adenocarcinoma (APBC). J Clin Oncol 26 (2008): 20s.
- Nehls O, Oettle H, Hartmann JT, et al. A multicenter phase II study of capecitabine plus oxaliplatin (CapOx) in advanced biliary system adenocarcinomas. J Clin Oncol 23 (2004): 336s.
- Sharma A, Mohanti B, Raina V, et al. A phase II study of gemcitabine and oxaliplatin (Oxigem) in unresectable gall bladder cancer. Cancer Chemother Pharmacol 65 (2010): 497-502.
- Moussata D, Bessayah A, Chauvenet M, et al. A pathologic complete response in the surgical specimen after systemic chemotherapy for a gallbladder carcinoma. Gastrointest Cancer Res 5 (2012): 106-108.
- Walker EJ, Simko JP, Nakakura EK, et al. A patient with cholangiocarcinoma demonstrating pathologic complete response to chemotherapy: exploring the role of neoadjuvant therapy in biliary tract cancer. J Gastrointest Oncol 5 (2014): E88-E95.
- Lim JH, Ryu JK, Choi YJ, et al. A case of common bile duct cancer that completely responded to combination chemotherapy of gemcitabine and TS-1. Gut Liver 7 (2013): 371-376.
- Takeshi W, Junji F, Naohiro O, et al. A pathological complete response after combined chemotherapy of gemcitabine and S-1 in advanced biliary tract cancer with para-aortic lymph nodes metastasis: a case report. Surg Case Rep 3 (2017): 26.
- Matsubara T, Nishida T, Tomimaru Y, et al. Histological complete response in a patient with advanced biliary tract cancer treated by gemcitabine/cisplatin/S-1 combination chemotherapy: A case report. Mol Clin Oncol 5 (2016): 757-761.
- Liu R, Cox K, Guthery SL, et al. Cholangiocarcinoma and high-grade dysplasia in young patients with primary sclerosing cholangitis. Dig Dis Sci 59 (2014): 2320-2324.
- Park J, Tadlock L, Gores GJ, et al. Inhibition of interleukin 6-mediated mitogen-activated protein kinase activation attenuates growth of a cholangiocarcinoma cell line. Hepatology 30 (1999): 1128-1133.
- Jaiswal M, LaRusso NF, Burgart LJ, et al. Inflammatory cytokines induce DNA damage and inhibit DNA repair in cholangiocarcinoma cells by a nitric oxide-dependent mechanism. Cancer Res 60 (2000):184-190.
- Malhi H, Gores GJ. Cholangiocarcinoma: Modern advances in understanding a deadly old disease. J Hepatol 45 (2006): 856-867.
- Lee J, Park SH, Chang HM, et al. Gemcitabine and oxaliplatin with or without erlotinib in advanced biliary-tract cancer: a multicentre, open-label, randomised, phase 3 study. Lancet Oncol 13 (2012): 181-188.
- Lubner SJ, Mahoney MR, Kolesar JL, et al. Report of a multicenter phase II trial testing a combination of biweekly bevacizumab and daily erlotinib in patients with unresectable biliary cancer: a phase II Consortium study. J Clin Oncol 28 (2010): 3491-3497.
- Malka D, Cervera P, Foulon S, et al. Gemcitabine and oxaliplatin with or without cetuximab in advanced biliary-tract cancer (BINGO): a randomised, open-label, non-comparative phase 2 trial. Lancet Oncol 15 (2014): 819-828.
- Sohal DP, Mykulowycz K, Uehara T, et al. A phase II trial of gemcitabine, irinotecan and panitumumab in advanced cholangiocarcinoma. Ann Oncol 24 (2013): 3061-3065.
- Zhu AX, Meyerhardt JA, Blaszkowsky LS, et al. Efficacy and safety of gemcitabine, oxaliplatin, and bevacizumab in advanced biliary-tract cancers and correlation of changes in 18-fluorodeoxyglucose PET with clinical outcome: a phase 2 study. Lancet Oncol 11 (2010): 48-54.
- Bekaii-Saab T, Phelps MA, Li X, et al. Multi-institutional phase II study of selumetinib in patients with metastatic biliary cancers. J Clin Oncol 29 (2011): 2357-2363.
- Solit DB, Garraway LA, Pratilas CA, et al. BRAF mutation predicts sensitivity to MEK inhibition. Nature 439 (2006): 358-362.
- Jusakul A, Cutcutache I, Yong CH, et al. Whole-genome and epigenomic landscapes of etiologically distinct subtypes of cholangiocarcinoma. Cancer Disc 7 (2017): 1116-1135.
- Javle M, Lowery M, Shroff RT. Phase II Study of BGJ398 in Patients With FGFR-Altered Advanced Cholangiocarcinoma. J Clin Oncol 36 (2018): 276-282.
- Goyal L, Arkenau HT, Tran B, et al. Early clinical efficacy of TAS-120, a covalently bound FGFR inhibitor, in patients with cholangiocarcinoma. Ann oncol 28 (2017): 137-149.
- Ghassan KA, Juan WV, Robin KK. A phase 3 multicenter randomized double-blind study of AG-120 versus placebo in patients with non-resectable or metastatic cholangiocarcinoma with an IDH1 mutation. J Clin Oncol 36 (2018).
- Lemery S, Keegan P, Pazdur R. First FDA approval agnostic of cancer site—when a biomarker defines the indication. N. Engl. J. Med 377 (2017): 1409-1412.
- Silva VW, Askan G, Daniel TD, et al. Biliary carcinomas: pathology and the role of DNA mismatch repair deficiency. Chin. Clin. Oncol 5 (2016): 62.


 Impact Factor: * 5.3
Impact Factor: * 5.3 Acceptance Rate: 75.63%
Acceptance Rate: 75.63%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 
