Grapefruit Juice Facilitates Cortisol Replacement Therapy: Role of CYP3A Variant Alleles
Marjolein Drent1-3*, Petal Wijnen2-4, Otto Bekers4, Aalt Bast1, 3, 5
1Department of Pharmacology and Toxicology, Faculty of Health, Medicine and Life Science, Maastricht University, Maastricht, The Netherlands
2ILD Center of Excellence, St. Antonius Hospital, Nieuwegein, The Netherlands
3ILD Care Foundation Research Team, Ede, The Netherlands
4Department of Clinical Chemistry, Central Diagnostic Laboratory, Maastricht University Medical Centre, Maastricht, The Netherlands
5Maastricht University, Venlo Campus, Venlo, The Netherlands
*Corresponding Author: Prof. Marjolein Drent, Department of Pharmacology and Toxicology, Faculty of Health, Medicine and Life Science, Maastricht University, Maastricht, The Netherlands
Received: 31 August 2021; Accepted: 07 September 2021; Published: 20 September 2021
Article Information
Citation: Marjolein Drent, Petal Wijnen, Otto Bekers, Aalt Bast. Grapefruit Juice Facilitates Cortisol Replacement Therapy: Role of CYP3A Variant Alleles. Archives of Clinical and Medical Case Reports 5 (2021): 656-664.
View / Download Pdf Share at FacebookAbstract
Glucocorticosteroid (GC) replacement therapy is required in several clinical situations. However, tapering down the GC doses may lead to a steroid withdrawal syndrome. We describe the case of a patient presenting with Cushing’s syndrome due to a pituitary adrenocorticotropic hormone (ACTH) producing adenoma. After successful surgery, cortisol replacement was started. Unfortunately, tapering the hydrocortisone (HC) dose failed. We hypothesized that the presence of cytochrome P450 (CYP)3A variant alleles might at least partly explain the inappropriate response to HC. The patient appeared to be a CYP3A5 intermediate metabolizer (*1/*3) and a slightly more rapid CYP3A4 metabolizer (*1A/*1B), both accounting for an increased HC metabolism. Cortisol is metabolized via various biotransformation pathways which could be affected by grapefruit juice. After the patient started to take the HC with grapefruit juice, she felt better, and it became easier to taper down the daily HC dose. The peak cortisol dose was the same with and without the use of grapefruit juice, whereas the cortisol level six hours after the morning dose of HC was 20% higher than in the situation without grapefruit juice. In conclusion, grapefruit juice enhances cortisol availability by increasing 11β-hydroxysteroid dehydrogenase (HSD) type 1 activity and simultaneously inhibiting cortisol metabolism by inhibiting the CYP3A enzymes. The presence of CYP polymorphisms appeared to be a substantial susceptibility risk factor in the cortisol metabolism and the development of a GC withdrawal syndrome.
Keywords
<p>Adrenal insufficiency; Cortisol; Cushing’s syndrome; CYP3A; Grapefruit; Hydrocortisone; Quality of life</p>
Article Details
1. Introduction
Overproduction of the adrenal hormone cortisol may lead to Cushing’s syndrome. The classic phenotype displays central obesity, prominence of dorsal, temporal, and supraclavicular fat pads, abdominal striae, hypertension, and edema [1, 2]. However, the clinical manifestation is variable. Overall, most characteristics appear to be related to energy and vitality, with a major impact on the quality of life [1-5]. Physical health seems to be more affected than mental health. The course is likewise unpredictable, leading to the view that Cushing may be a syndrome rather than a single disease. Attempts have been made to define distinct subgroups (“phenotypes”), to simplify predictions for individual patients and to homogenize groups for research purposes [6]. However, Cushing phenotypes have not often been used to predict prognosis or to cluster patients with similar outcomes. Phenotyping could also be used to stratify patients by clinical features such as extent of organ involvement or by perceived severity [6].
Adrenal cortisol production is regulated in conjunction with the pituitary, and pituitary adenomas may also lead to Cushing’s syndrome [2]. To date, pituitary adenomas are uncommon, difficult to diagnose tumors whose heterogeneity and low incidence complicate large-scale studies [7]. Endoscopic transsphenoidal pituitary surgery (TS) is a safe and effective treatment [8].
We present the case of a patient with Cushing’s syndrome caused by an adrenocorticotropic hormone (ACTH) producing pituitary adenoma. The patient demonstrated a steroid withdrawal syndrome (SWS) despite cortisol replacement with a daily dose of 30 mg orally after successful TS.
2. Case Report
A 62-year old female non-smoker, without a relevant medical history, presented with a 2-year history of slightly progressive lymphedema at both feet, joint effusion (hydrops; transudate) in both ankles and knees, a moon/Cushing face, muscle weakness, dyspnea at exertion, and fatigue. Physical exam revealed a body mass index (BMI) of 25.3 kg/m2. She had a normal fat distribution. No hirsutism, acne, bruising, or striae were noted. Thyroid examination was normal. The clinical suspicion of Cushing’s syndrome was low. Her blood pressure was 130/70 mm Hg.
She was investigated for Cushing’s syndrome following a mildly elevated random serum cortisol level of 0.63 µmol/L and an ACTH of 18.3 pmol/L. Work-up revealed a serum cortisol at 8 a.m. of 0.63 μg/dL after a 1 mg overnight dexamethasone suppression test (DST). Further work-up showed two consecutive elevated 24-h urinary free cortisol level (UFC; 633 μg/day and 578 μg/day, normal 20–90). Her diffusing capacity of the lungs for carbon monoxide (DLCO) was 70% of predicted. Though she did not show the classical Cushing symptoms like central obesity, prominence of dorsal, temporal, and supraclavicular fat pads, abdominal striae and hypertension, biochemical tests were positive for Cushing’s syndrome, including increased urinary excretion rates of free cortisol, decreased overnight suppression by dexamethasone (1 mg) and elevated midnight salivary cortisol values in addition to non-suppressed ACTH levels. The MRI of the pituitary demonstrated a microadenoma of 7x5 mm.
During successful surgery (transsphenoidal microadenomectomy), a 7 mm corticotroph adenoma was removed. Postoperatively, serum cortisol was undetectable (<20 nmol/L) and the patient commenced cortisol replacement therapy (the compound is indicated as hydrocortisone [HC] when given as medication) [1]. Initially she felt well, but about three weeks after surgery she started to feel lethargic. An insulin tolerance test showed a normal growth hormone response to hypoglycemia. There were no signs of other pituitary hormone deficiencies. Despite suppletion with HC 30 mg daily, she developed a cortisol withdrawal syndrome (CWS) with pain, severe fatigue, muscle weakness, weight loss, and exercise intolerance, probably explained by the rapid decrease of cortisol after the surgery.
After four months, an HC dose reduction was recommended (to 27.5 mg daily). During this period, she suffered from increasing muscle weakness, tendinopathy and vaginitis (M. Quervain on the left side and bicipital tendinitis on both sides), weight gain, pain, and decreased exercise tolerance. She also developed extreme fatigue, especially in the afternoon.
After one month, the HC dose was further reduced to 25 mg daily. This caused clinical deterioration, increased disability and low blood pressure (105/62) with major impact on the quality of life. Therefore, the HC dose was temporarily increased again, first to 50 mg daily for two days, upon which the burden of complaints decreased immediately. Hence, it was decided to continue with a dose of 30 mg for two months. Thereafter, we applied the same dose reduction as before. After reduction to 25 mg daily, however, the problems reoccurred substantially. She also developed a frozen shoulder on the left side. Clinically, there appeared to be insufficient effect of HC. Laboratory tests showed no signs of an auto-immune disorder. Her BMI had decreased from 25.3 to 22.6 kg/m2 eight months after surgery.
Since we have a long-standing interest in CYP enzymes and their determining role in drug action [9-11], we hypothesized that CYP3A variant alleles might at least partly explain the inappropriate response to HC. To test this hypothesis, CYP3A4 and CYP3A5 were genotyped. The patient appeared to be an intermediate metabolizer or heterozygote expressor for CYP3A5 (*1/*3) and a slightly more rapid CYP3A4 metabolizer (*1A/*1B), both accounting for an increased metabolism of HC compared to more than 80% of the general Caucasian population, who are poor metabolizers or non-expressors (CYP 3A5 *3/*3) [12]. Nine months after starting the HC replacement therapy, the patient started to take the HC with grapefruit juice in the morning, after which she felt much better and it became easier to taper down the daily HC dose. The peak cortisol concentration was the same with and without grapefruit juice being taken, whereas the cortisol level six hours after the morning dose of HC was 20% higher compared to the situation without grapefruit juice.
3. Discussion
The case presented here, of a 62-year-old woman with Cushing’s syndrome caused by an ACTH producing pituitary adenoma, demonstrated insufficient clinical response to HC suppletion after successful surgery (transsphenoidal microadenomectomy). As soon as she started taking HC with grapefruit juice, however, she felt better, and it became easier to taper down the daily HC dose. Hence, she was less hindered by side-effects of HC thanks to the “grapefruit effect”, which increases the bioavailability of certain pharmaceutical compounds including cortisol, and this improved her quality of life substantially [13-15]. The presence of CYP polymorphisms appeared to be a substantial susceptibility risk factor in the development of a GC withdrawal syndrome, by influencing the cortisol metabolism.
Current HC formulations do not fully replicate the normal circadian rhythm, thereby contributing to the increased morbidity and mortality observed [16]. In the absence of objective variables to measure replacement quality, the clinician has to rely primarily on clinical judgment [16]. Our hypothesis is that the difficulty of achieving appropriate HC suppletion in this case could at least partly be due to more rapid biotransformation of cortisol, which could be delayed by grapefruit juice. Cortisol is metabolized via various biotransformation pathways which can be inhibited by grapefruit juice (Figure 1).
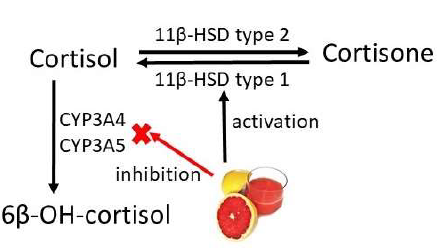
Figure 1: Schematic overview of the metabolism of cortisol. Oxidation to 6β-hydroxycortisol (6β-OH-cortisol) is catalyzed by cytochromes P450 3A4 and 3A5 (indicated as CYP3A4 and CYP3A5). Cortisone is converted into cortisol by hepatic 11β-hydroxysteroid dehydrogenase (11β-HSD) type 1. Grapefruit juice inhibits CYP3A4 and CYP3A5 and activates 11β-HSD type 1.
Cortisol, the main product of the hypothalamus–pituitary–adrenal (HPA) axis, is a crucial steroid hormone in the physiological stress response following homeostasis disturbance [16]. Van Keulen et al. emphasized the significant role of individual circumstances in the settings of the HPA axis [17]. They found that unlike cortisol production, the cortisol metabolism was considerably influenced by genetic constitution, suggesting distinct patterns of genetic and environmental contribution to the different metabolic pathways. Cortisol is in equilibrium with cortisone via the enzymes 11-beta-hydroxysteroid dehydrogenase (11-ß-HSD type 1 and HSD type 2) [17, 18]. 11ß-HSD type 1 is located mainly in liver, adipose tissue, and muscle, where its principal function is to reduce cortisone to cortisol. Hence, circulating cortisone serves as a depot that can be readily activated to form cortisol in the tissues [19]. This may explain to some extent the variety in individual susceptibility to the effects of glucocorticoids. Our case resembles patients described by Tomlinson et al., who failed to develop a classic Cushing’s phenotype despite the presence of hypercortisolemia and increased cortisol production rate [20]. This highlights that there is clear interindividual variability in the metabolism of cortisol and that this can have a profound impact upon the phenotypic effects of GC.
Drug metabolizing enzymes of the CYP3A subfamily play a role in cortisol metabolism [21]. GCs are metabolized by cytochrome P450 3A4 (CYP3A4). Hence, various CYP3A4 inhibitors may inhibit GC degradation, increase its accumulation, and may induce an iatrogenic Cushing’s Syndrome [22]. This pronounced role of CYP3A4 is clear, as the ratio of 6-β-hydroxycortisol to cortisol has been used as a marker of CYP3A4 activity [23]. The inhibition of CYP3A4 by grapefruit juice is well-known [18], and the influence of CYP3A5 polymorphisms on the 6-ß-hydroxycortisol/cortisol ratio has also been described [24]. The latter paper also mentioned the influence of the grapefruit–drug interaction in relation to genetic polymorphisms. Moreover, Methlie et al. found that licorice, and particularly grapefruit juice, increased cortisol availability to tissues in the hours following oral cortisone administration in patients with primary adrenal failure [18]. Compared with the usual treatment, they showed that the median AUC for serum cortisol increased under the influence of licorice and/or grapefruit juice intake [18]. Steroid profiling indicated that grapefruit juice increases hepatic 11β-HSD type 1 activity and enhances the early absorption of cortisone [18].
These findings suggest the possibility of attenuating the fluctuations of GC levels through the day in patients (with Addison’s disease) on replacement therapy. To date, conventional strategies for GC replacement (in Addison’s disease) fail to mimic the physiological diurnal rhythm of cortisol [16]. Multi-dosage regimens are usually preferred because circulating cortisol has a relatively short half-life [16]. Unfortunately, this renders the patient both over- and under-treated during the 24-h cycle. Grapefruit juice increases cortisol availability by increasing 11β-HSD type 1 activity and simultaneously inhibiting cortisol metabolism through CYP3A enzyme inhibition [18].
An alternative form of steroid withdrawal syndrome (SWS) can occur where patients experience symptoms of adrenal insufficiency despite acceptable serum cortisol levels. However, it is important to remember that SWS is a self-limiting state, and should it occur, its management should include a temporary increase in the dose of GC, followed by slow tapering to a maintenance dose [25, 26]. This regimen was followed in the present case as well. Today, GCs represent the standard therapy for reducing inflammation and immune activation in various diseases, including asthma, as well as allergic, rheumatoid, collagen, vascular, dermatological, inflammatory bowel and other systemic diseases, including sarcoidosis, and in ocular inflammatory diseases [16, 27, 28]. GCs have anti-inflammatory and immunosuppressive effects, but also potentially undesirable side-effects [27-30]. The risk/benefit ratio of GC therapy can be improved by careful monitoring and using appropriate preventive strategies to potentially minimize side-effects [16, 28, 30]. The primary aims of GC replacement therapy include (1) suppression of symptoms of GC deficiency; (2) prevention of acute adrenal crises; (3) avoidance of adverse effects of long-term GC therapy; and (4) restoration of quality of life [16].
The variability in drug response among patients is multifactorial. Both clinical and genetic risk stratification (pharmacogenomics) may lead to a more accurate drug management regime aimed at preventing unnecessary side-effects, increasing the efficacy of the drug, and improving quality of life. Current and future reports regarding the association of certain gene variants with the response to certain drugs should be validated before they are used in patient management [10]. As there is considerable variation in individual requirements for GC replacement, the balance between over- and under-replacement of GC is a significant clinical challenge [16]. Genotyping prior to drug prescription may be clinically relevant for the prediction of the response to GC, as well as for the prevention of side-effects and the development of a withdrawal syndrome. Both clinical and genetic risk stratification (pharmacogenomics) may lead to more accurate drug prescription [11].
Grapefruit juice contains many flavonoids [31]. It is known that flavonoids have direct anti-inflammatory effects [32]. Moreover, several flavonoids have been shown to prevent the potential resistance to GC, thus stimulating GC activity [32]. In case steroid-induced side-effects occur, careful tapering of the drug may be required, and other agents might be needed to achieve disease control. Frequently, as in our case study, GC withdrawal regimens are difficult to carry out. It is tempting to speculate that in other cases where difficulties arise with tapering GC, nutritional intervention may also be of help. A final word of caution is also appropriate here. Grapefruit juice might also interact with the biotransformation or transport of other concomitantly administered drugs, which should not be neglected.
4. Conclusion
In conclusion, the case presented here demonstrates that CYP3A variant alleles might explain, at least to some extent, an inappropriate response to HC and difficulties in tapering down the dose. Hence, this may explain the variety in individual susceptibility to the effects of GCs. Moreover, it shows, in accordance with earlier studies, that grapefruit juice enhances cortisol availability and thus facilitates the tapering of cortisol replacement therapy. It is tempting to speculate that these metabolic mechanisms also might explain, at least partly, the problems faced in a subgroup of patients treated with GCs when tapering the GC dose. Thus, we would like to challenge researchers to include this assumption in future studies to achieve a more personalized treatment strategy for GC and assess the role of nutrition in increasing the efficacy of drugs and reducing the side-effects.
Funding
No funding has been received for this work.
Conflicts of Interest
No conflict of interest was declared.
Informed Consent
Written permission was given by the patient to publish the data.
Author Contributions
M.D. wrote the first draft, collected clinical data, and organized the dietary management; M.D. and P.W. analysed the results; M.D. and A.B. supervised the clinical and nutritional management; M.D. P.W. O.B, and A.B. prepared the final version of the manuscript.
References
- Lacroix A, Feelders RA, Stratakis CA, et al. Cushing's syndrome. Lancet 386 (2015): 913-927.
- Pivonello R, De Martino MC, De Leo M, et al. Cushing's disease: the burden of illness. Endocrine 56 (2017): 10-18.
- Roerink SH, Cocks MS, Wagenmakers M, et al. Decreased Aerobic Exercise Capacity After Long-Term Remission From Cushing Syndrome: Exploration of Mechanisms. J Clin Endocrinol Metab 105 (2020): e1408-e1418.
- Wagenmakers MA, Netea-Maier RT, Prins JB, et al. Impaired quality of life in patients in long-term remission of Cushing's syndrome of both adrenal and pituitary origin: a remaining effect of long-standing hypercortisolism? Eur J Endocrinol 167 (2012): 687-695.
- Webb SM, Santos A, Resmini E, et al. Quality of Life in Cushing's disease: A long term issue? Ann Endocrinol (Paris) 79 (2018): 132-137.
- Braun LT, Riester A, Osswald-Kopp A, et al. Toward a Diagnostic Score in Cushing's Syndrome. Front Endocrinol (Lausanne) 10 (2019): 766.
- Luque RM, Ibanez-Costa A, Sanchez-Tejada L, et al. The Molecular Registry of Pituitary Adenomas (REMAH): A bet of Spanish Endocrinology for the future of individualized medicine and translational research. Endocrinol Nutr 63 (2016): 274-284.
- Wagenmakers MA, Boogaarts HD, Roerink SH, et al. Endoscopic transsphenoidal pituitary surgery: a good and safe primary treatment option for Cushing's disease even in case of macroadenomas or invasive adenomas. Eur J Endocrinol 169 (2013): 329-337.
- Bast A. Is formation of reactive oxygen by cytochrome P-450 perilous and predictable? Trends Pharmacol Sci 7 (1986): 266-270.
- Jessurun NT, Drent M, van Puijenbroek EP, et al. Drug-induced interstitial lung disease: role of pharmacogenetics in predicting cytotoxic mechanisms and risks of side effects. Curr Opin Pulm Med 25 (2019): 468-477.
- Wijnen PA, Drent M, Nelemans PJ, et al. Role of cytochrome P450 polymorphisms in the development of pulmonary drug toxicity: a case-control study in the Netherlands. Drug Saf 31 (2008): 1125-1134.
- Zeigler-Johnson C, Friebel T, Walker AH, et al. CYP3A4 CYP3A5 and CYP3A43 genotypes and haplotypes in the etiology and severity of prostate cancer. Cancer Res 64 (2004): 8461-8467.
- Rossi M, Aktar S, Davis M, et al. The Grapefruit Effect: Interaction between Cytochrome P450 and Coumarin Food Components Bergamottin Fraxidin and Osthole. X-ray Crystal Structure and DFT Studies. Molecules 25 (2020).
- Hu J, Shang D, Xu X, et al. Effect of grapefruit juice and food on the pharmacokinetics of pirfenidone in healthy Chinese volunteers: a diet-drug interaction study. Xenobiotica 46 (2016): 516-521.
- Kiani J, Imam SZ. Medicinal importance of grapefruit juice and its interaction with various drugs. Nutr J 6 (2007): 33.
- Dineen R, Martin-Grace J, Thompson CJ, et al. The management of glucocorticoid deficiency: Current and future perspectives. Clin Chim Acta 505 (2020): 148-159.
- van Keulen BJ, Dolan CV, Andrew R, et al. Heritability of Cortisol Production and Metabolism Throughout Adolescence. J Clin Endocrinol Metab 105 (2020).
- Methlie P, Husebye EE, Hustad S, et al. Grapefruit juice and licorice increase cortisol availability in patients with Addison's disease. Eur J Endocrinol 165 (2011): 761-769.
- Seckl JR, Walker BR. Minireview: 11beta-hydroxysteroid dehydrogenase type 1- a tissue-specific amplifier of glucocorticoid action. Endocrinology 142 (2001): 1371-1376.
- Tomlinson JW, Draper N, Mackie J, et al. Absence of Cushingoid phenotype in a patient with Cushing's disease due to defective cortisone to cortisol conversion. J Clin Endocrinol Metab 87 (2002): 57-62.
- Ferraris JR, Argibay PF, Costa L, et al. Influence of CYP3A5 polymorphism on tacrolimus maintenance doses and serum levels after renal transplantation: age dependency and pharmacological interaction with steroids. Pediatr Transplant 15 (2011): 525-532.
- Kedem E, Shahar E, Hassoun G, et al. Iatrogenic Cushing's syndrome due to coadministration of ritonavir and inhaled budesonide in an asthmatic human immunodeficiency virus infected patient. J Asthma 47 (2010): 830-831.
- Sychev DA, Ashraf GM, Svistunov AA, et al. The cytochrome P450 isoenzyme and some new opportunities for the prediction of negative drug interaction in vivo. Drug Des Devel Ther 12 (2018): 1147-1156.
- Li D, Abudula A, Abulahake M, et al. Influence of CYP3A5 and MDR1 genetic polymorphisms on urinary 6 beta-hydroxycortisol/cortisol ratio after grapefruit juice intake in healthy Chinese. J Clin Pharmacol 50 (2010): 775-784.
- Bhattacharyya A, Kaushal K, Tymms DJ, et al. Steroid withdrawal syndrome after successful treatment of Cushing's syndrome: a reminder. Eur J Endocrinol 153 (2005): 207-210.
- Hochberg Z, Pacak K, Chrousos GP. Endocrine withdrawal syndromes. Endocr Rev 24 (2003): 523-538.
- Pande A, Culver DA. Knowing when to use steroids immunosuppressants or biologics for the treatment of sarcoidosis. Expert Rev Respir Med 14 (2020): 285-298.
- Drent M, Crouser ED, Grunewald J. Challenges of sarcoidosis and its management. N Engl J Med 385 (2021): 1018-1032.
- Drent M, Proesmans VLJ, Elfferich MDP, et al. Ranking self-reported gastrointestinal side effects of pharmacotherapy in sarcoidosis. Lung 198 (2020): 395-403.
- Oray M, Abu Samra K, Ebrahimiadib N, et al. Long-term side effects of glucocorticoids. Expert Opin Drug Saf 15 (2016): 457-465.
- Zhang Y, Yu Y, Li H, et al. Effects of Citri Reticulatae Pericarpium and grapefruit juice on the pharmacokinetics of omeprazole in rats. J Food Biochem 10.1111/jfbc.13804 (2021): e13804.
- Bast A, Semen KO, Drent M. Nutrition and corticosteroids in the treatment of sarcoidosis. Curr Opin Pulm Med 24 (2018): 479-486.


 Impact Factor: * 5.3
Impact Factor: * 5.3 Acceptance Rate: 75.63%
Acceptance Rate: 75.63%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 
