Impact of free Fall Impact versus Treadmill Physical Exercise Programs: Which is Most Osteogenic?
Aveline PC1, Lespessailles E3,4, Best TM2, Cesaro A1, Toumi H3,4
1I3MTO, University d’Orléans, Orléans 45000, France
2UHealth Sports Medicine Institute, Department of Orthopedics, Division of Sports Medicine, U of Miami, USA
3Departement de Rhumatologie, Centre Hospitalier d’Orleans, Orléans, France
4Plateforme Recherché Innovation Medicale Mutualisée d’Orléans, CHR, Orleans, France
*Corresponding Author: Hechmi Toumi. Departement de Rhumatologie, Centre Hospitalier d’Orleans, Orléans, France.
Received: 13 December 2021; Accepted: 07 January 2022; Published: 14 February 2022
Article Information
Citation: Aveline PC, Lespessailles E, Best TM, Cesaro A, Toumi H. Impact of free Fall Impact versus Treadmill Physical Exercise Programs: Which is Most Osteogenic?. Archives of Clinical and Medical Case Reports 6 (2022): 91-104.
View / Download Pdf Share at FacebookAbstract
Exercise plays a key role for bone remodeling throughout our lives. Within bone cells, osteocytes have the ability to translate mechanical stimuli to bone anabolic cellular signaling. A variety of protocols have been used to apply both direct and indirect stimuli to bone. Herein, we compared running and free fall impact exercise protocols and their effects on markers of osteogenesis and bone strength.
Methods: 50 female Wistar rats (6 weeks-old) were randomly assigned to either a sedentary group (S) or one of 4 exercise groups: treadmill training (T) and 3 Free-fall (F) groups (F30, F45, F60 respectively fall of 30, 45 and 60cm; 5 days/week, 8 weeks). We evaluated BMD and BMC (by DXA), bone microarchitecture of the left femur and tibia (by μCT), mechanical strength of the left femur (three-point bending test), and bone marker levels (by ELISA).
Results: After 8 weeks of physical exercise (EXE), whole body BMD and BMC were both significantly higher from baseline in all EXE groups, with no difference between the 4 EXE groups. Left femur and tibia BMC and BMD significantly increased in the F45 and F60 groups compared to the S and T groups. BV/TV, Tb.Th and Tb.N were significantly higher in F45 and F60 compared to all other groups. Tb.N was significantly higher in F60 compared to F45. Yield point stress and Young modulus were significantly higher for F45 compared to S and T groups. Bone alkaline phosphatase and osteocalcin levels were significantly higher in the F45 group compared to the remaining groups. NTX level was significantly decreased in the F45 compared to the S and T groups.
Conclusion: Both treadmill and impact training protocols produced a benefit on BMD and BMC. Interestingly, impact mechanical stress was a better stimulus for bone trabecular structure than treadmil
Keywords
<p>Treadmill and free-fall impact exercise, female rats, microarchitecture, bending test</p>
Article Details
1. Introduction
It is well recognized that bone formation and Bone Mineral Density (BMD) are both enhanced by physical exercise (EXE) [1-4]. EXE is recommended both to improve bone accretion in young women and to decrease bone loss in the elderly [5]. It is well accepted that EXE increases mechanical stresses within bone, which in turn enhances osteoblastic activation [6] and decreases osteoclast resorption [7]. Mechanical stresses are primarily detected in bone by the osteocytes [6,8,9], referred to as the controller of bone remodeling [10]. Osteocytes modulate bone remodeling by converting mechanical stress into biological messengers, and directing the recruitment and differentiation of osteoclasts and osteoblasts [11]. Several studies have demonstrated that these effects are modulated through the Wnt/β-Catenin pathway through the regulation of sclerostin expression, an osteocyte product which plays a key role as a circulating inhibition of the Wnt-signaling pathways [12]. Mechanical stress resulting from EXE reduces sclerostin expression and thus promotes bone formation [13,14]. In addition, mechanical signals generated by EXE increase osteocalcin expression, and bone alkaline phosphatase (ALP), two bone formation markers, while decreasing N-terminal telopeptide (NTX), a bone resorption marker [9,15]. Similarly, EXE produces changes in circulating levels of growth hormone (GH) [16] and insulin-like growth factor (IGF-1), which together have an anabolic effect on both bone and skeletal muscle [17]. The optimal form and dose of exercise however remain unknown at this point in time.
Several forms of EXE have been observed to increase bone mass including high impact exercise (i.e jumping) and lower impact activities such as running 17-20. Treadmill exercise is often used [18-21] as a strategy to enhance bone remodeling given that the dose of exercise can be accurately estimated. Running has been shown to both increase bone formation and decrease bone resorption in weight bearing sites [20]. BMD and trabecular bone microarchitecture improve in growing rats subjected to running [19,20,22]. Interestingly, none of these studies detected changes in bone strength. Impact exercise constitutes an alternative model recognized to improve bone mass, BMD and trabecular microarchitecture (BV/TV, trabecular thickness and porosity) [23,24]. Jump exercise is known to generate high mechanical stress and significant anabolic effects [25]. Biomechanical bone properties are related to the amount of strain applied [26] and the loading rate [27]. It has also been shown that impact exercise significantly enhance femoral breaking load and cross-sectional moment of inertia [28]. Free-fall impacts are similarly an effective stimulus for enhancing bone formation through an increase in ultimate breaking force in the shaft of the forelimbs but not the hindlimbs [29]. Interestingly, only a few differences in BMD and Cross Sectional Area (CSA) have been observed when comparing drops from different heights [29].
Accordingly, the purpose of the current study was to compare various forms of low and high impact exercise and their effects on a comprehensive evaluation of bone strength, morphology, and biochemistry. To achieve our goal, we compared treadmill running exercise (T) and 3 free-fall impact (F) regimens from 30, 45 and 60cm in an effort to determine an optimal dose of exercise to influence bone heath status.
2. Materials and Methods
2.1. Animals and in vivo experimental design
All experiments were approved by the animal protection committee of the University of Orleans (CEEA VdL: 2011-11-2). Fifty female Wistar rats, aged 6 weeks-old, were purchased from Janvier Animal Production (Janvier Animal Production, Le Genest-St-Isle, France). After one week of acclimation, animals were randomly assigned to one of 5 groups: control / sedentary rats (S), impact exercises from a height of 30cm (F30), 45cm (F45) or 60cm (F60), and a treadmill group (T). DXA analysis was conducted at time 0 (W0) under anesthesia with ketamine-xylazine (80-10mg/kg, intraperitoneally, Panpharma). At the end of the study, all animals were euthanized with an intraperitoneal injection of pentobarbital sodium chloride (0.1mL/100g of body weight, Ceva santé animal) [25]. Animals were maintained in observation for a few minutes following cardiac exsanguination. The blood was centrifuged and the serum was frozen at −80°C. Left femurs were dissected free of connective and fat tissues and stored at −20°C and +4°C respectively.
2.2. Exercise protocols
Treadmill running (T): This protocol corresponded to a previously established and adapted method [30] of running at 8m/min, 1h per day, 5 days/week for 8 weeks (Figure 1). Free fall impact rats performed impact exercises for 8 weeks. During the acclimation period, rats were familiarized to the impact exercise protocol: initial height was 25cm, and this was gradually increased to 30cm, 45cm or 60cm. Rats were submitted to 10 drops per day at a frequency of 1 drop per 10sec, 5 days/week for 8 weeks from a height of 30cm (F30), 45cm (F45) or 60cm (F60) (Figure 1). As previously described, the rats were lifted horizontally for the drop thus causing them to land on all four feet [31].
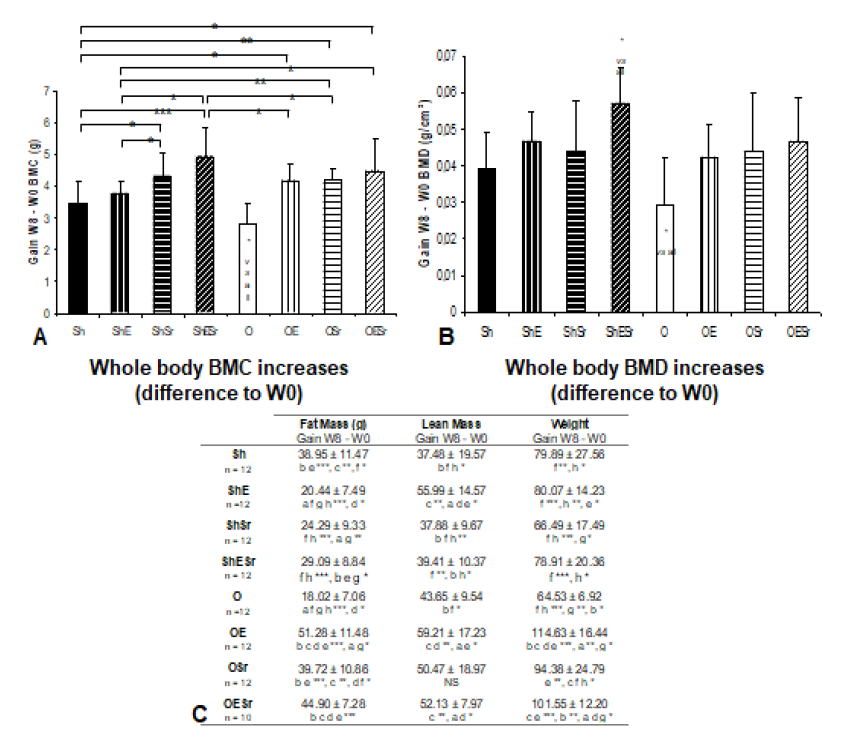
Figure 1: Densitometric characteristics and composition. A) Whole body BMC increases (difference to W0). B) Whole body BMD increases (difference to W0). C) Body composition: fat mass, lean mass and weight. The rats in O groups were ovariectomized. Rats in E groups were submitted to 10 impacts per day, 5 days a week during 8 weeks. Rats in Sr groups received 625 mg/kg/day of SrRan. The critical p-value were p=0.05: *, p<0.01: **, p<0.001: ***, NS: non significant and a: vs Sh, b: vs ShE, c: vs ShSr, d: vs ShESr, e: vs O, f: vs OE, g: vs OSr and h: vs OESr.
2.3. Bone densitometry measurements (DXA)
BMD, BMC and body composition were measured by DXA (Discovery, Hologic, Bedford, USA) using a specific small animal body composition mode [32]. Both measures were made once at week 0 (W0), week 4 (W4) and week 8 (W8) before sacrifice. The root-mean square coefficient of variation (CV) was 1.2% for WB BMD measurement. The root-mean square CV was determined from three repeated measures with repositioning 10 rats as previously described [33].
2.4. Morphological and topological characteristics of the trabecular bone
Trabecular microarchitecture of the distal metaphysis of the left femur was evaluated post-mortem using a µCT (Skyscan 1072, Skyscan, Belgium) as previously described [32-34]. Briefly, the X-ray source was set at 80kV and 100µA with an isometric pixel size 15.49µm. 225 slices were selected for each sample and analyzed by NRecon and CTan softwares (Skyscan 1072, Skyscan, Belgium). The following parameters were measured: Bone Volume/Tissue Volume BV/TV (%), Trabecular Number Tb.N (1/mm), and Trabecular Thickness Tb.Th (mm).
2.5. Morphological characteristics of cortical bone
Cortical bone of all left femurs was analyzed with the same acquisition characteristics as trabecular bone. Cortical porosity Ct.Po (%) (BV/TV equivalent) and pore number Po.N (1/mm) (Tb/N equivalent) were measured as previously described [35].
2.6. Bone mechanical testing
Mechanical properties of all left femurs were assessed by a three-point bending test with a universal testing machine (Instron 3343, Instron, Australia). Femurs were secured on the two lower supports separated by a distance of 20mm. At a loading rate of 1mm/min the following mechanical parameters were recorded: ultimate strength (N), stiffness (N/mm), yield point stress (N/mm²), moment of inertia (mm4) and Young’s modulus (MPa). This protocol was adapted from Maurel et al. [32].
2.7. Biochemical analysis
Bone turnover markers were analyzed at W0 and W8 (end of the protocol). Osteocalcin and bone alkaline phosphatase (ALP) were evaluated as markers of bone formation with N-terminal telopeptide of type I collagen (NTx) for bone resorption. ELISA kits were obtained from EIAab (China) and used according to the manufacturer’s instructions.
2.8. Statistical analysis
All numerical variables were expressed as mean ± SEM. A p-value of p≤0.05 was chosen for significance. Statistical analysis was performed using GraphPad Prism software (GraphPad Prism, USA). Physical exercise effects were analyzed using a nonparametric Mann-Whitney test to compare groups. The effects of time were analyzed with a 1-way repeated measures ANOVA.
3. Results
3.1. BMC and BMD (Table 1, Figure 2)
Whole body BMC and BMD (difference to W0) increased significantly in all groups at W4 and W8.
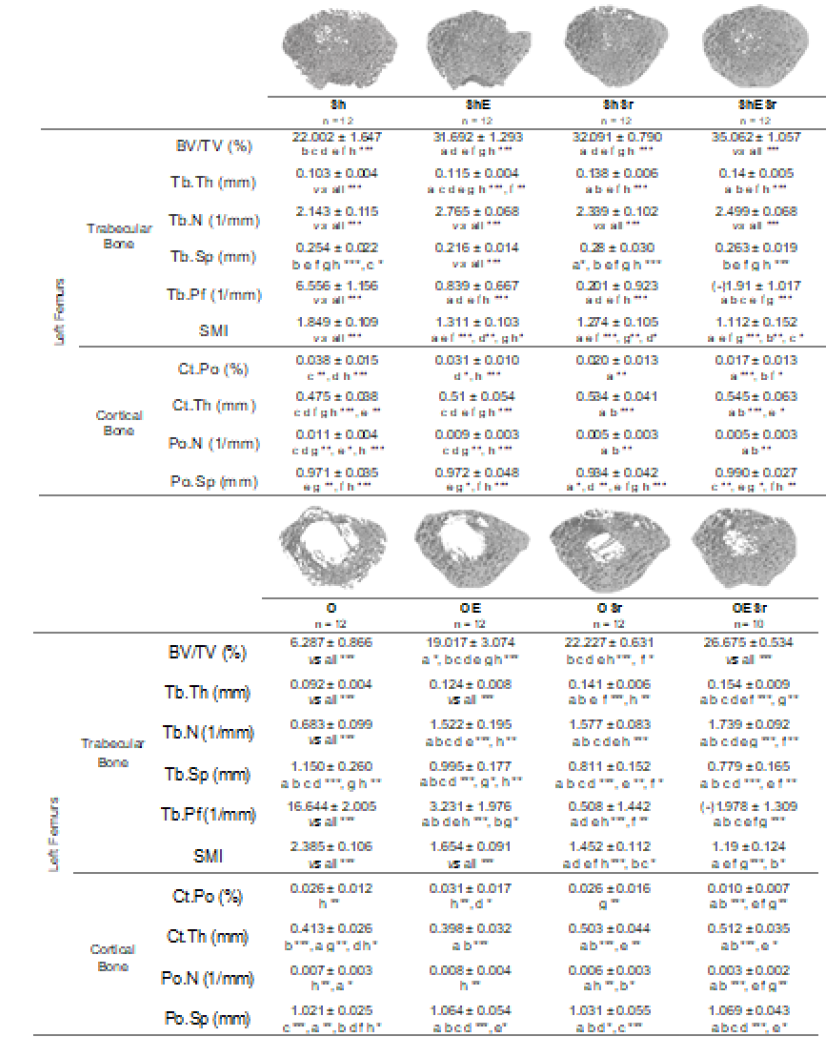
Figure 2: Microarchitecture of the trabecular and cortical bone at the left femurs of Wistar female rats. The rats in O groups were ovariectomized. Rats in E groups were submitted to 10 impacts per day, 5 days a week during 8 weeks. Rats in Sr groups received 625 mg/kg/day of SrRan. The critical p-value were p=0.05: *, p<0.01: **, p<0.001: ***, NS: non significant and a: vs Sh, b: vs ShE, c: vs ShSr, d: vs ShESr, e: vs O, f: vs OE, g: vs OSr and h: vs OESr.
3.2. Body weight, lean and fat mass (Table 1)
Weight decreased significantly only in the F30 compared to the S group (p = 0.050). Fat mass decreased significantly in all EXE groups compared to sedentary rats. Note that no differences were observed for lean body mass.
Table 1: Microarchitecture of the trabecular bone on vertebras of Wistar female rats. The rats in O groups were ovariectomized. Rats in E groups were submitted to 10 impacts per day, 5 days a week during 8 weeks. Rats in Sr groups received 625 mg/kg/day of SrRan. The critical p-value were p=0.05: *, p<0.01: **, p<0.001: ***, NS: non significant and a: vs Sh, b: vs ShE, c: vs ShSr, d: vs ShESr, e: vs O, f: vs OE, g: vs OSr and h: vs OESr.
3.3. Bone microarchitecture of the left femur (Figure 3)
BV/TV and Tb.Th increased significantly in the F45 and F60 groups compared to S, F30 and T. Tb.N increased significantly in F45 and F60 compared to all other groups. Tb.N was also significantly higher in F60 compared to F45. No difference was observed between S, F30 and T. Tb.Sp decreased significantly in F60 compared to all groups. There was no difference for Ct.Po, Ct.Th and Po.N.
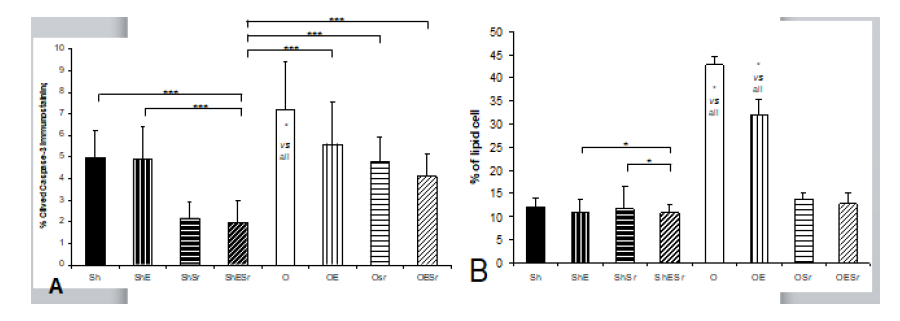
Figure 3: Osteocyte analysis. A) Osteocyte apoptosis represented by % of cleaved caspase-3 immunostaining. B) Lipid cell immunostaining. The rats in O groups were ovariectomized. Rats in E groups were submitted to 10 impacts per day, 5 days a week during 8 weeks. Rats in Sr groups received 625 mg/kg/day of SrRan. The critical p-value were p=0.05: *, p<0.01: **, p<0.001: ***, NS: non significant and a: vs Sh, b: vs ShE, c: vs ShSr, d: vs ShESr, e: vs O, f: vs OE, g: vs OSr and h: vs OESr.
3.4. Biomechanical analysis of the left femur (Table 2)
Yield point stress increased significantly in F45 compared to S, F60 and T (p = 0.016, p = 0.024 and p = 0.005 respectively). No difference was observed between S, F30, F60 and T. Young’s modulus significantly increased in F45 compared to S and T (p = 0.071 and p = 0.041). No difference was observed between the other groups. Moment of inertia decreased significantly in F45 groups compared to S and T (p = 0.032 and p = 0.036 respectively). No difference was observed between the other groups. CSA decreased significantly in F30 compared to S and F60 (p = 0.022 and p = 0.009 respectively). Concerning ultimate strength and stiffness, no difference was observed between all groups.
Table 2: Biomechanical testing at the left femurs of Wistar female rats. The rats in O groups were ovariectomized. Rats in E groups were submitted to 10 impacts per day, 5 days a week during 8 weeks. Rats in Sr groups received 625 mg/kg/day of SrRan. The critical p-value were p=0.05: *, p<0.01: **, p<0.001: ***, NS: non significant and a: vs Sh, b: vs ShE, c: vs ShSr, d: vs ShESr, e: vs O, f: vs OE, g: vs OSr and h: vs OESr.
3.5. Bone remodeling markers (Table 3)
ALP and OCN levels increased significantly in F45 compared to all groups. ALP level was lower in T compared to all groups. NTX level decreased significantly in F45 and F60 compared to S and T.
Table 3: Bone remodelling markers of Wistar female rats. Alkaline phosphatise (ALP) and ostéocalcine (OCN) were bone formation markers and NTX a bone resorption marker. The rats in O groups were ovariectomized. Rats in E groups were submitted to 10 impacts per day, 5 days a week during 8 weeks. Rats in Sr groups received 625 mg/kg/day of SrRan. The critical p-value were p=0.05: *, p<0.01: **, p<0.001: ***, NS: non significant and a: vs Sh, b: vs ShE, c: vs ShSr, d: vs ShESr, e: vs O, f: vs OE, g: vs OSr and h: vs OESr.
4. Discussion
Mechanical stress is an essential factor for maintaining overall bone health. During loading, bone receives diverse forms of mechanical stimuli resulting in complex cellular responses. These amount/forms of loading induce biological signals within osteocytes that result in various downstream signals and alteration/maintenance of bone homeostasis. In this study, the aim was to explore bone microstructure, biomechanical, and biomechanical responses to different forms and rates of physical exercise. We compared treadmill running to free fall impact and compared gradual increased heights of the latter (30, 45 and 60cm). Our results demonstrated that free fall impact exercises were more effective for enhancing bone microarchitecture, formation and strength than treadmill exercise. Second, bone formation was affected by the amount of applied mechanical stress. Both the F45 and F60 conditions produced greater advantages for bone remodeling compared to the F30 group. Biomechanical responses favored the F45 training condition.
Following 8 weeks of training, all forms of exercise decreased fat mass compared to the sedentary condition. Surprisingly, there was no exercise effect on lean body mass. Weight decreased significantly only for the F30 compared to the S condition (p = 0.050). This was likely due to a higher decrease in fat mass in this group compared to the other groups (-20%; Table 1).
Whole body BMC and BMD significantly increased at W8 compared to W0 for all groups. These findings are consistent with several previous reports. It has been shown that rats completing a 4 week interval-training program had higher femur BMD values compared to non-exercised controls [30]. In a tail-suspension-induced osteopenia model, Ju et al. have investigated the differential effects of jump exercise (10 jumps/day, 5 days/week, 40 cm of height for 5 weeks) compared to continuous running exercise on femoral BMD. They demonstrated that both jumping and running exercises significantly improved total femoral BMD [36]. At W8, BMC and BMD were significantly higher in the F45 and 60 groups compared to the S and T groups. Similar results were noted for trabecular bone microarchitecture of the left femur (BV/TV, Tb.Th, and Tb.N). Ju et al. have shown that jump exercise (10 jumps/day, 5 days/week for 5 weeks, 40 cm of height) contributed to an increase of Tb.Th and BV/TV (but not for Tb.N) in a tail suspension-induced osteopenia model. However, the continuous running exercises (25 m/min, 60 min a day, 5 days/week, for 5 weeks) increased BV/TV and Tb.N (but not Tb.Th). Consequently, different effects of these two exercises on trabecular bone microarchitecture were observed. Tb.Th was increased with jump exercise and Tb.N alone with treadmill running exercise [36]. In our protocol, Tb.Th increased in all free fall exercises and no modification was observed in treadmill exercise group. Yet Tb.TN significantly increased only in F45 and F60 and not in F30. Moreover, a significant advantage was observed in Tb.TN in F60 compared to F45.
It was also observed in the current study that yield point stress increased significantly in the F45 compared to the S, F60 and T groups while Young’s modulus increased in the F45 compared to the S and T conditions. Changes in the newly forming and pre-existing bone matrix probably related to type I collagen, which is known to affect the post-yield behavior of bone [37]. The significant increase in femur bone composition (increase in BMD, BV/TV and Tb.Th) impacts bone network structure, which probably explain the changes in femur biomechanical properties. Biochemical changes might have also interfered with bone matrix modification results from impact exercises notably at the collagen fiber level and thus improves bone microstructure. Both ALP and osteocalcin levels were significantly higher for all free fall groups compared to the T group. Interestingly, ALP and osteocalcin concentration were significantly higher in F45 compared to all groups. The increase in ALP and osteocalcin reflects greater osteoblastic activity induced by acute exercise but the response is known to be dependent on exercise intensity and modality but also to the age and sex [38]. The higher values of the F45 group as compared to the other exercise groups might reflect that ground reaction forces induced by the free fall impact (45 cm) would be more efficacious than those produced in other exercise groups in terms of bone formation [39]. However, the precise physiological function of ALP and osteocalcin in osteogenesis is still unclear. Osteocalcin a bone formation marker and identified as a late marker of osteoblastic activity. However it has a short half-life, accumulate in patients with severe renal failure and Vitamin K dependent [37]. Some studies highlight that osteocalcin is involved in bone mineralization rather than matrix and ALP is involved in osteoid formation and bone mineralization [40]. Regarding NTX, recognized as a bone resorption marker, they are generated from the amino terminus of the type 1 collagen by cleavage of N-terminal region by cathepsin K during the resorption phase of bone turnover. After 8 weeks of training NTX expression was significantly decreased in the F45 compared to the S and T groups. Although we do not assess directly the RANKL expression in our experiment [41], as the NTX production is a reflect of the resorption process, our results suggest that the mechanical signals induced by the F45 exercise on osteoclast gave a better limitation of bone resorption that those produced in the other groups. Thus,it may be hypothesized that changes in biochemical (NTX, ALP and osteocalcin) are sensitive to the amount of mechanical loadings on both osteoblast and osteoaclast (treadmill and F30 exercises are too low and F60 to high).
5. Conclusion
Both treadmill and impact training yielded positive benefits on BMD and BMC. However, free-fall exercise produced greater effects than treadmill running for several parameters of bone health. Both biochemical and biomechanical bone parameters were sensitive to the level of applied mechanical stress suggesting that this variable may be important in exercise prescription programs.
Author Contributions
Aveline PC, Lespessailles E, Best TM, Cesaro A, Toumi H.
Funding
This research was funded by Servier France.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Institutional Review Board of the of the uni-ver-sity of Orleans and all experiments were approved by the animal protection committee of the Univer-sity of Orleans, France (agreement n°: CEEA VdL; delivered: 2011-11-2).
Acknowledgments
The authors thank Dr. Laurent Benhamou for his support and Eric Dolleans from I3MTO laboratory, for animal experiment.
Conflicts of Interest
The authors declare no conflict of interest.
Reference
- Bass S, Pearce G, Bradney M, et al. Exercise before Puberty May Confer Residual Benefits in Bone Density in Adulthood: Studies in Active Prepubertal and Retired Female Gymnasts. J. Bone Miner. Res. Off. J Am Soc Bone Miner Res 13 (1998): 500-507.
- Blimkie CJ, Rice S, Webber CE, et al. Effects of Resistance Training on Bone Mineral Content and Density in Adolescent Females. Can. J Physiol Pharmacol 74 (1996): 1025-1033.
- Kannus P, Haapasalo H, Sankelo M, et al. Effect of Starting Age of Physical Activity on Bone Mass in the Dominant Arm of Tennis and Squash Players. Ann Intern Med 123 (1995): 27-31.
- Karlsson MK, Vergnaud P, Delmas PD, et al. Indicators of Bone Formation in Weight Lifters. Calcif. Tissue Int 56 (1995): 177-180.
- Maïmoun L, Sultan C. Effects of Physical Activity on Bone Remodeling. Metabolism 60 (2011): 373-388.
- Bonewald LF. The Amazing Osteocyte. J. Bone Miner. Res. Off. J Am Soc Bone Miner Res 26 (2011): 229-238.
- Robergs RA, Icenogle MV, Hudson TL, et al. Temporal Inhomogeneity in Brachial Artery Blood Flow during Forearm Exercise. Med. Sci. Sports Exerc 29 (1997): 1021-1027.
- Klein-Nulend J, Nijweide PJ, Burger EH. Osteocyte and Bone Structure. Curr. Osteoporos. Rep 1 (2003): 5-10.
- Klein-Nulend J, Bacabac RG, Veldhuijzen JP, et al. Microgravity and Bone Cell Mechanosensitivity. Adv. Space Res. Off. J. Comm. Space Res. COSPAR 32 (2003): 1551-1559.
- Verborgt O, Tatton NA, Majeska RJ, et al. Spatial Distribution of Bax and Bcl-2 in Osteocytes after Bone Fatigue: Complementary Roles in Bone Remodeling Regulation? J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res 17 (2002): 907-914.
- Dallas SL, Prideaux M, Bonewald LF. The Osteocyte: An Endocrine Cell ... and More. Endocr. Rev 34 (2013): 658-690.
- Thompson WR, Rubin CT, Rubin J. Mechanical Regulation of Signaling Pathways in Bone. Gene 503 (2012): 179-193.
- Robling AG, Niziolek PJ, Baldridge LA, et al. Mechanical Stimulation of Bone in Vivo Reduces Osteocyte Expression of Sost/Sclerostin. J Biol Chem 283 (2008): 5866-5875.
- Tu X, Rhee Y, Condon KW, et al. Sost Downregulation and Local Wnt Signaling Are Required for the Osteogenic Response to Mechanical Loading. Bone 50 (2012): 209-217.
- Klein-Nulend J, Bakker AD, Bacabac RG, et al. Mechanosensation and Transduction in Osteocytes. Bone 54 (2013): 182-190.
- Toumi H, Higashiyama I, Suzuki D, et al. Regional Variations in Human Patellar Trabecular Architecture and the Structure of the Proximal Patellar Tendon Enthesis. J Anat 208 (2006): 47-57.
- Hamrick MW. A Role for Myokines in Muscle-Bone Interactions. Exerc. Sport Sci Rev 39 (2011): 43-47.
- Hagihara Y, Nakajima A, Fukuda S, et al. Running Exercise for Short Duration Increases Bone Mineral Density of Loaded Long Bones in Young Growing Rats. Tohoku J Exp Med 219 (2009): 139-143.
- Iwamoto J, Takeda T, Sato Y. Interventions to Prevent Bone Loss in Astronauts during Space Flight. Keio J Med 54 (2005): 55-59.
- Iwamoto J, Shimamura C, Takeda T, et al. Effects of Treadmill Exercise on Bone Mass, Bone Metabolism, and Calciotropic Hormones in Young Growing Rats. J. Bone Miner. Metab 22 (2004): 26-31.
- Iwamoto J, Yeh, JK, Aloia JF. Differential Effect of Treadmill Exercise on Three Cancellous Bone Sites in the Young Growing Rat. Bone 24 (1999): 163-169.
- Joo YI, Sone T, Fukunaga M, et al. Effects of Endurance Exercise on Three-Dimensional Trabecular Bone Microarchitecture in Young Growing Rats. Bone 33 (2003): 485-493.
- Honda A, Sogo N, Nagasawa S, et al. High-Impact Exercise Strengthens Bone in Osteopenic Ovariectomized Rats with the Same Outcome as Sham Rats. J. Appl. Physiol. Bethesda Md 95 (2003): 1032-1037.
- Honda A, Umemura Y, Nagasawa S. Effect of High-Impact and Low-Repetition Training on Bones in Ovariectomized Rats. J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner Res 16 (2001): 1688-1693.
- Umemura Y, Ishiko T, Yamauchi T, et al. Five Jumps per Day Increase Bone Mass and Breaking Force in Rats. J. Bone Miner. Res. Off. J Am Soc Bone Miner Res 12 (1997): 1480-1485.
- Rubin CT, Lanyon LE. Regulation of Bone Formation by Applied Dynamic Loads. J. Bone Joint Surg. Am 66 (1984): 397-402.
- Turner AS, Alvis M, VanderVliet MM, et al. Heterogeneity of Bone Mineral Density in Ewes. Proc Orthop Res Soc New Orleans 441 (1994).
- Järvinen TLN, Kannus P, Sievänen H, et al. Randomized Controlled Study of Effects of Sudden Impact Loading on Rat Femur. J Bone Miner Res 13 (1998): 1475-1482.
- Welch JM, Weaver CM, Turner CH. Adaptations to Free-Fall Impact Are Different in the Shafts and Bone Ends of Rat Forelimbs. J Appl Physiol Bethesda Md 1985 97 (2004): 1859-1865.
- Chen X, Aoki H, Fukui Y. Effect of Exercise on the Bone Strength, Bone Mineral Density, and Metal Content in Rat Femurs. Biomed Mater Eng 14 (2004): 53-59.
- Nagasawa S, Honda A, Sogo N, et al. Effects of Low-Repetition Jump Exercise on Osteogenic Response in Rats. J. Bone Miner. Metab 26 (2008): 226-230.
- Maurel DB, Boisseau N, Ingrand I, et al. Combined Effects of Chronic Alcohol Consumption and Physical Activity on Bone Health: Study in a Rat Model. Eur. J Appl Physiol 111 (2011): 2931-2940.
- Hagihara Y, Fukuda S, Goto S, et al. How Many Days per Week Should Rats Undergo Running Exercise to Increase BMD? J Bone Miner Metab 23 (2005): 289-294.
- Achiou Z, Toumi H, Touvier J, et al. Sclerostin Antibody and Interval Treadmill Training Effects in a Rodent Model of Glucocorticoid-Induced Osteopenia. Bone 81 (2015): 691-701.
- Bouxsein ML, Boyd SK, Christiansen BA, et al. Guidelines for Assessment of Bone Microstructure in Rodents Using Micro-Computed Tomography. J Bone Miner Res Off J Am Soc Bone Miner. Res 25 (2010): 1468-1486.
- Ju YI, Sone T, Ohnaru K, et al. Differential Effects of Jump versus Running Exercise on Trabecular Architecture during Remobilization after Suspension-Induced Osteopenia in Growing Rats. J Appl Physiol Bethesda Md 1985 112 (2012): 766-772.
- Shetty S, Kapoor N, Bondu JD, et al. Bone Turnover Markers: Emerging Tool in the Management of Osteoporosis. Indian J Endocrinol Metab 20 (2016): 846-852.
- Smith C, Tacey A, Mesinovic J, et al. The Effects of Acute Exercise on Bone Turnover Markers in Middle-Aged and Older Adults: A Systematic Review. Bone 143 (2021): 115766.
- Prawiradilaga RS, Madsen AO, Jorgensen NR, et al. Acute Response of Biochemical Bone Turnover Markers and the Associated Ground Reaction Forces to High-Impact Exercise in Postmenopausal Women. Biol Sport 37 (2020): 41-48.
- Orimo H. The Mechanism of Mineralization and the Role of Alkaline Phosphatase in Health and Disease. J. Nippon Med. Sch. Nippon Ika Daigaku Zasshi 77 (2010): 4-12.
- Rubin J, Fan X, Biskobing DM, et al. Osteoclastogenesis Is Repressed by Mechanical Strain in an in Vitro Model. J Orthop Res Off Publ Orthop Res Soc 17 (1999): 639-645.


 Impact Factor: * 5.3
Impact Factor: * 5.3 Acceptance Rate: 75.63%
Acceptance Rate: 75.63%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 
