Psychotic Episode following Treatment with Hydroxychloroquine in a 17- Year-Old Female Adolescent with Cutaneous Lupus Erythematosus: A Drug Causality Supported by a Literature Review and a Worldwide Pharmacovigilance Database Search
Cora Cravero1*, Miguel Hie2, Stéphane Barete3, Solène Spiers1,4, Julie Brunelle1, Bénédicte Lebrun-Vignes5, 6, David Cohen1, 7
1Department of Child and Adolescent Psychiatry, Reference Centre for Rare Psychiatric Diseases, AP-HP.Sorbonne Université, Groupe Hospitalier Pitié-Salpêtrière, Paris, France
2Department of Internal Medicine, French National Referral Centre for Systemic Lupus Erythematosus, Antiphospholipid Antibody Syndrome and Other Autoimmune Disorders, AP-HP.Sorbonne Université, Groupe Hospitalier Pitié-Salpêtrière, Paris, France
3Unit of Dermatology, AP-HP.Sorbonne Université, Groupe Hospitalier Pitié-Salpêtrière, Paris, France
4Department of Child and Adolescent Psychiatry, Children Day Hospital “Les Alouettes”, Centre Hospitalier Le Vinatier, Lyon, France
5Department of Pharmacology, APHP.Sorbonne Université, Groupe Hospitalier Pitié-Salpêtrière, Paris, France
6Pitié and Saint-Antoine Pharmacovigilance Centres, APHP.Sorbonne Université, Paris, France
7CNRS UMR 7222, Institute for Intelligent Systems and Robotics, Sorbonne Université, Paris, France
*Corresponding Author: Cora Cravero, Department of Child and Adolescent Psychiatry, AP-HP.Sorbonne Université, Groupe Hospitalier Pitié-Salpêtrière, 47-83 bd de l’Hôpital, F-75651 Paris Cedex 13, France
Received: 19 October 2021; Accepted: 08 November 2021; Published: 22 November 2021
Article Information
Citation: Cora Cravero, Miguel Hie, Stéphane Barete, Solène Spiers, Julie Brunelle, Bénédicte Lebrun-Vignes, David Cohen. Psychotic Episode following Treatment with Hydroxychloroquine in a 17-Year-Old Female Adolescent with Cutaneous Lupus Erythematosus: A Drug Causality Supported by a Literature Review and a Worldwide Pharmacovigilance Database Search. Archives of Clinical and Medical Case Reports 5 (2021): 862-872.
View / Download Pdf Share at FacebookAbstract
Background: Hydroxychloroquine (HCQ), a useful treatment for chronic dermatologic or rheumatologic diseases, has recently gathered widespread attention as a possible treatment for COVID-19 infection. However, its rare et severe neuropsychiatric side effects (NSE), such as psychosis and suicidal tendencies, are poorly documented, especially in youths.
Case presentation: We present the first case on a 17-yearold girl of severe acute psychosis with a suicide attempt during HCQ treatment in association with thalidomide for chronic and refractory discoid lupus erythematosus. Drug causality was evaluated using the updated French causality assessment method. We performed a literature review and a worldwide pharmacovigilance database search on psychotic features after HCQ and thalidomide treatment. We found six cases in the literature and 53 cases (psychotic disorder: N=45, 3.7% and acute psychosis: N=8, 0.7%) in the pharmacovigilance database reporting the occurrence of psychotic symptoms under HCQ and none under thalidomide. The intrinsic and extrinsic imputability scores support the hypothesis that HCQ induced psychosis and suicide attempt in our patient. Withdrawing HCQ resulted in a dramatically improved situation, which remained perfectly stable after 3 years of follow-up.
Conclusion: In HCQ-induced psychosis, recovery may be obtained with HCQ withdrawal, no future HCQ reintroduction, and, for the most serious manifestations, a short period of antipsychotic medication. Clinicians need to be aware of the NSE of HCQ and the appropriate interventions to be carried out.
Keywords
<p>Psychosis; Suicide Attempt; Hydroxychloroquine; Drug Imputability; Case Report</p>
Article Details
1. Introduction
Hydroxychloroquine (HCQ), a derivative of chloroquine, is routinely used to treat uncomplicated malaria and inflame-matory autoimmune diseases, such as systemic lupus erythematosus or chronic discoid lupus erythematosus (DLE). During the coronavirus disease 2019 (COVID-19) pandemic, HCQ received attention as a possible treatment for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and concerns for serious side effects associated with HCQ emerged. Contraindications of HCQ in the case of conditions such as maculopathy, retinopathy and QTc prolongation are well-known, and its less severe adverse events (gastrointestinal disturbances and cutaneous manifestations) are recognized [1]. However, its neuro-psychiatric side effects (NSE) are less documented. Recent articles have been interested in the NSE of chloroquine and HCQ in the wake of COVID-19 [2-5]. Even so, NSE of HCQ are mainly presented in publications recording principally NSE of chloroquine [3, 6] and are described in the elderly [5, 7-9] but not in youths. The NSE of HCQ can include mild clinical manifestations, such as affective lability, irritability, insomnia/nightmares, and psychomotor agitation, or more severe and life-threatening conditions, such as psychosis, depression, and suicidal tendencies, whose early signs are sometimes subtle [10-12].
Here, we aim to present the first case on a 17-year-old girl displaying a psychotic episode with suicide attempt attributed to HCQ, during HCQ and thalidomide treatment for refractory DLE. We performed a literature review and a worldwide pharmacovigilance database search on psychotic features after HCQ and/or thalidomide treatment and assessed the imputability of adverse drug reactions in our patient.
2. Case Presentation
The patient, a 17-year-old adolescent girl, had a diagnosis of chronic DLE with Raynaud syndrome since 9 years of age (Fitzpatrick skin phototype IV-V). Following an exacerbation of DLE, she had been taking thalidomide for three and a half months. Concomitantly, she started treatment with HCQ 400 mg/d (body mass index: 20.6 kg/m2, 50th percentile). Two weeks later, she experienced asthenia, headaches, nightmares, sadness, and a sense of persecution. Then her mental condition worsened, with the emergence of psychotic symptoms. She thought her cat was a plush, once smelled a fragrance of ginger, said her phone and the computers at home were hacked. In one month, she lost 5 kg. Six weeks after the initial symptoms, on a hallucinatory injunction, she attempted suicide by severe self-inflicted drug intoxication, taking HCQ (45 tablets, i.e., 9 g), thalidomide (28 tablets) and alprazolam (30 tablets – from the family medicine cabinet). She was hospitalized for 3 days in the ICU for a cardiac arrest. She improved with hemodiafiltration. HCQ and thalidomide administration were stopped. She was referred to an adult psychiatric department. She presented with persecutory delusions, inappropriate laughter, thought blocking, and anxiety, which improved under risperidone and alprazolam. A diagnosis of first episode psychosis was made. She was discharged after 3 weeks with risperidone 8 mg/d, alprazolam 0.25 mg at night and tropatepine 20 mg/d. She was not in a condition to return to high school. Her parents were extremely worried and requested hospitalization in a university hospital for a second psychiatric and internist opinion.
Upon admission to the Department of Child and Adolescent Psychiatry, the patient was extremely slowed cognitively, had an obvious extrapyramidal syndrome, and a weight gain of 4 kg. She had no psychotic symptoms at home for 2 weeks. Alprazolam was stopped, and risperidone was reduced then stopped within two weeks. A drug chart and a timeline of events are presented in Figure 1. The etiological assessment of a first psychotic episode was carried out. Brain MRI showed an enlargement of the cortical grooves in the bilateral frontal regions and a discrete posterior ventricular dilatation that were linked to a history of prematurity. There were no signs of cerebral lupus. The EEG was strictly normal. The patient had a past use of cannabis (smoke inhalation) the previous year, with no history of heavy cannabis use nor other substance abuse; she was an active tobacco smoker. Urine toxicology was initially negative in the ICU and was not repeated. Metabolic, genetic (CGH-array) and biological tests were normal, except a hyperprolactinemia (248 µg/L under risperidone 8 mg/d), with normalization of prolactin (12 µg/L) within 2 months after stopping risperidone. There was no circulating native anti-DNA, anti-nucleosome, and anti-soluble nuclear antigen autoantibodies, normal C3 and C4, no proteinuria, no pleuropericarditis, and no synovitis. The internist and dermatological evaluations concluded a quiescent, nonsystemic chronic DLE.
The previous year, the patient started concomitant treatment with HCQ 400 mg/d and nifedipine 30 mg/d. We realized retrospectively that, at that time after a few days, she developed asthenia, vertigo and visual disturbances that lasted for 3 weeks. During this period, she reported a fall from a bicycle and a toe fracture. She later fell from her height, and spontaneously stopped her treatment after 3 months. The prescription of HCQ was renewed 6 months later in association with thalidomide (see Figure 1 for the chronology of events). The patient had no personal psychiatric history. She was born with a moderate premat-urity at 33 SA, with no sign of fetal distress. Puberty was normally reached at age 11. There was a history of posttraumatic stress disorder and depressive episode in a first degree relative, with no other family psychiatric history.
During hospitalization, there were no hallucinatory or delusional elements, and no behavioral oddities. Within 7 weeks of stopping the antipsychotic treatment, the patient told us “to be herself again”, went back to school and found pleasure in her usual activities. She was discharged after 9 weeks of hospitalization with no drug treatment (stopping smoking and photoprotection were recommended to reduce lupus flares). HCQ was lifelong contraindicated. Psychiatric, internist and dermatological follow-ups were maintained. After 3 years of follow-up, there was no resurgence of neuropsychiatric manifestations. The patient repeated her twelfth grade and graduated with honors the next year. She joined a Fashion Design School, and she is brilliantly completing her studies. Currently, she is undergoing DLE treatment with good response to lenalidomide 5 mg/d.
2.1 Causality assessment
The intrinsic imputability score for HCQ was I4 (C3, S1) (range 0-6) (Table 1), using the updated French causality assessment method [13]. The involvement of thalidomide alone was ruled out (I0 (C2, S0)). To assess the extrinsic imputability, we performed: (1) a literature review on Medline for articles published up to August 31, 2021 on the psychotic side-effects of HCQ and/or thalidomide. We found six cases reporting the occurrence of psychotic symptoms under HCQ [8, 9, 14-17] (Table 1), and none under thalidomide. (2) A worldwide pharmacovigilance database search on VigiBase, via VigiLyze online database, the WHO global database of individual case safety reports [18] up to September 21, 2020. NSE under HCQ were reported in 1226 cases, including psychotic disorder (N=45, 3.7%), acute psychosis (N=8, 0.7%, including one case corresponding to the literature), and suicidal ideation (N=29, 2.4%). Two cases in youths and adolescents were reported out of the 53 cases of psychotic manifestations (B2 level of extrinsic imputability; range 1-4). No suspected case of acute psychosis under thalidomide was reported. We conclude that HCQ likely induced the psychosis and suicide attempt in our patient.
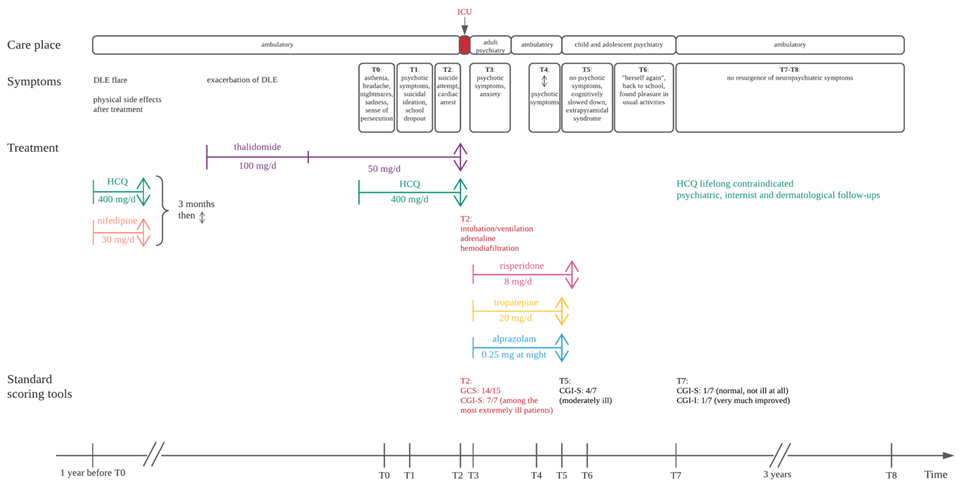
Figure 1: Drug chart and timeline of events. CGI-I: Clinical Global Impression – Improvement scale; CGI-S: Clinical Global Impression – Severity scale; DLE: discoid lupus erythematosus; GCS: Glasgow Coma Scale; HCQ: hydroxychloroquine; ICU: Intensive Care Unit; T0: initial presentation; T1: at 2 weeks; T2: at 6 weeks; T3: at 6.5 weeks; T4: at 3 months; T5: at 3.5 months; T6: at 4 months; T7: at 6 months; T8: after 3 years of follow-up; stop.
AP: antipsychotic; APS: antiphospholipid syndrome; DLE: discoid lupus erythematosus; EPLP: erosive plantar lichen planus; HCQ: hydroxychloroquine; mg/d: milligrams per day; NS: not specified; NSE: neuropsychiatric side effects; PD: psychiatric disorder; PTSD: post-traumatic stress disorder; SLE: systemic lupus erythematosus.
Table 1: description of the literature cases reporting psychotic symptoms under hydroxychloroquine
3. Discussion
Here, we report the first adolescent case of HCQ-induced psychosis and suicide attempt, that was successfully treated by a collaborative expert care. Competing contributory neuropsychiatric diagnoses were discussed during hospit-alization. Neuropsychiatric lupus and substance-induced psychotic disorder were rapidly excluded by both clinical and complementary examinations. No other psychiatric diagnoses were retained according to DSM-5: 1) for a major depressive disorder (MDD) with psychotic features, only four (depressed mood, significant weight loss, fatigue or daily loss of energy, and suicidal ideation with persecutive delusions) of the five required criteria out of nine were present. More importantly, persecutive delusions seem to have appeared quickly within 2 weeks, while the mood was not markedly depressed. MDD in adolescents is often attributable to dynamic contextual factors. It could also further follow a bipolar course [19]. We found no recent triggers that could have resulted in an MDD. There has also never been a manic, hypomanic or mixed episode, or prepubertal MDD that might have strongly suggested a bipolar disorder. 2) An early-onset schizophrenia: no history of developmental delays, a high premorbid level of functioning and no abnormal behavior, strong social skills, a remission of the psychotic symptoms after the discontinuation of antipsychotic medication. 3) A brief psychotic disorder: the psychotic behavior course was quick but not sudden and was preceded for almost two weeks by feelings of persecution; the psychotic episode was followed by complete functional remission but lasted more than 1 month (5 to 7 weeks); at last, the exclusion criteria were not met, as the psychotic behavior could have been a direct result of medication (HCQ).
Only six case reports concerning this drug-event couple we-
re previously published over the past 35 years (Table 1) [8, 9, 14-17]. Neuropsychiatric symptoms improved after the withdrawal of HCQ in all cases and upon the use of an antipsychotic medication during a short time period in most cases [9, 15-17]. Due to the long half-life of HCQ (> 3 weeks in blood and > 4 months in plasma for oral intake) and its ability to cross the blood-brain barrier, achieving tissue concentrations 10-20 times higher than plasma concentrations [20], NSE may continue for several weeks after the drug suspension. Several predictors of HCQ-induced NSE are established, such as personal or family history of psychiatric disorder, female gender, low body weight, alcohol intake, dose of HCQ > 6.5 mg/kg/day, concomitant administration of cytochrome P-450 (CYP) 3A4 inhibitors, concomitant administration of low-dose glucocorticoids, and polypharmacy [7]. Our patient took a high dose of HCQ of 7.3 mg/kg/d, which might have contributed to the occurrence of NSE. She did not have concomitant administration of glucocorticoids or CYP3A4 inhibitors. Among calcium-channel blockers, benzothiaze-pines (e.g., diltiazem) and phenylalkylamines (e.g., verapamil) are moderate inhibitors of CYP3A4, but dihydropyridines, such as nifedipine, are not. Thalidomide is associated with depressed-mood but not an increased risk of suicide attempt or psychosis. However, the combined use of thalidomide and HCQ likely increases the risk and severity of adverse effects [21].
Our patient had a high dose of risperidone. This dosage was probably not necessary to control the psychotic symptoms. She showed frequent side effects of second-generation antipsychotics despite an anticholinergic agent (tropate-pine): extrapyramidal syndrome, psychomotor slowdown/ somnolence, weight gain and hyperprolactinemia [22]. Conversely, in regard to rare side effects, the imputability of drug-related iatrogeny is more difficult to assess. We chose to use the recently updated French imputability method [13], mandatory for cases arising from spontaneous reporting [23]. Our patient presented a moderate to high intrinsic imputability score (I4; range 0-6). Unfortunately, no drug testing for HCQ has been performed over time.
The limitations to our case report include the notable challenges with toxicology screening tools, potential for substance to induce neurological changes, and effect of cardiac arrest on neurologic function. At all events, the patient experienced physical side effects of HCQ and nifedipine. Psychotic side effects appear to be associated with HCQ, while depressive symptoms might have been due to iatrogenic physical symptoms, DLE pain, and probably both HCQ and thalidomide medications. Our patient improved after the suspension of HCQ and thalidomide, and a short period of antipsychotic treatment. The improvement may have been delayed by the HCQ and thalidomide drug overdose that occurred during the suicide attempt and the clinical severity of the event.
4. Conclusion
This first adolescent case of possible HCQ-induced psycho-
sis and suicide attempt illustrates the complexity of differential diagnosis in situations with psycho-organic intrications, especially during adolescence. Clinicians should consider potential neuropsychiatric adverse drug reactions as part of every differential diagnosis. In case of suspected HCQ-induced NSE, prompt suspension of HCQ would likely resolve NSE, including psychotic symptoms and behavioral disorders. It is also worth reporting patients’ rare drug-related NSE and patients’ outcomes to pharma-covigilance databases. It would thereby facilitate the detection of NSE at an early stage and prevent any further serious adverse events, especially with use of HCQ during the COVID-19 pandemic.
Declarations
Consent for publication:
Written informed consent was obtained from the parents and from the patient for publication of this case report. A copy of the written consents is available for review by the Editor-in-Chief of this journal.
Conflicts of Interest:
The authors declare that they have no competing interests.
Funding statement:
No financial support was received for the conduct or preparation of this case report.
Acknowledgements:
The authors fully acknowledge the patient and her family for their contribution to this case report. They also thank American Journal Experts (AJE) for English language editing.
VigiBase data statement:
The information obtained from VigiBase/VigiLyze comes from a variety of sources, and the probability that the suspected adverse effect is drug-related is not the same in all cases. The information does not represent the opinion of the Uppsala Monitoring Centre or the World Health Organization.
References
- Tetu P, Hamelin A, Lebrun-Vignes B, et al. [Prevalence of hydroxychloroquine-induced side-effects in dermatology patients: A retrospective survey of 102 patients]. Ann Dermatol Venereol 145 (2018): 395-404.
- Hamm BS, Rosenthal LJ. Psychiatric Aspects of Chloroquine and Hydroxychloroquine Treatment in the Wake of Coronavirus Disease-2019: Psycho-pharmacological Interactions and Neuropsychiatric Sequelae. Psychosomatics (2020).
- Doyno C, Sobieraj DM, Baker WL. Toxicity of chloroquine and hydroxychloroquine following therapeutic use or overdose. Clin Toxicol (Phila) 59 (2021): 12-23.
- Garcia P, Revet A, Yrondi A, et al. Psychiatric Disorders and Hydroxychloroquine for Corona-virus Disease 2019 (COVID-19): A VigiBase Study. Drug Saf 43 (2020): 1315-1322.
- Lane JCE, Weaver J, Kostka K, et al. Risk of depression, suicide and psychosis with hydroxyl-chloroquine treatment for rheumatoid arthritis: a multinational network cohort study. Rheumatology (Oxford) 60 (2021): 3222-3234.
- Sato K, Mano T, Iwata A, et al. Neuropsychiatric adverse events of chloroquine: a real-world pharmacovigilance study using the FDA Adverse Event Reporting System (FAERS) database. Biosci Trends 14 (2020): 139-143.
- Mascolo A, Berrino PM, Gareri P, et al. Neuropsychiatric clinical manifestations in elderly patients treated with hydroxychloroquine: a review article. Inflammopharmacology 26 (2018): 1141-1149.
- Ferraro V, Mantoux F, Denis K, et al. Hallucinations during treatment with hydro-chloroquine. Ann Dermatol Venereol 131 (2004): 471-473.
- Altintas E. Hydroxychloroquine-induced acute psychotic disorder in a female patient with rheumatoid arthritis: a case report. Dusunen Adam The Journal of Psychiatry and Neurological Sciences 28 (2015): 369-373.
- Good MI, Shader RI. Behavioral toxicity and equivocal suicide associated with chloroquine and its derivatives. Am J Psychiatry 134 (1977): 798-601.
- Bitta MA, Kariuki SM, Mwita C, et al. Antimalarial drugs and the prevalence of mental and neurological manifestations: A systematic review and meta-analysis. Wellcome Open Res 2 (2017): 13.
- Pinho de Oliveira Ribeiro N, Rafael de Mello Schier A, Ornelas AC, et al. Anxiety, depression and suicidal ideation in patients with rheumatoid arthritis in use of methotrexate, hydroxyl-chloroquine, leflunomide and biological drugs. Compr Psychiatry 54 (2013): 1185-1189.
- Arimone Y, Bidault I, Dutertre JP, et al. Updating the French method for the causality assessment of adverse drug reactions. Therapie 68 (2013): 69.
- Ward WQ, Walter-Ryan WG, Shehi GM. Toxic psychosis: a complication of antimalarial therapy. J Am Acad Dermatol 12 (1985): 863-865.
- Hsu W, Chiu N, Huang S. Hydroxychloroquine-induced acute psychosis in a systemic lupus erythematosus female. Acta Neuropsychiatr 23 (2011): 318-319.
- Das P, Rai A, Chopra A, et al. Psychosis likely induced by hydroxychloroquine in a patient with chronic Q fever: a case report and clinically relevant review of pharmacology. Psychosomatics 55 (2014): 409-413.
- Gonzalez-Nieto JA, Costa-Juan E. Psychiatric symptoms induced by hydroxychloroquine. Lupus. 24. England (2015): 339-340.
- Uppsala Monitoring Centre (2020).
- Swann AC, Geller B, Post RM, et al. Practical Clues to Early Recognition of Bipolar Disorder: A Primary Care Approach. Prim Care Companion J Clin Psychiatry 7 (2005): 15-21.
- Rynes R. Antimalarials. In: Kelley W, Harris E, Ruddy S, Sledge C, editors. Textbook of Rheuma-tology. London, United Kingdom: Saunders Compagny (2001): 864-865.
- DrugBank Online. OMx Personal Health Analytics Inc (2020).
- Cohen D, Bonnot O, Bodeau N, et al. Adverse effects of second-generation antipsychotics in children and adolescents: a Bayesian meta-analysis. J Clin Psychopharmacol 32 (2012): 309-316.
- Journal officiel de la République française (JORF) (2020).


 Impact Factor: * 5.3
Impact Factor: * 5.3 Acceptance Rate: 75.63%
Acceptance Rate: 75.63%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 
