Surgical Intervention for Two Patients Suffering from Pituitary Apoplexy Associated with Covid-19
Mohamad Yazbeck1*, Elissa Abi Fadel2, Christin Berjaoui3, Gerard Abadjian4, Beatrice Akiki5, Youssef Comair6
1Departments of Neurosurgery, Lebanese University and Lebanese Hospital Geitaoui, Beirut, Lebanon
2Lebanese University faculty of Medical Sciences and Lebanese Hospital Geitaoui, Beirut, Lebanon
3Beirut Arab University, Faculty of Medicine, Beirut, Lebanon
4Department of Pathology, Lebanese Hospital Geitaoui, Beirut, Lebanon
5Department of Pathology, Lebanese University, Beirut, Lebanon
6Department of neurosurgery, Lebanese University, Lebanese Hospital Geitaoui, Beirut, Lebanon
*Corresponding Author: Dr. Mohamad Yazbeck, Lebanese University and Lebanese Hospital Geitaoui, Departments of Neurosurgery, Beirut, Lebanon
Received: 27 August 2021; Accepted: 14 September 2021; Published: 27 September 2021
Article Information
Citation: Mohamad Yazbeck, Elissa Abi Fadel, Christin Berjaoui, Gerard Abadjian, Beatrice Akiki. Surgical Intervention for Two Patients Suffering from Pituitary Apoplexy Associated with Covid-19. Archives of Clinical and Medical Case Reports 5 (2021): 665-671.
View / Download Pdf Share at FacebookAbstract
Background: Pituitary apoplexy (PA), which is mainly associated with pituitary adenomas, is a very rare disease. PA imposes high risk of hemorrhages and infarction, which in most cases; emergent surgical intervention would be needed. Moreover, SARS-CoV-2 is a newly emergent viral infection, which has shown several neurological manifestations, including intracranial hemorrhages. Yet, a definitive correlation between PA and COVID-19 still needs further investigations.
Observations: We report two cases of COVID-19 male patients, who presented with PA manifestations, mainly headache associated with ocular paresis and diplopia. Both patients underwent transsphenoidal resection of the sellar tumor, and both showed significant clinical improvement.
Conclusion: Pituitary apoplexy has been linked to COVID- 19 as a post-infection complication, with surgery being a successful treatment.
Keywords
<p>Pituitary apoplexy; COVID-19; Transsphenoidal resection</p>
Article Details
1. Introduction
Pituitary apoplexy is a rare disease, with prolactinomas being the most prevalent type of PA. Patients with pituitary apoplexy typically present with a sudden severe onset of thunderclap or migraine like headache, visual disturbances like diplopia, ocular paresis or even vision loss. Other symptoms may include altered mental status, and hormonal dysfunction such as hypopituitary disease, Addison disease, and, prolactinoma [1]. For the past year, a new emergent viral infection, SARS-CoV-2, spread worldwide, affecting millions of people. SARS-CoV-2 mainly attacks the lungs, resulting in severe respiratory distress symptoms; yet, several cases have been reported correlating some neurological manifestations with COVID-19 infection, such as headache, dizziness, and stroke. In addition, some cases have been reported linking COVID-19 as a possible precipitating factor for pituitary apoplexy [2]. In our article, we present 2 cases of young male patients, admitted with pituitary apoplexy, and who were covid-19 positive, for which we performed surgical resection of the mass.
2. Case Study
2.1 Cas
A 45 years old male with a past history of COVID-19 infection 3 weeks’ prior, presents to the ED with severe thunderclap headache associated with ocular paresis and blurred vision. He claimed that these symptoms started on the 10th day after being tested as COVID-19 positive, for which he got hospitalized for symptoms management. Upon physical examination, palsies for all of the cranial nerves 3, 4, and 6 were noted with mydriatic non-reactive pupils, left eye ptosis and decreased bilateral visual field acuity. MDCT of the sinus was performed with sagittal and coronal reconstruction, which showed a large lesion of 3.7 cm involving the sella turcica and eroding the clivus (Figure 1). In addition, invasion of the sphenoidal sinus was noted, with a possible extension into the posterior ethmoidal air cells suggestive of invasive macroadenoma (Figure 1). Neurosurgery was consulted, and transphenoidal resection of the sellar tumor was performed. A sample was sent to the ana-pathology which showed the following (Figure 1). The headache resolved immediately after surgery. Additionally, ptosis and ocular paresis resolved after 3 months, however panhypopituitary is present and is being treated with hydrocortisone, thyroid hormone and testosterone.
2.2 Case
A 28 years old male with COVID-19 infection upon admission presents to the ED with severe acute headache associated with a new onset of diplopia as well as esotropia of the left eye. He claimed that these symptoms started 2 days ago, prior to his admission. Cerebral MRI showed a lesion involving the sella turcica with c invasion of the para-sellar, supre-sellar and sphenoid sinus cavities (Figure 2). Transphenoidal resection of the sellar tumor was performed. A sample was sent to the anapath which showed the following (Figure 3). The headache resolved immediately after surgery. Additionally, both diplopia and esotropia resolved after 3 months.
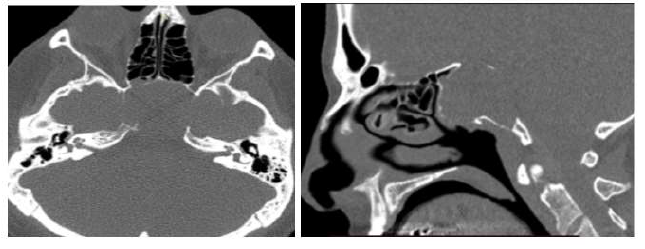
Figure 1: Brain CT scan showing (A) 3.7 cm large mass involving the sella turcica and eroding the medial wall of the petrous segment of the left and right internal carotid artery and the foramen lacerum. (B) the mass eroding the sellar floor and sphenoid sinus.
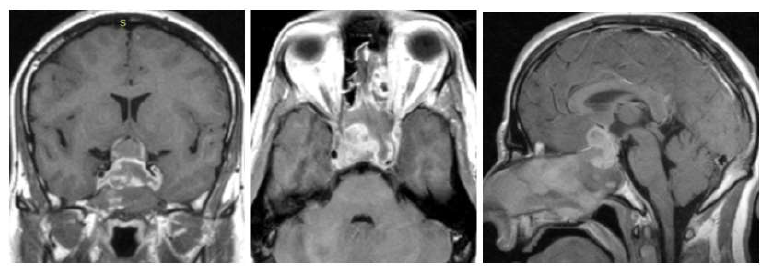
Figure 2: Brain MRI T1 sequence: (A) Coronal Cut, showing sellar tumor invading both cavernous bilateral sinuses and compressing and elevating the optic chiasm superiorly. (B) Axial cut, tumor encompassing the right and left internal carotid arteries. (C) sagittal section, tumor invading the sellar base and sphenoid sinus.
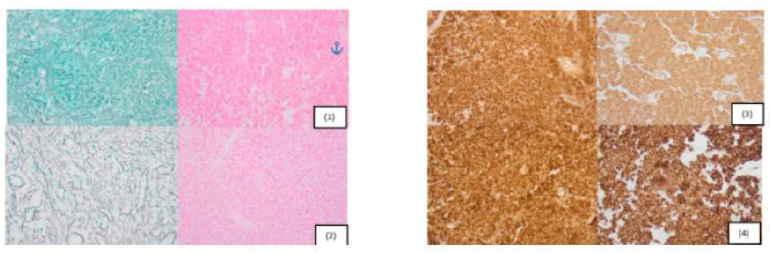
Figure 3: Pathological picture of the tumors for both patients (1) complete necrotic tissue, without nuclear details. Sheet like growth pattern illustrated in both patients (2) the endocrine pattern of the tumor necrosis revealed by a dense reticulin meshwork in both cases. (3) Intense and diffuse staining of tumor cell with the pancytokeratin. (4) Intense and diffuse staining of tumor cell with the synaptophysin.
(A) Complete necrotic tissue, without nuclear detail. Sheet like growth pattern illustrated in both patients. (Hematoxylin and eosin, original magnification x400). (B) The endocrine pattern of the tumor necrosis revealed by a dense reticulin meshwork in both cases. (Reticulin stain, original magnification x400). (C) Intense and diffuse staining of tumor cell with the pancytokeratin. (Original magnification x400). (D) Intense and diffuse staining of tumor cell with the synaptophysin. (Original magnification x400).
3. Discussion
3.1 Presentation
The incidence of symptoms affecting patients with PA vary, for instance, headache being the most common of all clinical presentations, following with Visual acuity loss, hypopituitarism, nausea, ocular paresis and diplopia and finally altered level of consciousness. Within our case, the most prevalent chief complaint was headache associated with ocular paresis and diplopia at a lesser prevalence [3].
3.2 Diagnosis
PA diagnosis is primarily based clinically, in accordance, with adequate history taking of the patient and symptoms evaluation, and followed by a thorough physical examination which must include neurological assessment, mainly targeting the cranial nerves from II to the VI, as well as a visual field exam and an OCT test. Fundoscopy could be performed in order to rule out papilledema of optic atrophy. In addition, lab tests should be taken, more importantly for the pituitary hormones. And finally yet importantly, an MRI with contrast should be done to confirm the definitive diagnosis of PA [1].
3.3 Management
PA primary management consists of hypothalamic-pituitary-adrenal axis maintenance using hydrocortisone. To note, in cases of hypopituitarism, the patient may suffer from Addisonian shock. So in order to avoid it, cortisol must be replaced 24 hrs. before the administration of thyroid hormones [4]. Subsequently, in the presence of macroprolactinoma, and even for all patients with PA up until their prolactin level is determined, cabergoline should be administered as first-line therapy, as suggested by Brisman et al [5]. Additionally, some patients may suffer from hyponatremia as a consequence of SIADH secondary to both hypocortisolism and hypothyroidism. Therefore, once the cortisol and thyroid hormones are corrected, then sodium will likewise be corrected. Nevertheless, if sodium concentrates are needed, administration should be done at a very slow rate (0.5 mEq/h or 12 mEq/d) to avoid the risk of either CPM or EPM leading to severe neurologic deficits [6].
The next step of the treatment is controversial among different guidelines. For instance, U.S guidelines prefer surgical intervention, however, on the contrary, European guidelines advise conservative management accompanied by follow up whenever it is needed. The goal of surgical resection differs between acute and sub-acute cases. While in acute presentation our primary goal is to decompress the optic apparatus, pituitary gland, and the cavernous sinus, in sub-acute case, the surgery’s goal is a maximal safe tumor resection [1]. In parallel, conservative approach is based on the fact that the mass resorbs by itself like in small intraparenchymal hematoma [1]. Choosing between surgery or conservative treatment is based on several criteria like time of presentation and severity of symptoms.
3.4 Outcome
Many studies have demonstrated that conservative management is as efficient as surgical intervention when it comes to headache and oculomotor dysfunction [7]. Nevertheless, it has been reported in one of the studies that surgical intervention, did in fact, provide better efficiency for oculomotor dysfunction [8]. This study goes in concordance with the results we faced in our study where both of our patients were cured from headache and oculomotor diseases after surgery. As a matter of fact, OMD resolved in almost 90% of the patients following a 1-year post-op [9]. Contrariwise, visual field and acuity only improved in about 74 to 94% of patients with a much lower rate of complete resolution, with both surgery and conservative management [3, 10, 11]. Additionally, recovery from hormonal deficiency appeared very low within both surgical and conservative management, with an improvement rate of approximately 20% and in dependence on the time of the operation [10, 11]. Despite that, better endocrine improvement was seen with subclinical cases over the acute ones [12].
Moreover, less severe symptoms and better outcomes were seen in infarction than hemorrhagic tumor [13]. Large or invasive tumors, severe vision loss and long use of anticoagulants were also associated with poor prognosis [7]. Regarding the risk of residual viable tumor, and recurrence of PA especially when these risk factors are present, surgery is often preferred [14]. This clarifies why for our two cases, surgical resection was performed despite the fact that the patients presented with sub-acute PA without any major neurological deficits, since within both of the cases, the tumor diagnosed was large in size as well as invasive, due to the hemorrhagic infarct. Therefore, surgery was done in order to secure a tumor resection with a maximal safe margin along with optic chiasm decompression.
3.5 Pituitary apoplexy and coronavirus (correlation with other case reports)
In analogy to our cases, we are correlating with 2 cases, who were COVID-19 positive and of whom were diagnosed with PA and subsequently, treated surgically. The first case corresponds to a 28 years old pregnant female; whose chief complaints were blurry vision associated with a left dilated pupil along with a headache that lasted for 4 days. In addition, her prolactin level was high and associated with hypopituitarism [15]. As for the second case, corresponds to a 47 years old male whose main chief complaint was left frontal headache associated with diplopia, ptosis of the left eye, as well as loss of visual acuity.16 In both cases, MRI was performed, and surgical transphenoidal resection was done with a 2 weeks’ delay for the pregnant female, and emergently done for the male for whom transsphenoidal hypophysectomy was performed. Both patients had a full recovery after the surgery [15, 16].
Furthermore, the results of our 2 cases and the timing of improvement were similar to other studies, complete resolution of headache and diplopia after 3 months. Not to mention that also the patient was on hormone replacement post-op.
3.6 Pituitary apoplexy & COVID-19 pathophysiology
To understand better the correlation between PA & COVID-19, the hypophyseal portal system of pituitary adenomas is much less vascularized, meaning of inhibited angiogenesis, leading to a compensation which is achieved by the direct blood supply from both capsular and loral arteries respectively [17]. To further elaborate, COVID-19 has been linked to several events of vascular thromboembolism, with venous being more prevalent than arterial in terms of vascular involvement. Thus, increasing the risk of hemorrhagic complication which in turn, would lead to PA [18]. Another perspective also associates acute necrotizing hemorrhagic encephalitis to the cytokine storm syndrome that might also be released by Coronavirus [19].
4. Conclusion
COVID-19 remains an emergent disease that needs further studies to understand its pathophysiology better. Yet, several neurological manifestations have been linked as post-covid-19 complications, with pituitary apoplexy being one. Moreover, surgical resection has been proven to be an effective treatment for pituitary apoplexy.
References
- Barkhoudarian G, Kelly DFJNC. Pituitary apoplexy. Neurosurg Clin N Am 30 (2019): 457-463.
- Bordes SJ, Phang-Lyn S, Najera E, et al. Pituitary apoplexy attributed to COVID-19 infection in the absence of an underlying macroadenoma or other identifiable cause Case Report 13 (2021).
- Briet C, Salenave S, Bonneville J-F, et al. Pituitary apoplexy. Endocr Rev 36 (2015): 622-645.
- Mattke AF, Vender JR, Anstadt MR. Pituitary apoplexy presenting as Addisonian crisis after coronary artery bypass grafting. Tex Heart Inst J 29 (2002): 193-199.
- Brisman MH, Katz G, Post KDJJon. Symptoms of pituitary apoplexy rapidly reversed with bromocriptine: Case report. J Neurosurg 85 (1996): 1153-1155.
- Laureno RJAoNOJotANA, Society tCN. Central pontine myelinolysis following rapid correction of hyponatremia. Ann Neurol 13 (1983): 232-242.
- Ayuk J, McGregor EJ, Mitchell RD, et al. Acute management of pituitary apoplexy–surgery or conservative management?. Clin Endocrinol (Oxf) 61 (2004): 747-752.
- Tu M, Lu Q, Zhu P, et al. Surgical versus non-surgical treatment for pituitary apoplexy: a systematic review and meta-analysis. J Neurol Sci 370 (2016): 258-262.
- Hage R, Eshraghi SR, Oyesiku NM, et al. Third, fourth, and sixth cranial nerve palsies in pituitary apoplexy. World Neurosurg 94 (2016): 447-452.
- Rutkowski MJ, Kunwar S, Blevins L, et al. Surgical intervention for pituitary apoplexy: an analysis of functional outcomes. J Neurosurg 129 (2017): 417-424.
- Gondim JA, de Albuquerque LAF, Almeida JP, et al. Endoscopic endonasal surgery for treatment of pituitary apoplexy: 16 years of experience in a specialized pituitary center 108 (2017): 137-142.
- Liu ZH, Chang CN, Pai PC, et al. Clinical features and surgical outcome of clinical and subclinical pituitary apoplexy 17 (2010): 694-699.
- Semple PL, Jane JA, Lopes MBS, et al. Pituitary apoplexy: correlation between magnetic resonance imaging and histopathological results 108 (2008): 909-915.
- Vicente A, Lecumberri B, Gálvez MÁ, et al. Clinical practice guideline for the diagnosis and treatment of pituitary apoplexy 60 (2013): 582. e581-582. e512.
- Chan JL, Gregory KD, Smithson SS, et al. Pituitary apoplexy associated with acute COVID-19 infection and pregnancy. Pituitary 23 (2020): 716-720.
- Santos CDSE, Santos CAT, Neill JS, Vale HF, Kurnutala LNJRBA. Pituitary tumor resection in a patient with SARS-CoV-2 (COVID-19) infection. A case report and suggested airway management guidelines. Braz J Anesthesiol (2020): 165-170.
- Turner HE, Nagy Z, Gatter KC, et al. Angiogenesis in pituitary adenomas and the normal pituitary gland. J Clin Endocrinol Metab 85 (2000): 1159-1162.
- Lodigiani C, Iapichino G, Carenzo L, et al. Venous and arterial thromboembolic complications in COVID-19 patients admitted to an academic hospital in Milan, Italy. Thromb Res 191 (2020): 9-14.
- Poyiadji N, Shahin G, Noujaim D, et al. COVID-19-associated acute necrotizing hemorrhagic encephalopathy: CT and MRI features (2020).


 Impact Factor: * 5.3
Impact Factor: * 5.3 Acceptance Rate: 75.63%
Acceptance Rate: 75.63%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 
