Systemic Amyloidosis Involving the Fallopian Tube
Vina P Nguyen, Lisa Mendelson, Michelle Kaku, John L Berk, Vaishali Sanchorawala*
Amyloidosis Center, Boston University School of Medicine and Boston Medical Center, Boston, MA
*Corresponding Author: Vaishali Sanchorawala, MD, Section of Hematology/Oncology, FGH 1001, 820 Harrison Avenue, Boston, MA 02118, USA
Received: 09 February 2022; Accepted: 18 February 2022; Published: 28 February 2022
Article Information
Citation: Vina P Nguyen, Lisa Mendelson, Michelle Kaku, John L Berk, Vaishali Sanchorawala. Systemic Amyloidosis Involving the Fallopian Tube. Archives of Clinical and Medical Case Reports 6 (2022): 110-115.
View / Download Pdf Share at FacebookKeywords
<p>Amyloidosis; Hydrosalpinx; Infertility; Light chain immunoglobulin; Transthyretin</p>
Article Details
1. Introduction
Systemic amyloidosis refers to a group of protein folding disorders characterized by extracellular tissue deposition of misfolded insoluble protein fibrils that demonstrate apple-green birefringence with polarized light microscopy of Congo red stained tissue [1]. There are numerous precursor amyloidogenic proteins, but two of the most common include the immunoglobulin light chain, causing AL amyloidosis and transthyretin, causing ATTR amyloidosis. The precursor protein is significant, because current therapy is aimed at reducing the production of the amyloidogenic protein to slow progression of disease. For example, AL amyloidosis is treated with therapy that targets the plasma cells of the bone marrow, where the monoclonal light chains are produced [2]. In ATTR amyloidosis, therapies include transthyretin tetramer stabilizers and transthyretin RNA interference (RNAi), otherwise known as gene silencers which halt the production of TTR by the liver [3]. The usual organs involved by systemic amyloidosis include the heart, kidney, nervous system, gastrointestinal tract, and liver. Involvement of the reproductive tract and the fallopian tubes is rare and, on our review of the literature, less than ten case reports have been published. Of these, many different types of precursor protein were identified, including four cases with untyped amyloidosis [4-6], and individual case reports involving secondary serum amyloid A (AA) amyloidosis [6], wild-type ATTR amyloidosis [7], hereditary ATTR amyloidosis [8], beta-2 microglobulin amyloidosis [9], and MALT lymphoma associated amyloidosis [10]. We did not find any reported cases associated with systemic AL amyloidosis. Here we present two patients with systemic amyloidosis and biopsy proven fallopian tube involvement, one with AL amyloidosis and the other with hereditary ATTR amyloidosis, who were evaluated at our tertiary referral amyloidosis center between January 1, 2015 and February 28, 2018. During this time period, we evaluated 255 patients with AL amyloidosis and 89 patients with hereditary ATTR amyloidosis.
2. Case Presentation
Case 1
A 44-year-old Japanese woman, gravida 2 para 2, with no past medical history developed progressively worsening shortness of breath and cough for a month. She was found to have large bilateral pleural effusions requiring repeated therapeutic thoracenteses and placement of a PleurX drainage catheter, as well as anasarca and ovarian cysts on CT of the chest, abdomen and pelvis. She denied a history of menometrorrhagia or infertility. Concern initially was for ovarian cancer given an elevated CA-125 of 307 U/mL; CEA and CA 19-9 were normal. She underwent laparoscopic abdominal surgery with bilateral salpingectomy and unilateral oophorectomy. Biopsies of the omentum and peritoneum were also taken given their abnormal gross appearance. Pathology showed amyloid deposits by Congo red stain in the peritoneum, omentum and bilateral fallopian tubes. Right ovary, as well as endometrial and endocervical biopsies were benign and without evidence of amyloid deposition. The omental biopsy was sent for laser capture microdissection and tandem mass spectrometry-based proteomics, which showed the amyloid to be AL-lambda type. Further testing showed a normal serum free kappa light chain level of 12.4 mg/L (range 3.3-19.4 mg/L), an elevated lambda serum free light chain level of 1046.9 mg/L (range 5.7-26.3 mg/L), and normal immunoglobulin levels. She also had a cardiac MRI that showed diffuse late gadolinium enhancement as well as moderately increased left ventricular wall thickness up to 15 mm, consistent with cardiac amyloidosis. She was then referred to our medical center, where she had a bone marrow biopsy that showed 15-20% monoclonal plasma cells with lambda light chain restriction and normal cytogenetics. Fat pad aspirate showed amyloid deposits by Congo red stain (Figure 1). Blood tests showed elevation in cardiac enzymes with a B-type natriuretic peptide of 240 pg/mL (range <100 pg/mL), troponin I of 0.044 ng/mL (range <0.013 ng/mL), and NT-proBNP of 866 pg/mL (range <300 pg/mL). Serum immunofixation electrophoresis showed a trace free lambda monoclonal protein and urine immunofixation electrophoresis showed no monoclonal immunoglobulin. Urine total protein excretion was not significantly elevated at 100 mg/24 hour. She was diagnosed with systemic AL amyloidosis involving the heart, peritoneum, omentum, and fallopian tubes. At the time of initial evaluation, she was not a candidate for high dose chemotherapy and autologous stem cell transplantation due to decompensated heart failure so she was started on treatment with cyclophosphamide, bortezomib, and dexamethasone and achieved only a partial hematologic response after four cycles. She was subsequently treated with single agent daratumumab achieving a complete hematologic response and organ response as demonstrated by her NTproBNP decreasing from 866 pg/mL at baseline to 189 pg/mL.
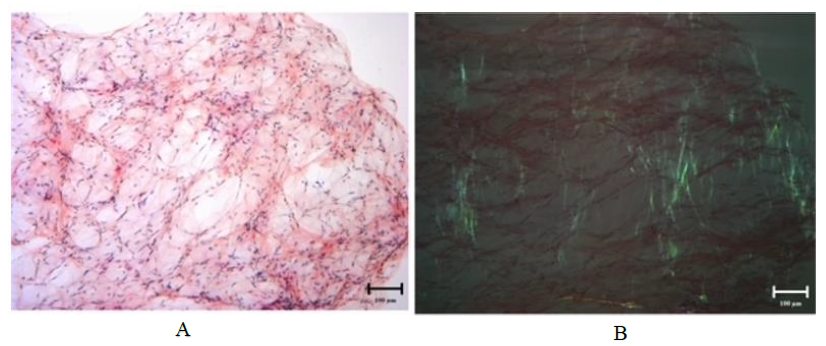
Figure 1: Congo red stain on fat pad aspirate for Case 1 demonstrating amyloid deposits on light microscopy at 100x magnification (A) and exhibiting apple-green birefringence under polarized light at 100x magnification (B).
Case 2
A 33-year-old Caucasian woman, gravida 1 para 1, was initially diagnosed with hereditary E42G ATTR genopositivity by genetic testing at age 26. Her mother, who also had the disease, developed peripheral neuropathy in her 20s and underwent a liver transplant at age 41. The patient developed symptoms of carpal tunnel syndrome bilaterally, which was likely related to amyloidosis, and underwent bilateral carpal tunnel release surgery at age 33. Subsequently, a fat pad aspirate showed amyloid deposits on Congo red stain, confirming active disease, although she had no other overt symptoms and only mildly decreased sensation to pinprick in her feet on neurologic examination. At the same time, she underwent workup for secondary infertility due to failure to become pregnant after two years of regular intercourse despite normal ovulation. She already had a healthy 4-year-old child with no postpartum complications or history of sexually transmitted diseases. A hysterosalpingogram showed right hydrosalpinx with occlusion, a uterine fundal endometrial polyp, and normal appearing left fallopian tube. Other infertility testing of her and her husband was normal. She then underwent a hysteroscopic polypectomy and laparoscopic right salpingectomy. Pathology of the right fallopian tube showed amyloid deposits, and the endometrial polyp was benign. Six months later, she proceeded with in vitro fertilization with preimplantation genetic diagnosis, and gave birth by caesarean section to a healthy baby. She was then started on diflunisal 250 mg twice daily, an NSAID that has been shown to inhibit neurologic disease progression and improve quality of life in patients with ATTR amyloidosis [11]. Eventually she went on to participate in a clinical trial evaluating efficacy and safety of ALN-TTRSC02 (now known asVutrisiran) in patients with hereditary ATTR amyloidosis. Vutrisiran is a RNAi therapeutic designed to block the production of wild-type and mutant TTR protein.
2. Discussion
In systemic amyloidosis, the insoluble extracellular protein fibrils can deposit in any organ, but documented amyloid deposition in the fallopian tubes is rare. Here we present two cases of patients with systemic amyloidosis and biopsy proven amyloid deposits in the fallopian tubes. In the first case, amyloid deposits were simultaneously also found in the omentum and peritoneum at diagnosis, and on further evaluation cardiac involvement was discovered as the cause of most of the patient’s symptoms. She originally underwent evaluation for ovarian cancer given her young age and presentation with bilateral pleural effusions, but was found instead to have systemic AL amyloidosis. Amyloid deposits in the fallopian tubes did not cause any known issues or recognized infertility. On the other hand, in the second case, the patient was of reproductive age and, after her first pregnancy, suffered from secondary infertility. In the workup of her infertility, she was found to have one fallopian tube blocked by amyloid deposits, and required in vitro fertilization for her second pregnancy. She had a hereditary form of ATTR amyloidosis, and thus presented earlier in life. In comparison, the first patient had AL amyloidosis, where the median age at diagnosis is around 60 years [11]. Thus, it is possible that more patients with systemic AL amyloidosis have fallopian tube amyloid deposits but never manifest any clinical symptoms, such as infertility, given the later age of onset. After demonstrating amyloid deposits, typing the amyloid to find the amyloidogenic precursor protein is of vital importance in order to formulate an appropriate treatment plan, offer genetic counseling when relevant, and discuss prognosis. There are over 36 different types of systemic amyloidosis, each with different therapeutic options. Only systemic AL amyloidosis is treated with chemotherapy, monoclonal antibodies, immunomodulatory drugs, and other agents aimed at the underlying monoclonal plasma cell [12]. In contrast for hereditary TTR therapeutics include transthyretin tetramer stabilizers like tafamidis [13], RNAi silencers like patisiran or vutrisiran [14], and antisense oligonucleotides (ASO) like inoteresen [15]. Amyloid deposits in the fallopian tubes have been found in patients with beta-2 microglobulin dialysis related amyloidosis [16], AA amyloidosis6, wild-type ATTR amyloidosis [7], hereditary ATTR amyloidosis [8], and now we also present a case associated with systemic AL amyloidosis. Hence, the location of the amyloid deposit does not inevitably suggest the type of amyloid, and specific typing must be performed in order to tailor treatment for the patient. Typing can be performed by immunoflurorescence, immunohistochemistry, and immunogold electron microscopy, but the most advanced technique is laser microdissection followed by mass spectrometry [1], as was used with the first patient. In conclusion, fallopian tube involvement is a rare but possible complication of different types of systemic amyloidosis. It can be associated with infertility especially if it presents during the reproductive years. In our case, fertility treatment with in vitro fertilization ended with a successful pregnancy. Overall, treatment is aimed at the underlying systemic disease, depending on the type of amyloid. While incurable, many treatment options exist for AL amyloidosis, and several new therapies for ATTR amyloidosis are currently in clinical trials.
Consent for Publication
Written informed consent was obtained from the patients for publication of this Case report and any accompanying images. A copy of the written consent is available for review by the Editor of this journal.
Availability of Data and Materials
NA
Conflicts of interest
The authors have no potential conflicts of interest to declare.
Funding
NA
Authors' Contributions
VPN did literature search and manuscript draft. LM, MK, JLB, VS interpreted the patient's data and assisted with manuscript critique. All authors read and approved the manuscript before submission.
Acknowledgments
The authors would like to acknowledge Haili Cui for obtaining the images.
References
- Muchtar E, Dispenzieri A, Magen H et al. Systemic amyloidosis from A (AA) to T (ATTR): a review. J Intern Med 289 (2021): 268-292.
- Merlini G, Dispenzieri A, Sanchorawala V, et al. Systemic immunoglobulin light chain amyloidosis. Nat Rev Dis Primer 4 (2018): 38.
- Koike H, Katsuno M. Transthyretin Amyloidosis: Update on the Clinical Spectrum, Pathogenesis, and Disease-Modifying Therapies. Neurol Ther 9 (2020): 317-333.
- Copeland W, Hawley PC, Teteris NJ. Gynecologic amyloidosis. Am J Obstet Gynecol 153 (1985): 555-556.
- Jongen VH, Grond AJ, Van Veelen H, et al. Uterine amyloidosis in menopause. Br J Obstet Gynaecol 105 (1998): 362-364.
- Yue CC, Lampman JH, Park CH, et al. Secondary amyloidosis: diagnosis from an endometrial biopsy. Arthritis Rheum 26 (1983): 1295-1296.
- Pinney JH, Whelan CJ, Petrie A, et al. Senile systemic amyloidosis: clinical features at presentation and outcome. J Am Heart Assoc 2 (2013): e000098.
- Arpa Gutiérrez J, Morales C, Lara M, et al. Type I familial amyloid polyneuropathy and pontine haemorrhage. Acta Neuropathol (Berl) 86 (1993): 542-545.
- Mount SL, Eltabbakh GH, Hardin NJ. Beta-2 microglobulin amyloidosis presenting as bilateral ovarian masses: a case report and review of the literature. Am J Surg Pathol 26 (2002): 130-133.
- Mehta N, Schöder H, Chiu A, et al. Adnexal mass secondary to extranodal marginal zone lymphoma of mucosa-associated lymphoid tissue (MALT lymphoma) with associated amyloid deposition. BMJ Case Rep 12 (2014) 1889-1895.
- Merlini G, Stone MJ. Dangerous small B-cell clones. Blood 108 (2006): 2520-2530.
- Abdallah M, Sanchorawala V. Update on the contemporary treatment of light chain amyloidosis including stem cell transplantation. Am J Med 63 (2022): 211-216.
- Maurer MS, Schwartz JH, Gundapaneni B, et al. Tafamidis treatment for patients with transthyretin amyloid cardiomyopathy. N Engl J Med 379 (2018): 1007-1016.
- Adams D, Gonzalez-Duarte A, O’Riordan WD, et al. Patisiran, an RNAi Therapeutic, for hereditary transthyretin amyloidosis. N Engl J Med 379 (2018): 11-21.
- Benson MD, Waddington-Cruz M, Berk JL, et al. Inotersen treatment for patients with hereditary transthyretin amyloidosis. N Engl J Med 379 (2018): 22-31.
- Mount SL, Eltabbakh GH, Hardin NJ. Beta-2 microglobulin amyloidosis presenting as bilateral ovarian masses: a case report and review of the literature. Am J Surg Pathol 26 (2002): 130-133.


 Impact Factor: * 5.3
Impact Factor: * 5.3 Acceptance Rate: 75.63%
Acceptance Rate: 75.63%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 
