The Dilemma of Regularly Missed Diagnoses: ADTKD
Karl X. Knaup1, Maike Büttner-Herold2, Bernt Popp3, Johanna Stoeckert1, Mario Schiffer1, Markus Schueler1, André Reis3, Kerstin Amann2, Arif B. Ekici3, Michael S. Wiesener1
1Department of Nephrology and Hypertension, University Hospital Erlangen, Friedrich-Alexander-University Erlangen-Nürnberg, Erlangen, Germany
2Department of Nephropathology, Institute of Pathology, University Hospital Erlangen, Friedrich-Alexander-University Erlangen-Nürnberg, Erlangen, Germany
3Institute of Human Genetics, University Hospital Erlangen, Friedrich-Alexander-University Erlangen-Nürnberg, Erlangen, Germany
*Corresponding Author: Prof. Dr. Michael S. Wiesener, Department of Nephrology and Hypertension, University Hospital Erlangen, Friedrich-Alexander-University Erlangen-Nürnberg, Ulmenweg 18, 91054 Erlangen, Germany
Received: 02 August 2019; Accepted: 26 August 2019; Published: 25 November 2019
Article Information
Citation: Knaup KX, Büttner-Herold M, Popp B, Stoeckert J, Schiffer M, Schueler M, Reis A, Amann K, Ekici AB, Wiesener MS. The Dilemma of Regularly Missed Diagnoses: ADTKD. Archives of Clinical and Medical Case Reports 3 (2019): 500-507.
View / Download Pdf Share at FacebookAbstract
An ill-defined number of patients with chronic kidney disease (CKD) receive an incorrect diagnosis. Numerous diseases are rather unspecific in terms of clinical appearance and histological characteristics, particularly hereditary kidney diseases. Autosomal dominant tubulointerstitial kidney diseases (ADTKD) can be seen as a paradigm of the diagnostic challenge, where only molecular genetics can assure the diagnosis.
We report a young lady with CKD who historically received a diagnosis of “biopsy-proven” IgA nephropathy (IgAN). She received 8 months of oral steroids, with side effects but further renal deterioration. Despite a positive family history with CKD over at least three generations, this clue was not considered. We established a doubtless diagnosis of MUC1-associated ADTKD by a heterozygous frameshift-causing mutation by SNaPshot minisequencing in our index patient and her affected father. Furthermore, the mucin 1 frameshift protein was readily detectable in the 10 year old kidney biopsy. We hypothesize that similar diagnostic failures are frequent in the current routine. Interestingly, the misdiagnosis of IgAN may be an underestimated problem since a substantial fraction of the healthy population may show mesangial proliferation and IgA deposition. The correct diagnosis of hereditary diseases has several clinical implications and should be the ultimate aim of precision nephrology.
Keywords
<p>MCKD; Interstitial nephritis; TIN; IF/TA; CKD</p>
Article Details
Abbreviations:
WES- Whole Exome Sequencing; ESRD- End-Stage Renal Disease; CKD- Chronic Kidney Disease; CNV- Copy Number Variations; ADTKD- Autosomal Dominant Tubulointerstitial Kidney Diseases; IF/TA- Tubular Atrophy and Interstitial Fibrosis; IgAN- IgA nephropathy; NSAR- Non-Steroidal Antirheumatics; VNTR- Variable Number of Tandem Repeats; MUC1-fs- MUC1 frameshift protein.
1. Introduction
The potential of high throughput sequencing by exon panels or whole exome sequencing (WES) has increasingly moved our focus recently towards hereditary kidney diseases. Several hundred monogenic kidney diseases are meanwhile being discussed, which most frequently affect children. However, some diseases or the effect of phenotype variabilities may lead to onset of disease as late as adult age [1, 2]. Population based studies have clearly shown that the occurrence of end-stage renal disease (ESRD) in an individual, positions family members to a significantly higher risk to also develop ESRD [3, 4]. Indeed, incident dialysis patients show family members with ESRD possibly exceeding 30%, which we feel strongly exceeds the current clinical perception [5]. Accordingly, recent studies investigating different cohorts of patients with chronic kidney disease (CKD) by WES have yielded unexpectedly high amounts of disease causing mutations [6-9], where complex mutations, such as copy number variations (CNV) and inversions/deletions, as well as variants of unknown significance have yet to be evaluated.
A particularly difficult group of hereditary kidney diseases to diagnose clinically are the Autosomal Dominant Tubulointerstitial Kidney Diseases (ADTKD [10]). These diseases are characterized by progressive decline in kidney function, reaching ESRD usually in mid adulthood. No specific clinical or morphological findings can be named. Furthermore, routine renal histology merely shows chronic degenerative changes such as tubular atrophy and interstitial fibrosis (IF/TA) and glomerulosclerosis, where immunohistochemistry and electron microscopy remain negative or inconclusive, respectively. However, the disease can be suspected in such adult patients where the pedigree shows several affected family members in more than one generation. In these cases only molecular genetics can secure the diagnosis, where numerous subtypes need to be considered and respective genes tested: UMOD, MUC1, HNF1B, REN and SEC61A1 [10, 11]. All listed genes can be analyzed by standard Sanger sequencing and/or massive parallel sequencing, with exception of the crucial region of MUC1. In the latter, a highly complex and repetitive structure impedes any standard nucleic acid amplification [12]. Therefore, to date, detection of the pathogenic mutations of MUC1 is only possible in few specialized laboratories world-wide. Should the family history of CKD in any given patient with ADTKD not be recognized or a patient shows sporadic disease by a de novo mutation, a reliable diagnosis on clinical or histological grounds will not be possible. Thus, many patients receive an erroneous diagnosis. In view of the strongly improved diagnostic potential of molecular genetics this circumstance is rather unfortunate and should be improved in future clinical nephrology.
2. Material and Methods
2.1 PAS staining
Periodic acid-Schiff reaction was performed after de-waxing and rehydration with graded ethanol, applying periodic acid (Merck, Darmstadt, Germany) for 5 minutes and Schiff Reagent (Sigma-Aldrich, Steinheim, Germany) for 7 minutes. Counterstaining was performed with Mayer`s Haematoxylin solution (Merck, Darmstadt, Germany) for 30 seconds and section were finally dehydrated.
2.2 Sirius red staining
Staining sections were rehydrated, then Weigert`s Haematoxylin A+B (Waldeck, Münster, Germany) was applied for 5 minutes and after rinsing Sirius Red F3B (Waldeck, Münster, Germany) was added for 30 minutes. Finally, section were rehydrated.
2.3 Immunofluorescent stainings for IgA and C3c
For IgA and C3c-immunofluorescence stainings FITC-conjugated antibodies were applied for 30 minutes (both 1:10, Dako, polyclonal, Glostrup, Denmark) after incubation in aceton for 10 minutes.
2.4 Immunohistochemistry
Paraffin sections (2 µm) were dewaxed in xylene and rehydrated in a series of ethanol washes. For antigen retrieval before staining of MUC1-fs protein, all slides were cooked in a microwave 20 min in 0.1 M citrate puffer. Endogenous peroxidase activity was blocked by incubation for 10 min with Peroxidase Real (DAKO). All blocking steps were performed at room temperature.
Sections were incubated with primary antibodies diluted in Antibody Diluent (Dako) overnight at 4°C. For MUC1-fs protein staining, slides were incubated with ImmPRESS Reagent Kit, Anti-mouse, -rabbit Ig for 30 min. Finally, AEC+ solution (DAKO) was used as chromogen according to the manufacturer’s instructions. All incubations were performed in a humidified chamber. Between incubations, specimens were washed three times in Tris-buffered saline (50 mmol/L Tris-HCl and 136 mmol/L NaCl, pH 7.4). Samples were processed in parallel throughout. Finally, the sections were counterstained with hematoxylin solution according to Mayer (DAKO) and analyzed with a Leica DMRB microscope (Leica, Bensheim, Germany).
3. Case Report
The patient reported is a female, currently 35 years of age. She received a preemptive kidney transplantation by a live donation from her husband in 2012, when her serum creatinine peaked 12 mg/dl. The course of kidney transplantation has been unremarkable with a creatinine of currently below 1 mg/dl and no proteinuria. She gave birth to a healthy boy in 2015, after developing preeclampsia and receiving a caesarean section at gestational week 35. In her clinical transplant records, her underlying renal disease was documented as IgA nephropathy (IgAN). In 2009, at 26 years of age, she was transferred from her general practitioner because of an elevated serum creatinine of 2.2 mg/dl. She visited him because of unspecific indisposition, which triggered a routine blood check, including the serum creatinine. She had no known pre-existing diseases, or any medication. There was no preceding infection, or any temporary medication, specifically no use of non-steroidal antirheumatics (NSAR). Physical examination was unremarkable. Blood pressure was moderately elevated up to 160/95 mmHg on repeated measurements. Urine investigations showed no proteinuria. Very few eumorphic erythrocytes in the urinary sediment were described. No clinical or laboratory investigation suggested the existence of an inflammatory/autoimmune disease. Ultrasound was unremarkable, apart from small singular cysts in both kidneys, measuring 1.8 cm in the largest diameter. A renal biopsy was performed and showed focal tubulointerstitial inflammation with moderate IF/TA and weak mesangial hypercellularity (Figure 1A and B). The immunofluorescence staining displayed a moderate granular positivity in the mesangium for IgA (Figure 1C) and stronger for C3c (Figure 1D). Electron microscopy showed weak mesangial cellular and matrix expansion with traces of osmiophilic deposits in the mesangial space (data not shown). The pathological summary at the time suggested IgAN with a low risk for progression, alongside tubulointerstitial nephritis.
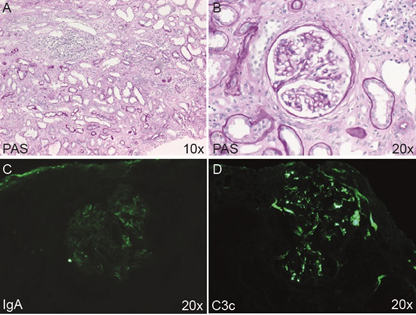
Figure 1: Routine processing of kidney biopsy. (A and B) PAS staining showing mild to moderate tubulointerstitial inflammation and tubulointerstitial scarring. (A). Glomeruli showed no signs of intracapillary or extracapillary proliferative glomerulonephritis (B). The immunofluorescence staining showed a moderate granular positivity in the mesangium for IgA (C), and slightly stronger for C3c (D). Magnifications as indicated.
The patient was dismissed with a triple antihypertensive therapy and a therapy with prednisolone 1mg/kg was initiated, which persisted for 6 weeks. The prednisolone was then tapered over 6 further months. The patient complained of numerous side effects of the steroids and moderately gained weight. During the 7.5 months of steroid treatment, the serum creatinine further increased beyond 3 mg/dl. In the numerous clinical reports it was repeatedly mentioned that the father of the patient also suffered from ESRD, with the reason being unknown. No family member was sent to genetic consultation. We previously identified the father from a tissue array of his previous tumor nephrectomy by immunohistochemistry for the frameshift protein of mutated mucin 1 (MUC1-fs) [13]. Molecular genetics subsequently confirmed that he suffered from ADTKD-MUC1. Therefore, we investigated whether our patient inherited the disease. Staining of the kidney biopsy with Sirius Red showed moderate lamellation and splicing of the tubular membranes, which is a compatible but rather unspecific sign of tubular disease (Figure 2A). Immunohistochemistry for MUC1-fs showed a clearly positive and cytoplasmic staining pattern in distal tubules (Figure 2B), as described in other ADTKD-MUC1 samples before [13]. Patients with ADTKD-MUC1 most frequently display a duplication of a cytosine base (dupC) in any one of the variable number of tandem repeats (VNTR), where 7 cytosines are usually situated [12]. We previously established a SNaPshot minisequencing protocol which allows the base specific detection of the mutation in the affected repeat [14] and which confirmed the dupC germline mutation in our patient (Figure 2C).
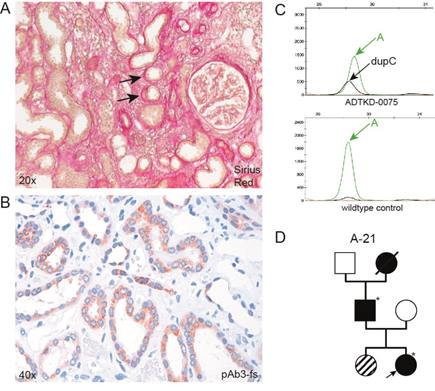
Figure 2: Identification of ADTKD-MUC1 as leading cause of CKD. (A) Sirius Red staining and (B) immunohistochemical staining of MUC1-fs with pAb3-fs[13]. Arrows in (A) indicate lamillation and splicing of tubular basal membrane. (C) Electropherogram of SNaPshot minisequencing of leucocyte DNA, showing the pathogenic dupC mutation (black curve) in the crucial region of the MUC1 VNTR. The wild-type allele displays an adenine at the respective position (green curve). (D) Pedigree, where the arrow indicates the reported individual. Asterisks indicate individuals with performed and confirmed SNaPshot minisequencing for dupC MUC1. The individual with the dashed symbol has moderate renal insufficiency (serum creatinine, 1.4 mg/dl), but was not yet tested for dupC MUC1.
Having established the molecular diagnosis of ADTKD-MUC1, we re-evaluated the family pedigree (Figure 2D). The father of our patient reached ESRD at the age of 44 and is meanwhile transplanted successfully. His mother died at the age of 68 due to unknown kidney failure. The sister of our patient shows an elevated serum creatinine of 1.4 mg/dl, but did not yet wish to perform the genetic test. Her clinical data in concert with the knowledge of the familial disease strongly suggests that she is also affected by ADTKD-MUC1.
4. Discussion
The discussed case herein is a fine example of the diagnostic challenge that a kidney biopsy can pose. A number of findings in the renal histology are completely unspecific in nature, such as IF/TA, glomerulosclerosis, vascular sclerosis and hyalinosis, as well as tubulointerstitial inflammation. Different diseases or conditions do in fact merely show such features, which are hypertensive nephropathy, toxicity of NSAR or calcineurin inhibitors or tubulointerstitial hereditary diseases and nephritis. Very often, the pathological report in these cases can solely be descriptive and a correct diagnosis is only possible in concert with a correct clinical description and history taking.
This situation is clearly different in inflammatory glomerular diseases, where glomerular (ultrastructural) morphology, together with characteristic immunodetection of immunoglobulins and/or complement in the context of the clinical findings usually secures the diagnosis. However, this may be different in IgAN, which is the most frequent type of glomerulonephritis [15]. Mesangial deposition of immunoglobulin A is a finding not so uncommon in the general population, where some individuals never develop IgAN. Accordingly, early autopsy studies showed that 4.8% of cases displayed diffuse mesangial IgA deposits, where the great minority had any signs of renal disease such as microscopic hematuria or proteinuria [16]. Similar, but quantitatively diverse findings were reported by 1.3% of autopsies of trauma victims and suicides in Finland [17], as well as from 15.6% of deceased donor candidates in Japan [18], showing mesangial IgA deposition, respectively. The great variation in suspected IgAN may indeed reflect the global variability of the disease. However, biopsy practice patterns and technological aspects, as well as population screening may profoundly change these figures [15, 19]. Hence, we hypothesize that there is some potential of wrongly classifying renal injury as IgAN, where the true cause may not be correctly recognized.
The lady reported here has had a diagnosis of IgAN with concurrent interstitial nephritis for a decade. The fact that her pedigree clearly indicated an autosomal dominant disease was never acknowledged. We cannot completely negate that influences of IgAN and/or interstitial nephritis may have contributed to the progression of her kidney disease. However, the lack of proteinuria and a bland urinary sediment argue against a dominant effect. Indeed, inheritance of a mutation of ADTKD alone is usually sufficient to reach ESRD in mid adulthood, which results from the almost complete penetrance of the disease [11, 10]. Since neither the clinical feature in any single patient, nor the histological findings can secure the diagnosis, family history and molecular genetics are of paramount importance. The timely diagnosis of this disease ensures best counseling of patients and families, secures living related kidney donation and prevents unnecessary diagnostic procedures. Finally, the correct diagnosis of ADTKD will avoid immunosuppressant medication, since there are no data to support such an approach which might even harm patients.
Acknowledgements
Funded by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation), project number 387509280, SFB 1350, C4, as well as DFG WI 1581/4-1.
Conflict of Interest
The authors declare that they have no competing interests.
References
- Vivante A, Hildebrandt F. Exploring the genetic basis of early-onset chronic kidney disease. Nature reviews Nephrology 12 (2016):133-146.
- Devuyst O, Knoers NV, Remuzzi G, et al. Rare inherited kidney diseases: challenges, opportunities, and perspectives. Lancet 383 (2014): 1844-1859.
- O'Dea DF, Murphy SW, Hefferton D, et al. Higher risk for renal failure in first-degree relatives of white patients with end-stage renal disease: a population-based study. American journal of kidney diseases : the official journal of the National Kidney Foundation 32 (1998): 794-801.
- Skrunes R, Svarstad E, Reisaeter AV, et al. Familial clustering of ESRD in the Norwegian population. Clinical journal of the American Society of Nephrology: CJASN 9 (2014): 1692-1700.
- Freedman BI, Volkova NV, Satko SG, et al. Population-based screening for family history of end-stage renal disease among incident dialysis patients. Am J Nephrol 25 (2005): 529-535.
- Lata S, Marasa M, Li Y, et al. Whole-Exome Sequencing in Adults With Chronic Kidney Disease: A Pilot Study. Annals of internal medicine 168 (2018): 100-109.
- Groopman EE, Marasa M, Cameron-Christie S, et al. Diagnostic Utility of Exome Sequencing for Kidney Disease. The New England journal of medicine 380 (2019): 142-151.
- Mann N, Braun DA, Amann K, et al. Whole-Exome Sequencing Enables a Precision Medicine Approach for Kidney Transplant Recipients. J Am Soc Nephrol 30 (2019): 201-215.
- Connaughton DM, Kennedy C, Shril S, et al. Monogenic causes of chronic kidney disease in adults. Kidney Int (2019).
- Eckardt KU, Alper SL, Antignac C, et al. Autosomal dominant tubulointerstitial kidney disease: diagnosis, classification, and management-A KDIGO consensus report. Kidney Int 88 (2015): 676-683.
- Bleyer AJ, Kidd K, Zivna M, et al. Autosomal Dominant Tubulointerstitial Kidney Disease. Adv Chronic Kidney Dis 24 (2017): 86-93.
- Kirby A, Gnirke A, Jaffe DB, et al. Mutations causing medullary cystic kidney disease type 1 lie in a large VNTR in MUC1 missed by massively parallel sequencing. Nat Genet 45 (2013): 299-303.
- Knaup KX, Hackenbeck T, Popp B, et al. Biallelic Expression of Mucin-1 in Autosomal Dominant Tubulointerstitial Kidney Disease: Implications for Nongenetic Disease Recognition. J Am Soc Nephrol 29 (2018): 2298-2309.
- Ekici AB, Hackenbeck T, Moriniere V, et al. Renal fibrosis is the common feature of autosomal dominant tubulointerstitial kidney diseases caused by mutations in mucin 1 or uromodulin. Kidney Int 86 (2014): 589-599.
- Rodrigues JC, Haas M, Reich HN. IgA Nephropathy. Clinical journal of the American Society of Nephrology: CJASN 12 (2017): 677-686.
- Waldherr R, Rambausek M, Duncker WD, et al. Frequency of mesangial IgA deposits in a non-selected autopsy series. Nephrology, dialysis, transplantation: official publication of the European Dialysis and Transplant Association - European Renal Association 4 (1989): 943-946.
- Varis J, Rantala I, Pasternack A, et al. Immunoglobulin and complement deposition in glomeruli of 756 subjects who had committed suicide or met with a violent death. Journal of clinical pathology 46 (1993): 607-610.
- Suzuki K, Honda K, Tanabe K, et al. Incidence of latent mesangial IgA deposition in renal allograft donors in Japan. Kidney Int 63 (2003): 2286-2294.
- Schena FP, Nistor I. Epidemiology of IgA Nephropathy: A Global Perspective. Seminars in nephrology 38 (2018): 435-442.


 Impact Factor: * 5.3
Impact Factor: * 5.3 Acceptance Rate: 75.63%
Acceptance Rate: 75.63%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 
