A Case of Marjolin’s Ulcer – High-Resolution Ultrasound Findings with a Review of Literature
Article Information
Jeena Bordoloi Deka1*, Ritu Shah2, Mohit Veer Kumar Shah3, Miguel Jimenez Fermín4, Nirvikar Dahiya5, Fernando Jiménez Díaz6
1Dispur Polyclinic & Hospitals Pvt Ltd. Guwahati, Assam, India
2Seth G S Medical College and King Edward Memorial Hospital, Mumbai, India
3Abhipraay, Centre for Advance Ultrasound-guided interventions and Genetic Clinic, Mumbai, India
4Orthopedic Surgery Department, Hospital Asepeyo, Coslada Madrid, Spain
5Radiology Department, Mayo Clinic, Phoenix, USA
6Director of International Chair of MSK Ultrasound, San Antonio University of Murcia (UCAM), Spain
*Corresponding Author Jeena Bordoloi Deka, MD Radiology, Dispur Polyclinic & Hospitals Pvt Ltd. Guwahati, Assam, India
Received: February 26, 2024; Accepted: March 13, 2024;Published: March 28, 2024
Citation:
Jeena Bordoloi Deka, Ritu Shah, Mohit Veer Kumar Shah, Miguel Jimenez Fermín, Nirvikar Dahiya, Fernando Jiménez Díaz. A Case of Marjolin’s Ulcer – High-Resolution Ultrasound Findings with a Review of Literature. Archives of Clinical and Medical Case Reports. 8 (2024): 55-58.
View / Download Pdf Share at FacebookAbstract
Marjolin’s ulcer is a cutaneous malignancy that arises from previously damaged skin, long-standing scars, chronic wounds such as burns, chronic venous ulcers, pressure ulcers, chronic osteomyelitis, or sinuses. Burn scars are the most common lesion leading to this malignancy. Marjolin’s ulcer affects 1% to 2% of all burn scars. We present a case of Marjolin’s ulcer occurring in an old burn scar in a 46-year-old female, focusing on the high-resolution ultrasound features and its role in its management. Ultrasound was found to be helpful not only in evaluating the extent and depth of the lesion, but also in indicating the likelihood of malignant transformation. Histopathology confirmed it to be a squamous cell carcinoma. A wide excision with skin grafting was done. To the best of our knowledge, there is no literature reporting the ultrasound evaluation and findings of Marjolin’s ulcer.
Keywords
Marjolin; Burn scar; High-resolution ultrasound; Malignancy, Squamous cell carcinoma
Article Details
Introduction
Marjolin’s ulcer is a cutaneous malignancy arising from previously damaged skin, long-standing scars and chronic wounds. Malignant transformation occurs in 1% to 2% of all burn scars allowed to heal by secondary intention. The development of a non-healing ulcer in a chronic wound or scar should raise suspicion of malignant degeneration to a Marjolin’s ulcer (MU). High- resolution ultrasound can play a role in the evaluation of these ulcers. It can be used both as an indicator of the presence and type of malignancy and also as an indicator of its extent and depth. A biopsy is needed for confirmation of diagnosis.
Case Presentation
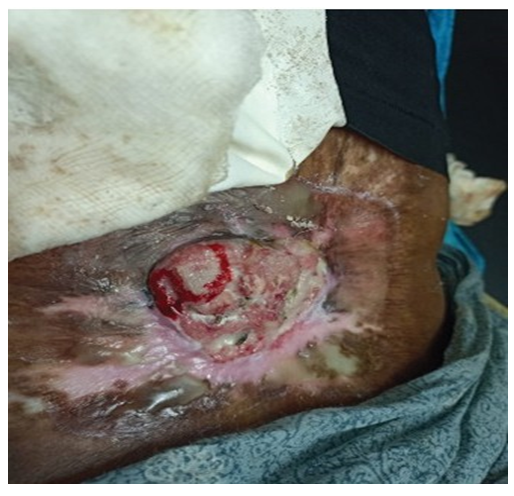
A 46-year-old female presented with history of a large, non-healing, ulcerating, exophytic mass with bleeding for 8 months. The ulcer was present in an old burn scar in the region of her left lower back (Figure 1). She had a history of flame-associated burn injury in the area approximately 42 years back. Initially, the scar was dry; however recently, she had been experiencing intermittent ulcerations in the scar. Relapses and remissions had been occurring for the past 20 years. A clinical diagnosis of Marjolin’s ulcer was made.
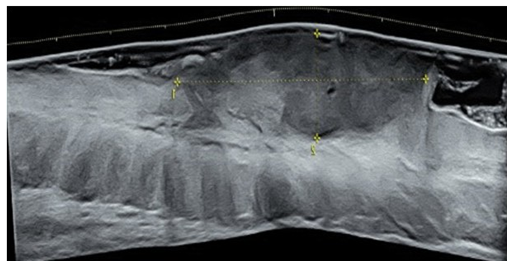
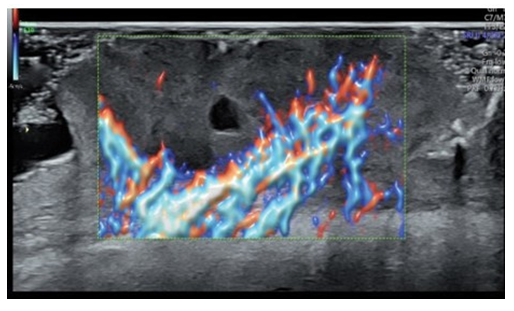
Patient was sent for pre-operative ultrasound imaging to evaluate for vascularity, extent and depth of the tumor. High-resolution ultrasound was done with a Voluson® E10 (GE Healthcare, Milwaukee, WI, USA) linear matrix transducer (6-15 Mhz). Ultrasound demonstrated a fairly well-defined, homogenously hypoechoic lesion 50 mm in cross-section and 20mm in depth. The lesion involved the skin and subcutaneous soft tissues of the left lower back (Figure 2). It had an irregular surface margin, showing ulcerations with rolled-out everted edges. The deeper margin was confined to the hypodermis and approximately 30mm away from the underlying muscles. The lesion was accompanied by thickening of the surrounding skin. Color doppler revealed increased vascularity of the lesion - predominantly at the base and in the underlying subcutaneous tissue. The pattern of vascularity was better depicted on power doppler study. A type 2 pattern of vascularity was evident from the linear, twisting, branching pattern covering more than 50% of the area of the lesion [1]. These ultrasound findings were strongly indicative of a squamous cell carcinoma (Figure 3).
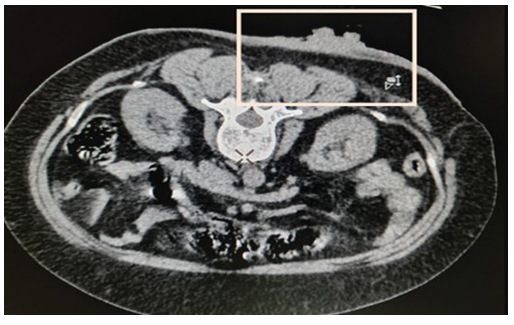
Computed tomography (CT) scan correlation was done subsequently to evaluate for visceral or bony metastasis (Figure 4). CT Scan images corroborated with the ultrasound findings. In fact, ultrasound delineation of the actual tumor from the surrounding thickened dermis and subcutaneous soft tissue was higher resolution than that of CT. The ulcer was surgically excised with a rim measuring 2 cm, and a skin graft was performed. Follow-up after 1 year showed no evidence of recurrence.
Biopsy with histopathological correlation confirmed diagnosis to be squamous cell carcinoma (SCC).
Figure 2: Short axis and long axis grayscale ultrasound of the mass showing a hypoechoic fairly well-defined homogenous mass lesion of size 48 x18 mm involving the skin and part of the subcutaneous soft tissues of the left lower back. The lesion had an irregular superficial margin due to ulceration.
Discussion
Historically, Marjolin’s ulcers are named after the French surgeon, Jean Nicolas Marjolin and were first described in 1828 as ulcerations with dense villi arising within a burn cicatrix [2]. It was Dupuytren, 2 years later, who noted that the lesions were malignant [3, 4]. Approximately 1.7% of chronic wounds undergo malignant transformation, and the incidence in burn scars specifically is 0.77-1 to 2.0%.3,4 Burn scars are the most common lesion causing this malignancy [5]. 75% of Marjolin’s ulcers occur in old burn scars - particularly deep partial thickness burns and full thickness burns that heal by secondary intention [4, 6]. Flame burns are more predisposed to the same [6]. Other chronic inflammatory etiologies that can lead to Marjolin’s ulcers are traumatic wounds, venous stasis ulcers, osteomyelitis, pressure ulcers, radiation dermatitis, stings, bites, hidradenitis suppurativa. Immunocompromised people are at increased risk [2]. The exact mechanism is still unknown, and the aetiology can be multifactorial with environmental and genetic factors. Chronic irritation, infection and immune deficiency are common factors [6-8]. The release of toxins by damaged, nutritionally deficient tissue is responsible for neoplastic change [4, 7]. An elevated expression of proto-oncogenes plays a role [4, 8]. Marjolin’s ulcers account for 0.5 % of all skin cancers [3]. The most common histologic variant of MU is SCC (71 %). Other manifestations include basal cell carcinoma (BCC), malignant melanoma, angiosarcoma, fibrosarcoma, dermatofibrosarcoma protruberans, osteosarcoma, malignant fibrous histiocytoma, liposarcoma, leiomyosarcoma and malignant schwanomma [9]. A study by Fei Xiang et al reported SCC to be the commonest histological variety in lower limbs, followed by BCCs in the face. Marjolin’s ulcers in the trunk were mainly found to be dermatofibrosarcoma protruberans [6]. The latent period for development of Marjolin’s ulcer is an average of 30-35 years, although cases as early as 4 weeks have been reported [2, 3]. They can be classified into acute or chronic; Acute ulcers occur within 12 months and are more commonly BCCs. SCCs are prevalent in scars and chronic wounds [3]. BCCs are commoner in scars resulting from superficial burn injuries [9]. There is an inverse relationship between patient age and the length of the latency period [4].
Diagnosis
Diagnosis of Marjolin’s ulcer consists of a multidisciplinary approach. A non-healing ulcer in an area of scar or abnormal skin should be considered a Marjolin’s ulcer unless proven otherwise [4]. Multiple four-quadrant biopsies remain the gold standard for diagnosing Marjolin's ulcer and should be performed for all suspicious lesions [7]. After histologic confirmation of malignancy, it is important to stage the tumor and look for metastasis [4]. Imaging plays a role in evaluating the extent of infiltration and metastasis. Radiography can depict periosteal reaction and bony destruction in the underlying bone [8]. Contrast-enhanced MRI is the imaging technique of choice for assessing a Marjolin's ulcer [5]. MRI provides excellent soft tissue detail, like tumor extent, depth, margins, any underlying bone cortical or marrow involvement or the involvement of adjacent neurovascular structures [8, 9]. CT along with three-dimensional CT (3D-CT) has an advantage over MRI for determining the relationship between the soft-tissue tumor and adjacent bone. It detects cortical destruction and provides better anatomical resolution for delineating the nature and extent of the bony involvement [5]. There are no case reports that mention ultrasound findings of Marjolin’s ulcer. High-resolution US in general can indicate the possibility of malignancy in cutaneous tumors [10]. It is often difficult to distinguish between a SCC and BCC based on US findings. A recent study by Zi-Tong Chen et al gave a discriminatory model based on intralesional grayscale and color Doppler flow imaging features which may be useful differentiate between SCC and high-risk BCC [10]. Imaging features included lesion size thickness, internal hyperechoic spots, posterior acoustic shadowing on grayscale and the vascularity pattern on color Doppler [10].
A SCC depicts a homogenous hypoechoic lesion, with pattern 3 vascularity (twisted and dense linear blood flow signals covering more than 50% of the lesion) [1]. This was seen in our case. A BCC is more heterogenous, with hyperechoic areas. The vascularity depicts spotty blood flow signals (pattern 1) or slender arborizing blood flow signals (pattern 2). A hyperechoic spot is a useful imaging sign. In general, cutaneous SCC lesions are found to be larger and thicker than high-risk BCC lesions. Homogeneous echogenicity and lesion surface do not serve as reliable evidence to distinguish high-risk BCC from cutaneous SCC. Metastasis is the most important prognostic factor. It is regional metastases in 20-60% and distant in 14% of cases (lungs & brain) [3]. In general, a considerable time elapses between the time of onset and occurrence of metastasis in Marjolin’s ulcers. The lymphatic system is the primary pathway [6]. Marjolin’s ulcers are more aggressive and have a higher metastasis rate of 27% compared to the 3% metastasis rate of squamous cell carcinoma of a different etiology [2, 3]. A distant metastatic workup may be obtained with positron emission tomography (PET -CT), chest and brain CT scan and abdominal ultrasound [4]. No separate TNM staging is used for Marjolin’s ulcer [2]. The tumor staging protocol of the American Joint committee on cancer (AJCC) for non- melanoma skin cancers is used [7].
Management
Prevention of Marjolin’s ulcer is the best management. Early reconstruction of burn injuries at the acute stage is an effective prophylactic management to avoid malignant transformation; any changes in an old post-burn scar of more than 10 years should alert the medical personnel taking care of patients with chronic wounds [3]. Wide local excision with a surgical margin of at least 2cm combined with skin grafting is the treatment of choice for a marjolin ulcer [5]. A 3cm safety margin is also recommended compounded [7]. Further interventions like lymph node dissection, adjuvant radiotherapy, and systemic treatment are considered based on the case scenarios [3].
Prognosis
Prognosis is poor even after treatment. The recurrence rate after surgical resection is up to 50%. The 5-year survival rate is a mere 40-60%. High-grade carcinoma (grII) and the presence of nodal metastasis indicate a worse prognosis [2, 6]. No significant statistical correlation is seen between staging & prognosis [7]. Close follow-up after surgery in the first year is paramount. After that, quarter-yearly visits are recommended for 5 years [7].
Conclusion
Marjolin’s ulcer is an aggressive cutaneous malignancy that can arise from burn scars. Squamous cell carcinoma is the most common type of malignant transformation. It is imperative to promptly investigate a non-healing ulcer in the region of a burn scar. Histopathology is the gold standard for diagnosis. Imaging modalities are used to evaluate the extent and metastasis of these malignancies. Ultrasound can be used to evaluate depth and extent of a Marjolin’s ulcer. Doppler examination to assess the type of vascularization allows us to distinguish between SCC and BCC. It is also indicative of the malignant transformation of an ulcerating scar.
Certain discriminatory features - especially the patterns of vascularity can help to predict the type of cutaneous malignancy.
Ethical considerations
Ethical approval: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent: Informed consent was obtained from the individual included in the study.
Conflict of interest: The authors have nothing to disclose
References
- Shin JH, Baek JH, Chung J, et al. Korean Society of Thyroid Radiology (KSThR) and Korean Society of Radiology. Ultrasonography Diagnosis and Imaging- Based Management of Thyroid Nodules: Revised Korean Society of Thyroid Radiology Consensus Statement and Recommendations. Korean J Radiol 17 (2016): 370-395.
- Shah M, Crane JS. Marjolin Ulcer. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing (2022).
- Bazalinski D, Przybek-Mita J, Baranska B,. Marjolin's ulcer in chronic wounds - review of available literature. Contemp Oncol (Pozn) 21 (2017): 197-202.
- Khan Kamran, Schafer Charles, Wood, et al. Marjolin Ulcer: A Comprehensive Review. Advances in Skin & Wound Care 33 (2020): 629-634.
- Jang Gyu Cha, Jae Ho Yoo, Hee Kyung Kim, et Imaging of a Marjolin’s Ulcer: A Case Report, Musculoskeletal Radiology Case Report Journal of the Korean Society of Radiology 64 (2011): 593-598.
- Fei Xiang, Hua-Pei Song and Yue-Sheng Clinical Features and Treatment of 140 Cases of Marjolin's Ulcer at a Major Burn Center In Southwest China. Experimental and Therapeutic Medicine 17 (2019): 3403-3410.
- Ahmed Mousa, Anwar A. Elshenawy, Salah M. Maklad, et al. Post-burn scar malignancy: 5-year management review and experience, Int Wound J 19 (2022): 895-909.
- Sukhpal Sawhney, Rajeev Jain, Anupam Kakaria, et al. Marjolin's Ulcer: Radiographic and magnetic resonance appearances in two cases. Sultan Qaboos Univ Med J 9 (2009): 162-166.
- Tobin C, Sanger Marjolin’s ulcers: a case series and literature review. Wounds 26 (2014): 248-254.
- Zi-Tong Chen, Jian-Na Yan, An-Qi Zhu, et al. High- frequency ultrasound for differentiation between high- risk basal cell carcinoma and cutaneous squamous cell carcinoma. Skin Res Technol 28 (2022): 410-418.


 Impact Factor: * 3.1
Impact Factor: * 3.1 CiteScore: 2.9
CiteScore: 2.9  Acceptance Rate: 11.01%
Acceptance Rate: 11.01%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 
