Immunoassay Interference due to Macro-TSH: A Case Study of a Pediatric Patient with Hashimoto's Thyroiditis
Article Information
Anil K Chokkalla1,2, David L Paul3, Estella Tam2, and Sridevi Devaraj1,2*
1Department of Pathology and Immunology, Baylor College of Medicine, Houston, TX, USA
2Department of Pathology, Texas Children's Hospital, Houston, TX, USA
3Section of Diabetes and Endocrinology, Department of Pediatrics, Texas Children's Hospital, Baylor College of Medicine, Houston, TX, USA
*Corresponding Author: Sridevi Devaraj, Professor of Pathology& Immunology, Baylor College of Medicine, Medical Director, Clinical Chemistry and POCT, Texas Children's Hospital, 6621 Fannin Street, West Tower, Pathology BB110.06. Houston TX 77030, USA.
Received: 17 February 2023; Accepted: 28 February 2023; Published: 16 March 2023
Citation:
Anil K Chokkalla, David L Paul, Estella Tam, and Sridevi Devaraj. Immunoassay Interference due to Macro-TSH: A Case Study of a Pediatric Patient with Hashimoto's Thyroiditis. Archives of Clinical and Medical Case Reports. 7 (2023): 124-128.
View / Download Pdf Share at FacebookAbstract
Thyroid-stimulating hormone (TSH) measurement is central to the diagnostic workup of thyroid diseases. TSH activity is inverse loglinearly related to thyroxine (T4) hormone levels. An isolated elevation of TSH with normal T4 is defined as subclinical hypothyroidism, which is treated by hormone replacement therapy. TSH immunoassays are prone to various interferences, including biotin, heterophile antibodies and macro-TSH complexes. These interferences often lead to misdiagnosis of thyroid dysfunction, potentiating unnecessary clinical investigation and management. This case reports a spuriously elevated TSH in a clinically euthyroid 17-year-old female with a past medical history of Hashimoto’s thyroiditis. By performing a series of troubleshooting experiments such as serial measurement, dilution tests and polyethylene glycol precipitation, we demonstrated potential interference due to the macro-TSH complex.
Keywords
Hypothyroidism; Immunoassay; Immunoglobulin; Interference; Macro-TSH
Article Details
1. Introduction
Thyroid homeostasis is driven by the inverse log-linear relationship between thyroid-stimulating hormone (TSH) and free thyroxine (T4) [1]. TSH concentration above the upper reference limit and free T4 concentration within the reference range is defined as subclinical hypothyroidism [1]. The prevalence of this condition was estimated to be ~4.3% among the US population (age >12 years) [2]. Patients with subclinical hypothyroidism are usually treated with low-dose levothyroxine (25-75 µg/day), depending on the extent of TSH elevation (cutoff >10 µIU/mL) [1]. However, the false elevation of TSH due to assay interference should be considered before initiating hormone replacement therapy. Common interferences affecting TSH immunoassays include heterophile antibodies, biotin, rheumatoid factor and macro-TSH complexes [3]. Macro-TSH is a large (>150 KDa) inactive form of TSH complexed to anti-TSH autoantibodies, mainly IgG and IgA [3]. The prevalence of macro-TSH was estimated to be ~1% in adult patients and not known in pediatric patients [4]. Because of its size, macro-TSH is predominantly confined to the intravascular compartment and displays poor renal clearance [3]. TSH immunoassays have evolved significantly over time, with functional sensitivity improved by two orders of magnitude [3]. Despite this, all the currently available TSH immunoassay platforms show cross-reactivity with macro-TSH [5]. Of note, Architect (Abbott Laboratories, USA) immunoassay platform is comparatively less reactive to macro-TSH than Elecsys (Roche Diagnostics, Switzerland) or Centaur (Siemens Healthineers, Germany) immunoassay platforms [5]. It is crucial to recognize macro-TSH interference to avoid unnecessary clinical investigation and management. This case report investigates spuriously elevated TSH in the context of normal free T4 in a clinically euthyroid 17-year-old female with a past medical history of Hashimoto’s thyroiditis.
2. Case Presentation
A 17-year-old female with a past medical history of Down syndrome, congenital heart disease (atrial and ventricular septal defects status post repair) and Hashimoto’s thyroiditis presented to the emergency department with acute hypoxemic and hypercapnic respiratory failure and septic shock secondary to Influenza A and multi-drug resistant E.coli bacteremia. The initial SpO2 was 18%, and the chest radiograph revealed multifocal pneumonia. The patient became progressively ill in the intensive care unit (ICU) with multiorgan failure requiring extracorporeal membrane oxygenation and continuous renal replacement therapy. Over the next few days, she also received multiple packed red blood cell transfusions, broad-spectrum antibiotics and electrolyte replacement. Thyroid function was closely monitored, given a significant history of autoimmune thyroid disease. The patient was diagnosed with Hashimoto’s thyroiditis at 6 years of age due to symptoms related to hypothyroidism like fatigue, constipation, weight gain, cold intolerance, and concomitant high thyroid peroxidase (TPO) antibodies (655.4 IU/mL). She was being managed with oral levothyroxine (150 µg/day) therapy and remained euthyroid with good compliance. At 12 days post-ICU admission, the thyroid function test was abnormal, with very high TSH (176 µIU/mL), normal free T4 (1.9 ng/dL), normal total T4 (11 µg/dL) and low triiodothyronine (T3) (65 ng/dL) (Table 1). Interestingly, there were no signs of goiter, thyromegaly or thyroid tenderness. The patient received levothyroxine (150 µg/day) intravenously every day in the ICU. Two years prior to this emergency episode, the patient's thyroid function test was unremarkable (Table 1). Acutely elevated TSH along with normal FT4, good compliance with levothyroxine and clinical euthyroid state made the endocrine team suspect false TSH results. Consequently, a care team member contacted the laboratory to investigate potential interferences affecting the TSH immunoassay.
|
Analyte |
During Diagnosis |
Before Hospitalization |
ICU Admission |
Reference Range |
|
TSH (µIU/mL) |
559 |
0.86 |
175.8 |
0.5-3.4 |
|
Free T4 (ng/dL) |
- |
1.2 |
1.9 |
0.8-2.0 |
|
Total T4 (µg/dL) |
2.3 |
- |
11 |
6.4-13.3 |
|
T3 (ng/dL) |
- |
- |
65 |
115-195 |
|
TPO antibody(IU/mL) |
655.4 |
- |
- |
<20 |
|
Tg antibody (IU/mL) |
<20 |
- |
- |
<20 |
Table 1: Thyroid function test results.
TSH- Thyroid-Stimulating Hormone; T4- Thyroxine; T3- Triiodothyronine; TPO- Thyroid Peroxidase; Tg- Thyroglobulin.
3. Discussion
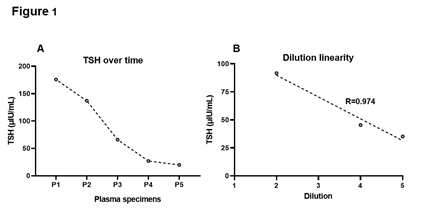
TSH levels were assessed in all the subsequent samples sent to the laboratory by chemiluminescent microparticle immunoassay on the Architect i2000SR analyzer (Abbott Laboratories, USA). It is a two-step, sandwich immunoassay employing an anti-β TSH capture antibody and anti-α TSH detection antibody conjugated to acridinium. The analytical measurement range of this assay is 0.01-100 µIU/mL, and an auto-dilution of 1:5 expands the reportable range to 0.01-500 µIU/mL. Interestingly, TSH values tapered over time (Figure 1A). Compared to the reported result (175.8 µIU/mL), specimens collected after 2h, 12h, 24h and 48h showed TSH of 137, 66, 27 and 20 µIU/mL, respectively (Figure 1A). This indicates the transient activity of an interfering substance. The three major interferences that distort TSH immunoassays include biotin, heterophile antibodies and macro-TSH complexes [3]. Using the patient’s pooled plasma collected within 48h post-discordant TSH result, we investigated the potential role of these interfering agents.
3.1 Biotin is an Unlikely Source of Interference
Biotin, also known as vitamin B7, is an essential enzyme co-factor for several metabolic reactions and is frequently supplemented in patients with diabetes, peripheral neuropathy, lipid disorders, carboxylase and biotinidase deficiencies [6]. High circulating biotin (>10 ng/mL) seen in ~7% of emergency room patients substantially interferes with the biotinylated TSH immunoassays [3]. The current guidelines recommend collecting specimens at least 8 hours after abstaining from the supplements [6]. Biotin is the most unlikely cause for spuriously elevated TSH in our patient for the following reasons: 1) Architect TSH immunoassay is devoid of biotin-streptavidin affinity enrichment, 2) no known history of biotin supplementation and 3) biotin promotes negative interference in the sandwich immunoassays [7]. Moreover, the free and total T4 measured with our Vitros XT 7600 analyzer (Ortho Clinical Diagnostics, USA) also do not employ a biotin-streptavidin system, precluding the interference due to biotin in the current thyroid function panel.
3.2 Dilution Test ruled out the Heterophile Antibody Interference
Heterophile antibodies, including human anti-animal antibodies, are nonspecific antibodies that interact weakly with the Fc region of the immunoassay antibodies, generating either falsely high or low signals [3]. These antibodies can remain in the bloodstream for up to 12 months and traverse the placenta to newborns [3]. The incidence of heterophile antibody interference in TSH immunoassays was estimated to be ~0.4% [8]. This type of interference is prevalent among patients undergoing animal-derived monoclonal antibody therapy, transfusions and prolonged animal exposure [3]. Dilution test is the gold standard for investigating heterophile antibody interference, in which a non-linear change in the analyte concentration with dilution is indicative of positive assay interference [3]. A total of three auto-dilutions (1:2, 1:4, and 1:5) made using the plasma sample with discrepant TSH value were examined. Diluted samples showed a linear decrease (R=0.974) in the TSH concentration (Figure 1B). The average bias between each dilution was 5 µIU/mL, which is well within the CAP recommended total allowable error of 3SD (11.8 µIU/mL). Clearly, discrepant TSH elevation was not due to heterophile antibodies. An alternate strategy is to employ a heterophilic blocking tube that contains a blocking reagent with specific binders to inactivate heterophile antibodies [3]. Due to insufficient quantity, we next tested for the presence of macro-TSH complex using polyethylene glycol (PEG).
Figure 1: Troubleshooting of spuriously elevated TSH. TSH levels dropped gradually with time (A). P1 is the plasma specimen of interest. P2, P3, P4 and P5 were collected at 2h, 12h, 24h and 48h after P1, respectively. Dilution studies revealed a linear decrease in the TSH concentration (B). TSH, thyroid-stimulating hormone.
3.3 PEG Precipitation is Suggestive of Interference due to Macro-TSH
Circulatory macro-complexes form predominantly due to the binding of immunoglobulins with proteins. Notable examples include macro-amylase, macro-creatine kinase, macro-prolactin and macro-TSH [9]. Previous studies reported positive interference due to macro-TSH in adult female patients with thyroid disease [10, 11]. Troubleshooting macro-TSH interference involves precipitation with PEG, followed by measurement of monomeric TSH in the supernatant. Briefly, 100 µL of PEG 8000 solution (250 g/L) was mixed with 200 µL of pooled patient plasma and incubated at 370C for 30 mins. The macro-TSH complex was precipitated by centrifuging at 13,000 rpm for 3 mins, and the supernatant was analyzed. TSH concentration of the pre-PEG treated sample was 47 µIU/mL, whereas the post-PEG treated sample was 19 µIU/mL. Importantly, the recovery of TSH [(post-PEG TSH/pre-PEG TSH) x100] after PEG treatment was only 40%. To further investigate the type of immunoglobulins (Ig) forming macro-TSH, the serum levels of IgG, IgA, IgM and IgE were measured on a BN ProSpec analyzer (Siemens Healthineers, Germany). Serum IgA showed marked elevation, whereas IgG, IgM and IgE levels remained within the reference range at two different time points tested (Table 2). Altogether, these findings are most consistent with the macro-TSH interference. The patient did not receive intravenous immunoglobulin therapy suggesting that autoantibodies likely contributed to the formation of macro-TSH. Consistent with our data, previous reports also observed linear response upon dilution in the presence of macro-TSH [12, 13]. It is also important to note that the cutoff for % recovery post-PEG precipitation varied from 25–40% in the literature [3]. Hence, gel filtration chromatography (GFC) remains the mainstream method for macro-TSH investigation as it can physically resolve high molecular weight complexes. A major limitation of this report is the lack of confirmatory evidence by GFC, which is an expensive and less accessible technique. Interestingly, TSH levels in our patient decreased substantially within 12h post-discrepant value. Although the half-life and turnover kinetics of macro-TSH is not known, a recent study showed that macro-TSH from patient serum injected into the right jugular vein of rats disappeared within 2h [14]. Furthermore, our patient was critically ill and therefore, conditions mimicking subclinical hypothyroidism, like TSH resistance and the recovery phase of nonthyroidal illness, may not be completely ruled out.
|
Antibody |
Specimen#1 |
Specimen#2 |
Reference Range |
|
IgA |
327 mg/dL |
339 mg/dL |
66-295 mg/dL |
|
IgG |
1140 mg/dL |
1150 mg/dL |
641-1353 mg/dL |
|
IgM |
97 mg/dL |
96 mg/dL |
40-180 mg/dL |
|
IgE |
15 mg/dL |
22 mg/dL |
<100 mg/dL |
Table 2: Immunoglobulin test results.
4. Conclusion
TSH immunoassay interferences due to biotin, heterophile antibodies and macro-TSH complex should be ruled out before initiating hormone replacement therapy in clinically euthyroid patients with sudden isolated TSH elevation. Typical laboratory workflow for investigating spurious TSH results may include method comparison with an alternate immunoassay platform, biotin withdrawal, dilution test, heterophile antibody blockers, PEG precipitation and gel filtration chromatography [3]. Proper communication and follow-up between the clinical care team and laboratorians are crucial for minimizing falsely reported thyroid function test results.
Credit Author Statement
Anil K Chokkalla: Investigation, Formal analysis, Writing - Original Draft. David Paul: Conceptualization; Writing- Review and Editing; Estella Tam: Investigation. Sridevi Devaraj: Conceptualization, Investigation, Formal analysis, Writing – Review & Editing, Supervision.
Conflicts of Interest
None.
Acknowledgments
Anil K Chokkalla was supported by Ching Nan Ou Fellowship Endowment in Clinical Chemistry.
References
- Garber JR, Cobin RH, Gharib H, et al. Clinical practice guidelines for hypothyroidism in adults: cosponsored by the American Association of Clinical Endocrinologists and the American Thyroid Association. Thyroid 22 (2012): 1200-1235.
- Hollowell JG, Staehling NW, Flanders WD, et al. Braverman, Serum TSH, T(4), and thyroid antibodies in the United States population (1988 to 1994): National Health and Nutrition Examination Survey (NHANES III). J Clin Endocrinol Metab 87 (2002): 489-499.
- Favresse J, Burlacu MC, Maiter D, et al. Interferences with Thyroid Function Immunoassays: Clinical Implications and Detection Algorithm, Endocr Rev 39 (2018): 830-850.
- Hattori N, Matsuda T, Chihara K, et al. SAT-426 Macro-Thyroid Stimulating Hormone (TSH) in Children. Journal of the Endocrine Society 4 (2020).
- Hattori N, Ishihara T, Shimatsu A. Variability in the detection of macro TSH in different immunoassay systems. Eur J Endocrinol 174 (2016): 9-15.
- Li D, Ferguson A, Cervinski MA, et al. AACC Guidance Document on Biotin Interference in Laboratory Tests. J Appl Lab Med 5 (2020): 575-587.
- Piketty ML, Polak M, Flechtner I, et al. False biochemical diagnosis of hyperthyroidism in streptavidin-biotin-based immunoassays: the problem of biotin intake and related interferences. Clin Chem Lab Med 55 (2017): 780-788.
- Ismail AA, Walker PL, Barth JH, et al. Wrong biochemistry results: two case reports and observational study in 5310 patients on potentially misleading thyroid-stimulating hormone and gonadotropin immunoassay results. Clin Chem 48 (2002): 2023-2029.
- Bürki C, Volleberg M, Blomgren L, et al. Reference ranges for the polyethylene glycol (PEG) precipitation activity (%PPA) of eight routine enzyme activities. Pract Lab Med 33 (2023): e00304.
- Nkuna X, Dire Z, Khoza S. A Macro-TSH: A Clinical Diagnostic Dilemma. Ejifcc 33 (2022): 317-324.
- Pluta D, Bedkowska-Szczepanska A, Madej P, et al. Macro-TSH - tips and tricks for gynecologists. Ginekol Pol (2022).
- Newman JD, Bergman PB, Doery JC, et al. Factitious increase in thyrotropin in a neonate caused by a maternally transmitted interfering substance, Clin Chem 52 (2006): 541-542.
- Hattori N, Ishihara T, Yamagami K, et al. Macro TSH in patients with subclinical hypothyroidism. Clin Endocrinol (Oxf) 83 (2015): 923-930.
- Yamada A, Hattori N, Matsuda T, et al. Clearance of macro-TSH from the circulation is slower than TSH. Clin Chem Lab Med 60 (2022): e132-e135.


 Impact Factor: * 3.1
Impact Factor: * 3.1 CiteScore: 2.9
CiteScore: 2.9  Acceptance Rate: 11.01%
Acceptance Rate: 11.01%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 
