Evaluation of a Novel External Neuromodulation Device (iCLEAR™) for the Acute Relief of Rhinitis and Rhinosinusitis Symptoms
Article Information
Mark Shusterman, MD 1, Michael E. Gertner, MD1, Anita Carmen Choy, MD2*, Jacob Johnson, MD3, Neil J. Friedman, MD4
1Olympic Ophthalmics, Inc. Issaquah, WA, USA
2Welch-Pasteur Allergy Medical Group, Palo Alto, CA, USA
3San Francisco Otolaryngology, San Francisco, CA; UCSF School of Medicine, San Francisco, CA, USA
4Mid-Peninsula Ophthalmology Medical Group, Menlo Park, CA; Stanford University School of Medicine, Palo Alto, CA, USA
*Corresponding Author: Anita Carmen Choy, Welch-Pasteur Allergy Medical Group, Palo Alto, CA, USA.
Received: 03 February 2023; Accepted: 14 February 2023; Published: 28 February 2023
Citation: E Mark Shusterman, Michael E Gertner, Anita Carmen Choy, Jacob Johnson, Neil J Friedman. Evaluation of a Novel External Neuromodulation Device (iCLEAR™) for the Acute Relief of Rhinitis and Rhinosinusitis Symptoms. Archives of Clinical and Medical Case Reports. 7 (2023): 104-113.
View / Download Pdf Share at FacebookAbstract
Purpose: To evaluate the efficacy and safety of a novel portable sonic external neuro-stimulation device (iCLEAR) in alleviating symptoms of chronic rhinitis and rhinosinusitis.
Methods: This was a multicenter, open-label, single-arm, clinical trial which included adult patients with nasal obstructive symptoms due to chronic rhinitis/rhinosinusitis and a minimum Visual Analog Scale (VAS) score of 25 mm and/or a minimum raw Nasal Obstruction Symptom Evaluation (NOSE) score of 2 on any of items 1, 2, 3, or 5. Enrolled subjects were instructed to apply the study device bilaterally to the external nasal nerve at least three times per day for 30 seconds until the 2-week followup, then twice per day and as needed thereafter. Subjects were evaluated at Days 3, 14, and 30 following the initial baseline visit. The primary efficacy endpoint was the change in nasal obstructive symptoms from baseline to Day 30 as measured by the VAS and NOSE instruments. Safety was assessed by the incidence of device-related Adverse Events (AEs).
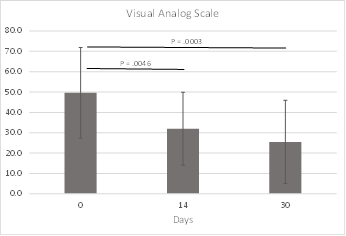
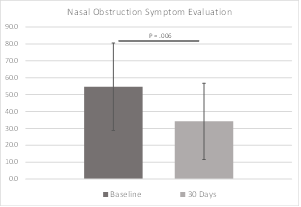
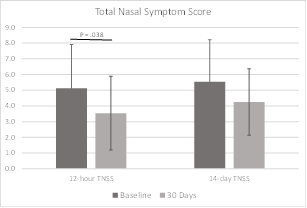
Results: In this pilot study of the iCLEAR device, efficacy was indicated by the improvement in rhinitis and rhinosinusitis symptoms on five different scales: Visual Analog Scale, Nasal Obstruction Symptom Evaluation, Total Nasal Symptom Score (TNSS), the Global Assessment of Change (GAC), and the Sino-Nasal Outcome Test (SNOT-20©). On average, the symptom assessment scores showed clinically significant improvement. Mean VAS score decreased to 32.0 (Standard Deviation [SD] = 17.9) from a baseline of 49.6 at 14 days (P = 0.0046) and to 25.5 (20.4) at 30 days. Mean NOSE score decreased to 34.2 (22.5) from 54.6 (25.9) at 30 days (P = 0.006). Mean 12-hour TNSS score decreased to 3.5 (2.3) from 5.1 (2.8) at 30 days (P = 0.038) and mean 14-day TNSS score decreased to 4.3 (2.1) from 5.5 (2.7). Mean GAC
Keywords
Neurostimulation; Rhinitis; Rhinosinusitis
Neurostimulation articles; Rhinitis articles; Rhinosinusitis articles
Article Details
Translational Relevance:
External nasal neurostimulation has the potential to improve symptoms of rhinitis/rhinosinusitis.
1. Introduction
Rhinitis, in its various forms, is a common and bothersome condition that has been estimated to affect up to 20% of the world’s population, with direct and indirect associated annual costs exceeding tens of billions of dollars [1,2]. It is defined by the presence of at least one of the following symptoms: congestion, rhinorrhea, sneezing, nasal itching, and nasal obstruction [2]. Other symptoms may occur, including headaches, facial pain, ear pain, itchy throat and palate, snoring, and sleep disturbances [3].
There are two main variants of rhinitis: allergic and non-allergic [2,4]. Although the etiology of the conditions differs, the symptoms are essentially the same. Nasal obstruction constitutes the most bothersome problem and has been proposed as the key symptom in allergic rhinitis [5], a condition which greatly impacts productivity, with 3.5 million lost workdays and two million lost school days [2]. This productivity loss exceeds that of asthma, diabetes, and coronary heart disease [2]. Treatments for rhinitis include the use of various symptom-relieving medications, such as oral and topical antihistamines, decongestants, intranasal corticosteroids, and others, as well as nasal saline lavage, and surgery.
Chronic rhinosinusitis is related to rhinitis [4]. It is an inflammatory disease of the paranasal sinuses that results in symptoms similar to rhinitis, including facial pain/pressure, nasal airway obstruction, and others [6]. Rhinosinusitis is defined by the presence of at least two the following four cardinal symptoms: facial pain/pressure, hyposmia/anosmia, nasal drainage, and nasal obstruction of at least twelve consecutive weeks’ duration [7]. Like rhinitis, rhinosinusitis has been shown to exert a significant negative overall health impact, including reduced quality of life, exacerbation of asthma and additional pulmonary conditions such as cystic fibrosis, and others [7,8,9].
Treatment of chronic rhinosinusitis is directed at improving sinus drainage and mucociliary clearance, mitigating local infection and inflammation, and improving delivery of topical medications [10]. Therapy consists of medical management, and endoscopic sinus surgery if necessary [10]. There are several non-surgical modalities that are commonly used, including nasal irrigation, intranasal and oral corticosteroids, and antibiotics [6,7]. More recently, new technologies have been described to treat nasal congestion that utilize various forms of vibrational energy. [11,12,13].
As previously noted, obstructive nasal symptoms constitute one of the most bothersome aspects of rhinitis and rhinosinusitis and are usually associated with engorgement of the nasal mucosa [1]. The regulatory mechanism of nasal mucosal swelling and the resulting nasal congestion is complex and involves both the sympathetic and the parasympathetic nerve systems, although mucosal vasal tone is determined primarily by sympathetic discharge [1]. Research has shown that withdrawal of the vasoconstricting effect mediated by sympathetic stimulation may play a key role in the development of cognitive symptoms, together with decreased mucociliary clearance [1]. Mucociliary clearance is defined as cleaning of the upper and lower airway by interaction of nasal mucus and ciliary beating and is also partly mediated by the activity of the autonomic nervous system [14]. Autonomic innervation of the nasal cavity is supplied via the posterior ethmoidal nerve, which is a branch of the nasociliary nerve [14]. The anterior ethmoidal branch of the nasociliary nerve, which is indirectly connected to the posterior ethmoidal branch, provides sensory innervation to the nasal cavity, and emerges between the inferior border of the nasal bone and the lateral cartilages as the external nasal nerve [15]. Although the external nasal nerve has been typically considered to be entirely sensory, it does communicate with the nasociliary nerve, and prior work has demonstrated that its stimulation can activate the nasal-lacrimal reflex pathway [16]. We hypothesize that stimulation of the external nasal nerve could also stimulate the sympathetic pathways innervating the nasal mucosa to bring about relief of nasal obstructive symptoms via the vasoconstrictive mechanism described above.
Neuromodulation is “the process of inhibition, stimulation, modification, regulation or therapeutic alteration of activity, electrically or chemically, in the central, peripheral or autonomic nervous systems” [17]. It has widespread uses throughout medicine in the treatment of various conditions, ranging from diabetes to neurological diseases such as epilepsy, to psychological disorders such as depression, to chronic pain and other long-term disabilities [17]. Fundamentally, the therapeutic effects of neuromodulation are achieved by increasing the frequency and/or intensity of whatever neural activity is already regulating the target physiologic process.
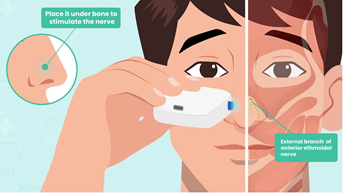
Olympic Ophthalmics, Inc. (Issaquah, WA) developed a novel portable external neuromodulation device, the iCLEAR, which is designed to stimulate the external nasal nerve (Figures 1 and 2). This device has been previously shown to provide a safe and effective method for treating dry eye disease and has been cleared for marketing by the U.S. Food and Drug Administration for that indication under the name iTEAR®100 [18]. In a study of 101 subjects followed for 30 days, conducted for regulatory clearance, the device was shown to increase tear production and improve symptoms of dry eye with minimal adverse effects [16]. Many patients using the device for dry eye also reported improvement in nasal congestion. There is a significant association between dry eye and allergic rhinitis, as the nasal ocular reflex creates an interaction between the nose and the eye [19]. The anecdotal findings from the dry eye study prompted this pilot study, where we aimed to evaluate the safety, efficacy, and usability of the iCLEAR device for alleviating the symptoms of chronic rhinitis and rhinosinusitis.
2. Methods
2.1 Study Design
This study was a multicenter, prospective, open-label, single-arm design assessing the effectiveness, tolerability, and safety of the iCLEAR device in adult subjects with nasal obstructive symptoms related to chronic rhinitis and/or rhinosinusitis. Thirty-one (31) patients participated at two sites in the United States between 26 September 2018 and 27 February 2020. The study’s duration was 30 days, and subjects were permitted to continue with their typical medication regimens, in addition to using the device. (Clinical Trial Registration at www.clinicaltrials.gov, identifier: NCT03682432; accessed 14 June 2021).
Device performance was evaluated by its ability to improve symptoms of rhinitis/rhinosinusitis, as measured by the Visual Analog Scale (Figure 3), the Nasal Obstruction Symptom Evaluation, Total Nasal Symptom Score, and the Sino-Nasal Outcome Test, which are commonly used and well-validated rhinological instruments [5,20,21]. The VAS included both nasal and ocular symptoms: nasal itching, runny nose, and sneezing; and itching, watering, and red eyes. The scale ranged from 0 cm/not at all bothersome, to 10 cm/very bothersome. Additionally, a Global Assessment of Change tool [22] was used to evaluate the subjects’ overall symptom change (Figure 4).
Device safety was assessed by the incidence of device-related Adverse Events and Serious Adverse Events. Device usability and satisfaction with treatment were evaluated using a Treatment Tolerability Scale (Figure 5), which is a modified version of the Numeric Pain Rating Scale[i] and a satisfaction survey (Figure 6).
Key eligibility criteria were age of at least 21 years, presence of nasal obstructive symptoms due to any type of rhinitis and/or rhinosinusitis, and a minimum VAS score of 25 mms and/or a minimum raw NOSE score of 2 on any of items 1, 2, 3, or 5. Initial diagnosis was allowed during screening and/or baseline visits, although subjects had to be on stable therapy for at least two weeks preceding enrollment. Complete inclusion and exclusion criteria are listed in Table 1.
|
Inclusion Criteria |
Exclusion Criteria |
|
1. Twenty-one (21) years of age and older |
1. History of rhinoplasty or other surgery involving nasal, maxillary, or orbital bones |
|
2. Diagnosed with chronic rhinitis of any etiology (i.e., allergic, seasonal, perennial, or non-allergic, including vasomotor), and/or rhinosinusitis |
2. Septal deviation and/or nasal polyps that result in >50% obstruction of the nasal airway |
|
3. Nasal obstructive symptoms (e.g., nasal airway congestion) associated with the rhinitis/rhinosinusitis diagnosis |
3. History of facial nerve palsy |
|
4. On stable therapy, without any changes during the 2 weeks preceding enrollment |
4. History of neuromuscular disorders |
|
5. Minimum VAS score of 25 mms and/or a minimum raw NOSE score of 2 on any of items 1, 2, 3, or 5 |
5. Presence of uncontrolled serious systemic disease (per PI’s determination) |
|
6. Willing and able to provide written Informed Consent |
6. Presence of clinically significant nasal/facial skin conditions (e.g., infection, ulceration, wound) |
|
7. Able to safely use the study device and be free of any condition that, in the opinion of the investigator, could impair study participation |
7. Under arrest or otherwise in custody |
|
8. Any other condition, which in the judgment of the PI would prevent a potential subject from safely completing the study or tolerating device use, such as mental illness, dementia, severe agitation, et cetera |
Table 1: Inclusion and Exclusion Criteria.
The study was conducted in compliance with Good Clinical Practice guidelines and in accordance with the principles of the Declaration of Helsinki. The protocol was approved as a non-significant risk study by the Quorum Review Institutional Review Board (now Advarra™; Seattle, WA). Enrolled patients were required to sign a consent form prior to treatment.
2.2 Study Treatment
Subjects were trained to use the iCLEAR device during their baseline visit and asked to perform the treatment three times per day until the 2-week follow-up, then twice per day and as needed for the remainder of the study. Assessments were conducted in person at Days 0 and 30, telephonically at Day 3, and telephonically or optionally in person at Day 14. Subjects were additionally required to keep diaries documenting device usage, treatment effects/concerns (if any), and symptom severity by means of the VAS. Subjects were also asked to provide treatment tolerability ratings using the TTS. Change in overall symptoms and satisfaction with treatment were measured using the GAC tool and a satisfaction survey, respectively. The complete Study Schedule of Assessments is provided in Table 2.
|
Assessment |
Schedule |
|||
|
Screening/Day 0a |
Day 3 |
Day 14 |
Day 30 |
|
|
Eligibility screening |
X |
|||
|
Demographic information |
X |
|||
|
Focused rhinitis/rhinosinusitis history |
X |
|||
|
Nasal Obstruction Symptom Evaluation |
X |
X |
X |
|
|
Visual Analog Scale |
X |
X |
X |
X |
|
Sino-Nasal Outcome Test |
X |
X |
X |
|
|
Total Nasal Symptom Score |
X |
X |
X |
|
|
Global Assessment of Change |
X |
X |
||
|
Treatment Tolerability Scale |
X |
|||
|
Subject Satisfaction Survey |
X |
|||
Table 2: Schedule of Assessments and Procedures.
a Screening and Day 0 were the same.
The trial utilized an early variant of the iCLEAR device which was functionally equivalent to the iTEAR100 model currently marketed for an ophthalmic indication. The device contained a unidirectionally oscillating tip placed against the skin of the nose around the junction of the nasal cartilage and the nasal bone at the location of the external nasal nerve (Figure 2). The frequency, force, geometry, and durometer of the tip were designed to optimize treatment effect and to minimize potential for complications such as skin and nerve injury. An integrated data logger was used to monitor device usage.
2.3 Statistical Methods
Data analyses occurred after the included subjects exited the study and all their study data were collected and locked. Study close-out was not required to evaluate the data; interim analyses were performed as warranted. Subject disposition (the number of subjects enrolled and completing the study), subject discontinuations and reasons for discontinuation, eligibility criteria exceptions and other major protocol deviations, are summarized. The demographic, medical, and other relevant history of the study subjects are presented descriptively. For continuous variables such as age, the mean and standard deviation are provided.
The principal statistical tests are descriptive, addressing the device’s ability to improve symptoms of rhinitis/rhinosinusitis, as measured by the VAS, NOSE, SNOT-20, and GAC instruments. The means and standard deviations (SDs) are provided. Student-t tests (α = 0.05) were used to determine the statistical significance of outcome score changes. As this was an exploratory study, no a priori outcome criteria were defined. Investigational device safety was evaluated by assessing the incidence of related AEs and SAEs. All events possibly, probably, or definitely related to the study device were tabulated descriptively. All adverse events are summarized by presenting the percentages of subjects with each event. No formal statistical analyses were conducted for this feasibility study. There was no imputation of missing data.
3.Results
3.1 Enrollment
Between September 26, 2018, and January 31, 2020, 31 subjects were enrolled at two sites. Of the 31 subjects, 24 (77%) reached the primary endpoint at Day 30, with 3 dropping out due to difficulty tolerating neurostimulation, and 4 lost to follow-up. The study population at study completion was 62.5% male and 37.5% female (Table 3). Mean age was 50.8 years, ranging from 21 to 78 years. A variety of races/ethnicities were represented; however, the subjects were mainly Caucasian (58%) or Asian (38%). Medication use at baseline is shown in Table 4. Corticosteroids and antihistamines were the most used medications.
|
Summarya |
All Subjects (N=24) |
|
Sex, n (%) |
|
|
Female |
9 (37.5%) |
|
Male |
15 (62.5%) |
|
Age (Years) |
|
|
Mean (SD) |
50.8 (17.5) |
|
<30, n (%) |
4 (16.7%) |
|
30-50, n (%) |
7 (29.2%) |
|
50-70, n (%) |
9 (37.5%) |
|
>70, n (%) |
4 (16.7%) |
|
Ethnicity, n (%) |
|
|
Hispanic or Latino |
0 (0.0%) |
|
Not Hispanic or Latino |
23 (95.8%) |
|
Not Applicable |
1b (4.2%) |
|
Race, n (%) |
|
|
Asian |
9 (37.5%) |
|
Black |
1 (4.2%) |
|
Caucasian |
14 (58.3%) |
|
Other |
0 (0.0%) |
Table 3: Demographics and Baseline Information of Completed Subjects.
a Percentages may not sum to 100% because of rounding.
b Ethnicity was not recorded for one subject.
|
Medication |
Subjectsa, N (%) |
|
Antihistamine |
10 (41.7%) |
|
Corticosteroid |
14 (58.3%) |
|
Decongestant |
2 (8.3%) |
|
Leukotriene Inhibitor |
5 (20.8%) |
|
Nasal Saline Lavage |
2 (8.3%) |
|
None |
5 (20.8%) |
Table 4: Rhinitis Medication Use at Baseline (N=24).
aTotal N exceeds the number of completed subjects, as some used more than one type of medication. Additionally, combination products such as nasal spray corticosteroids/antihistamines were included in both categories.
There were 12 protocol deviations within a group of nine subjects. Seven were late or missed follow-up visits, one was for a visit performed by phone rather than in person, one was a subject who should have been excluded due to history of rhinoplasty or nasal surgery, and three were allowances for subjects to extend their time in the study.
3.2 Outcomes
Overall outcome data are summarized in Table 5.
|
Outcome |
Baseline |
14 Days |
30 Days |
|
Visual Analog Scale Scale 0 to 100 mm |
49.6 (22.3) |
32.0 (17.9) P = 0.0046 |
25.5 (20.4) P = 0.0003 |
|
Nasal Obstruction Symptom Evaluation Score 0 to 100 |
54.6 (25.9) |
-- |
34.2 (22.5) P = 0.006 |
|
Total Nasal Symptom Score 12-Hour a Score 0 to 15 |
5.1 (2.8) |
-- |
3.5 (2.3) P = 0.038 |
|
Total Nasal Symptom Score 14-Dayb Score of 0 to 15 |
5.5 (2.7) |
-- |
4.3 (2.1) P = 0.069 |
|
Global Assessment of Change Scores range from -4 to 4 |
-- |
1.5 (0.9) |
1.4 (1.2) P = 0.613 |
|
Sino-Nasal Outcome Test Score of 0 to 100 |
33.0 (14.3) |
-- |
21.3 (13.4) P = 0.005 |
Table 5: Summary of iCLEAR Outcome Data - Mean (Standard Deviation).
a TNSS score for the preceding 12-hour period.
b TNSS score for the preceding 14-day period.
The Visual Analog Scale was used to monitor how bothersome rhinitis was to each subject at different timepoints. Improvements in VAS score were statistically significant at both 14-days (P = 0.0046) and 30-days (P = 0.0003) from baseline. VAS data were available for all subjects at baseline and 30 days, and for 23 of 24 subjects at 14 days. VAS results are included in Figure 7 and Table 5.
The Nasal Obstruction Symptom Evaluation scale ranges from 0, or not a problem, to 4, a severe problem, for each of five conditions involving patient congestion and nasal blockage. The sum of scores is then multiplied by five, allowing a best possible score of 0 and a worst of 100. Patients are categorized as follows: mild (range, 5-25), moderate (range, 30-50), severe (range, 55-75), extreme (range, 80-100) nasal obstruction. The NOSE scores in this study decreased significantly from the severe range at baseline to the moderate range at 30 days (P = 0.006). NOSE data were available for all subjects at baseline and 30 days. NOSE data are shown in Figure 8 and Table 5.
The Total Nasal Symptom Score includes rankings of symptoms related to nasal congestion, runny nose, nasal itching, sneezing, and difficulty sleeping. Symptoms are rated as they have been experienced in the last 12 hours and in the last two weeks. Each symptom is ranked from 0/none to 3/severe. The best possible score was 0 and the worst was 15 for each time interval. TNSS scores for the 12 hours prior to filling out the questionnaire improved significantly at 30 days (P = 0.038). Scores for the two weeks prior to filling out the questionnaire also improved, although did not reach statistical significance (P = 0.069). TNSS data were available for all subjects at baseline and 30 days. TNSS data are shown in Figure 7 and Table 5.
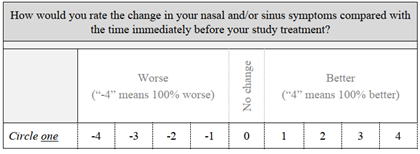
The Global Assessment of Change assesses how subjects rank the change in their nasal and/or sinus symptoms compared with the time immediately before their study treatment. A score of 0 indicates no change. If the symptoms are better or worse, they are scored from 1 to 4 better or from -1 to -4 worse, where scores of 4/-4 mean 100% better or worse. The mean scores at 14 and 30 days were 1.5 and 1.4, respectively. This loosely correlates to a 36% mean improvement, although this did not reach statistical significance (P = 0.613). GAC data were missing for two subjects at 14 days and one subject at 30 days. GAC data are included in Table 5. The Sino-Nasal Outcome Test is used to monitor symptoms and social/emotional consequences of the subjects’ rhinosinusitis. There are 20 axes, and each is ranked from 0/no problem to 5/problem as bad as it can be. The best possible score is 0 and the worst is 100. The SNOT-20 scores for this study population decreased significantly from baseline to 30 days (P = 0.005). SNOT-20 data were available for all subjects at baseline and 30 days. These data are included in Table 5.
3.3 Safety and Compliance
As previously noted, 24 of 31 enrolled subjects completed the study, with 3 dropping out due to difficulty tolerating treatment, and 4 lost to follow-up. A total of 17 Adverse Events were recorded, of which one was adjudicated to not be an AE following review by the Medical Monitor. The remaining 16 AEs are listed in Table 6. These occurred in 14 subjects, with two subjects having two AEs each. All AEs were of mild severity, and all resolved. Six AEs in six subjects were possibly related to the device and three of these subjects exited the study early and were not included in the completed data analysis. Of note, one of these exited subjects had a punctal plug procedure on the same day of the reported AE - discomfort in the eye following use of the device - prompting the decision to leave the study. The most common adverse event was common cold symptoms and/or congestion, occurring in approximately 33% of subjects, unrelated to the study device. There were no SAEs or UADEs.
Table 6: Adverse Events.
a This subject had a punctal plug procedure on the same day that the eye irritation was reported.
b AEs reported by same subject.
c Detailed AE report unavailable; subject noted to have had cold symptoms during final week of study.
d AEs reported by same subject.
3.4 Patient Satisfaction and Usability
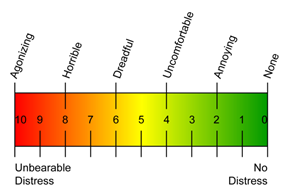
The Treatment Tolerability Scale was used to assess each subject's tolerance of the iCLEAR device. The scale ranges from 0/no distress to 10/agonizing/unbearable distress. The mean (SD) at 3 days was 3.9 (±2.1). TTS data were missing for 4 of 24 subjects.
The Subject Satisfaction Survey posed three questions to each subject. Most subjects (67%) were very or somewhat satisfied with the iCLEAR device. Subjects’ level of satisfaction with the study treatment is summarized in Table 7. When asked if subjects would use the device again, 71% said yes, 25% said no, and one did not fill out the survey. When subjects were asked if they would recommend the device to others, 67% said yes, 29% said no, and one did not fill out the survey.
|
Satisfaction Level |
Subjects, n (%) |
|
Very satisfied |
3 (12.5%) |
|
Somewhat satisfied |
13 (54.2%) |
|
Neither satisfied nor dissatisfied |
5 (20.8%) |
|
Dissatisfied |
1 (4.2%) |
|
Very dissatisfied |
0 (0.0%) |
Table 7: Subject Satisfaction with the Study Device Treatment (N=24a).
a Data were missing for two of 24 subjects.
4. Discussion
Several therapeutic approaches are employed for relief of symptoms of allergic and non-allergic rhinosinusitis, as noted in Table 8. Treatment involves various medical alternatives, as well as possible surgery to alleviate obstruction if other therapies fail [2].
|
Medication/Intervention |
AR |
NAR |
|
Intranasal corticosteroid |
X |
X |
|
Oral antihistamine |
X |
|
|
Topical antihistamine |
X |
X |
|
Decongestants (oral/topical) |
X |
|
|
Intranasal cromones |
X |
|
|
Ipratropium bromide |
X |
|
|
Leukotriene receptor antagonists |
X |
|
|
Immunotherapy |
X |
|
|
Nasal saline |
X |
X |
|
Surgery |
X |
Table 8: Treatment regimens for allergic and non-allergic rhinitis.
The most common treatment for allergic rhinitis is oral antihistamines. These are relatively inexpensive, easy to take, work quickly and can be taken on an as-needed basis, but they cause sleepiness in some patients [24]. Oral decongestants, with or without oral antihistamines, are also used for short-term treatment of acute symptoms of rhinitis, but these medications have several side effects (insomnia, headaches, elevated blood pressure, rapid heart rate and nervousness) and should not be used by people who are pregnant or who have underlying cardiovascular or cerebrovascular disease [28].
Intranasal corticosteroids are the first line of therapy for perennial and moderate to severe persistent allergic rhinitis and have been found to be excellent at controlling the symptoms. However, they need to be used daily for best results and may cause nasal irritation and epistaxis [28]. Nasal irrigation with saline is often effective in treating rhinitis symptoms like postnasal drip, sneezing, nasal dryness, and congestion. This treatment is simple, inexpensive, and readily available, but has not shown the same level of effectiveness as nasal corticosteroids in treating rhinitis [25]. Intranasal cromones, like cromone sodium, are also used to treat the symptoms of allergic rhinitis but are less effective than other treatments and work better if used before symptoms begin, limiting their overall effectiveness [29].
Leukotriene receptor antagonists are approved for treatment of allergic rhinitis but have been found to be less effective than oral antihistamines and nasal corticosteroids. They take several days to start working and may cause headaches, abdominal pain, and fatigue. Due to a black box warning regarding the risk of mental health side effects of leukotriene blockers, the FDA stresses that they should only be used as a last option when other treatments have failed [29].
Immunotherapy, through oral medications or injections, is an attractive treatment method for patients who have a poor response to other medications or want to minimize the number of medications they need long-term, but this treatment option could be expensive and is time-consuming [29]. Surgery may also be an option for some rhinitis patients, particularly in cases where sinus obstructions are present and/or other treatments have failed.
A new treatment for rhinitis and rhinosinusitis that avoids the shortcomings of the other management modalities would be desirable to patients. Neurostimulation may offer this attractive alternative. In this pilot study, the iCLEAR device has been shown to be safe, easy to use, and effective in alleviating the symptoms of chronic rhinitis and rhinosinusitis, with a convenient treatment profile, employing an approximately 30-second nasal vibration two to three times per day. Most subjects did well with neurostimulation, with only three (10%) dropping out due to difficulty tolerating treatment. This was somewhat below the expected number (approximately 15%) seen in the dry eye investigations [16], and it should be noted that there were very few adverse events, all of which were mild and resolved during the study. Two thirds of the study subjects were satisfied with the treatment and would recommend it to others. Additionally, VAS, TNSS, NOSE, GAC and SNOT-20 scores improved with iCLEAR use to an extent that surprised the investigators.
The TNSS rankings of symptoms related to nasal congestion, runny nose, nasal itching, sneezing, and difficulty sleeping improved 31% as experienced in the last 12 hours and 22% as experienced in the last two weeks, respectively. Similar improvements in TNSS were seen in children with allergic rhinitis treated with either 200 µg fluticasone propionate (FP) nasal spray or FP plus saline irrigation, where scores improved 41% for the FP group and 54% for the FP plus saline irrigation group [24]. iCLEAR TNSS scores showed better improvement than that for the saline irrigation only group (21%) in this study, suggesting that neurostimulation with iCLEAR can provide a better alternative to nasal lavage, with a more convenient use profile. Furthermore, patients exhibited higher baseline scores than those in this study, giving them more room for improvement. Findings of a Cochrane Database Systematic Review of saline irrigation for allergic rhinitis published in 2018 additionally support the conclusion that the effects of the iCLEAR device are at least comparable to that of nasal lavage [27].
iCLEAR VAS scores improved 35% from baseline to 14 days of treatment and 49% from baseline to 30 days of treatment. Comparatively, in a study of 101 patients with vasomotor rhinitis, Lin, et.al., found no significant change (4%) in VAS after one month for a group of patients treated with 3% saline nasal irrigation [28]. In the same study, the patient group treated with budesonide nasal spray improved 35% and the patient group treated with both saline irrigation and nasal spray improved 37%.
In this pilot study, the iCLEAR device was demonstrated to be safe, easy to use and to provide improvement in symptoms of rhinitis and rhinosinusitis at least equal to that shown for nasal irrigation and/or corticosteroids in other studies. A larger, randomized, controlled trial is needed to address the efficacy of the iCLEAR device for the management of congestive symptoms related to rhinitis and rhinosinusitis. A study of patients with higher baseline VAS, NOSE, TNSS, and SNOT-20 scores would also be interesting to determine how much improvement of symptoms would be observed.
5. Conclusions
In this pilot study of the iCLEAR device, efficacy was indicated by the improvement in rhinitis and rhinosinusitis symptoms on five validated, standardized scales: Visual Analog Scale, Nasal Obstruction Symptom Evaluation, Total Nasal Symptom Score, the Global Assessment of Change, and the Sino-Nasal Outcome Test. Patients on average rated the device tolerability as acceptable. Most were satisfied, would use the device again, and would recommend it to others. The iCLEAR has been shown to be safe in this study, as demonstrated by the small number of Adverse Events that were possibly related to the device, all of mild severity, and all resolved. The most common AE was common cold symptoms and/or congestion, occurring in approximately 33% of subjects, unrelated to the study device. There were no Serious Adverse Events or Unanticipated Adverse Device Effects.
Acknowledgement
We thank the patients for their participation in and commitment to the study. We also thank Irwin & Associates and Kari Zimmers for their technical assistance with the manuscript.
Funding
Funding for this study provided by Olympic Ophthalmics.
Commercial Relationships Disclosure
EMS- consulting fees; MG- inventor, founder, CEO, salary support, equity; ACH- none; JJ- none; NJF: consulting fees, equity.
References
- Lung MA. The role of the autonomic nerves in the control of nasal circulation. Biol Signals 4 (1995): 179-185.
- Tran NP, Vickery J, Blaiss MS. Management of rhinitis: allergic and non-allergic. Allergy Asthma Immunol Res 3 (2011): 148-156.
- Spector SL, Nicklas RA, Chapman JA, et al. Symptom severity assessment of allergic rhinitis: part 1. Ann Allergy Asthma Immunol 91 (2003):105-114.
- ENT Health (2018).
- Ciprandi G, Mora F, Cassano M, et al. Visual analog scale (VAS) and nasal obstruction in persistent allergic rhinitis. Otolaryngology - Head and Neck Surgery 141 (2009): 527-529.
- Sedaghat AR. Chronic Rhinosinusitis. Am Fam Physician 96 (2017): 500-506.
- Pynnonen MA, Mukerji SS, Kim HM, et al. Nasal saline for chronic sinonasal symptoms: a randomized controlled trial. Arch Otolaryngol Head Neck Surg 133 (2007): 1115-1120.
- Godoy JM, Godoy AN, Ribalta G, et al. Bacterial pattern in chronic sinusitis and cystic fibrosis. Otolaryngol Head Neck Surg 145 (2011): 673-676.
- Jarvis D, Newson R, Lotvall J, et al. Asthma in adults and its association with chronic rhinosinusitis. Allergy 67 (2012): 91-98.
- Rosenfeld RM, Piccirillo JF, Chandrasekhar SS, et al. Clinical practice guideline (update): adult sinusitis. Otolaryngol Head Neck Surg 152 (2015): S1-S39.
- Soler ZM, Nguyen SA, Salvador C, et al. A novel device combining acoustic vibration with oscillating expiratory pressure for the treatment of nasal congestion. Int Forum Allergy Rhinol 10 (2020): 610-618.
- Khanwalkar A, Johnson J, Zhu W, Johnson E, Lin B, Hwang PH. Resonant vibration of the sinonasal cavities for the treatment of nasal congestion. Int Forum Allergy Rhinol. 2022 Jan;12(1):120-123. doi: 10.1002/alr.22877. Epub 2021 Aug 6. PMID: 34355851.
- Phillips KM, Roozdar P, Hwang PH. Applications of vibrational energy in the treatment of sinonasal disease: A scoping review. Int Forum Allergy Rhinol. 2022 Nov;12(11):1397-1412. doi: 10.1002/alr.22988. Epub 2022 Mar 9. PMID: 35218159; PMCID: PMC9790470.
- Beule AG. Physiology and pathophysiology of respiratory mucosa of the nose and the paranasal sinuses. GMS Current Topics in Otorhinolaryngology, Head and Neck Surgery 9 (2010).
- Standring S. Gray’s Anatomy 41th Edition (2016).
- Ji MH, Moshfeghi DM, Periman L, et al. Novel Extranasal Tear Stimulation: Pivotal Study Results. Transl Vis Sci Technol 9 (2020): 23.
- Elliot SK, Hunter PP, Ali RR, eds. Neuromodulation. Academic Press 1-2 (2009): 1-1200.
- S. Food and Drug Administration, Center for Devices and Radiological Health. iTEAR DEN190026clearance letter (2020).
- Yenigun A, Elbay A, Ozdem A, et al. Dry Eye and Dry Nose Caused by the Effect of Allergic Rhinitis on Tear and Nasal Secretion Osmolarity. Ear, Nose & Throat Journal 100 (2021): 808S-812S.
- Stewart MG, Witsell DL, Smith TL, et al. Development and validation of the nasal obstruction symptom evaluation (NOSE) scale. Otolaryngology - Head and Neck Surgery 130 (2004): 157-163.
- Doulaptsi M, Prokopakis E, Seys S, et al.Visual analogue scale for sino-nasal symptoms severity correlates with sino-nasal outcome test 22: paving the way for a simple outcome tool of CRS burden. Clin Transl Allergy 8 (2018):
- Kamper SJ, Maher CG, Mackay G. Global Rating of Change Scales: A Review of Strengths and Weaknesses and Considerations for Design. The Journal of Manual and Manipulative Therapy 17 (2009): 163-170.
- Williamson A, Hogart B. Pain: a review of three commonly used pain rating scales. Issues in Clinical Nursing 14 (2005): 798-804.
- Verywell Health. Treatment of Allergic Rhinitis (2022).
- Patient Education: Allergic Rhinitis (Beyond the Basics) (2022).
- Chen JR, Jin L, Li XY. The effectiveness of nasal saline irrigation (seawater) in treatment of allergic rhinitis in children. International Journal of Pediatric Otorhinolaryngology 78 (2014): 1115-1118.
- Head K, Snidvongs K, Glew S, et al. Saline irrigation for allergic rhinitis. Cochrane Database Syst Rev 6 (2018): CD012597.
- Lin L, Lu Q, Tang XY, et al. Nasal irrigation for the treatment of vasomotor rhinitis: a pilot study. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke za Zhi Chinese Journal of Otorhinolaryngology Head and Neck Surgery 52 (2017): 446-452.





 Impact Factor: * 3.1
Impact Factor: * 3.1 CiteScore: 2.9
CiteScore: 2.9  Acceptance Rate: 11.01%
Acceptance Rate: 11.01%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 
