A Novel de novo Translocation (derXt(X;13) (q25;q12.11)dn) Manifesting As A Phenotypic Overlap with A Glycosylation Disorder: A Case Report
Caroline Hart1,2, Ellen Crushell1, Padraic Curran3, Clare Brenner4, Bryan Lynch5, Dirk J. Lefeber6, Sally Ann Lynch7,8, Patricia Fitzsimons9, Ina Knerr1,8*
1National Centre for Inherited Metabolic Disorders, Children’s Health Ireland at Temple Street, Dublin, Republic of Ireland
2Royal Belfast Hospital for Sick Children, Belfast, Northern Ireland (UK)
3Portiuncula Hospital, Ballinasloe, County Galway, Republic of Ireland
4Department of Radiology, Children’s Health Ireland at Crumlin, Dublin, Republic of Ireland
5Department of Neurology, Children’s Health Ireland at Temple Street, Dublin, Republic of Ireland
6Department of Neurology, Translational Metabolic Laboratory, Donders Institute for Brain, Cognition and Behavior, Radboud University Medical Centre, Nijmegen, The Netherlands
7Department of Genetics, Children’s Health Ireland at Crumlin, Dublin, Republic of Ireland
8University College Dublin, Dublin, Republic of Ireland
9Metabolic Laboratory, Department of Paediatric Laboratory Medicine, Children’s Health Ireland at Temple Street, Dublin, Republic of Ireland
*Corresponding Author: Prof. Ina Knerr, National Centre for Inherited Metabolic Disorders, Children’s Health Ireland (CHI) at Temple Street, Temple Street, Dublin, D01 YC67, Ireland
Received: 09 February 2023; Accepted: 03 May 2023; Published: 15 August 2025
Article Information
Citation: Caroline Hart, Ellen Crushell, Padraic Curran, Clare Brenner, Bryan Lynch, Dirk J. Lefeber, Sally Ann Lynch, Patricia Fitzsimons, Ina Knerr. A Novel de novo Translocation (derXt(X;13) (q25;q12.11)dn) Manifesting As A Phenotypic Overlap with A Glycosylation Disorder: A Case Report. Archives of Clinical and Medical Case Reports. 9 (2025): 171-174.
View / Download Pdf Share at FacebookAbstract
We describe a novel de novo translocation (derXt(X;13)(q25;q12.11)dn) manifesting as a phenotypic overlap with a Congenital Disorder of Glycosylation (CDG) in a 7-year-old girl. We characterize the clinical, biochemical and neuro-radiological phenotype of this unique and hitherto undescribed translocation, and discuss etiologic aspects in the context of a phenotypic overlap with a CDG.
Keywords
<p>(X;13) translocation; CDG; Glycosylation defect; Rare diseases</p>
Article Details
Abbreviations: CDG: congenital disorders of glycosylation; XIC: X-inactivation centre; IEM: Inborn errors of metabolism
1. Introduction
This paper describes a unique de novo X autosome translocation in a 7-year old girl who was found to have a persistent mildly abnormal serum transferrin glycosylation profile. We present this case to elucidate the clinical, biochemical and neuro-radiological phenotype of this de novo pathogenic genetic variant which has not been previously reported.
Congenital disorders of glycosylation (CDGs) are inborn errors of metabolism (IEM) characterised by glycosylation defects of proteins and lipids, which present with a variety of clinical features along with diagnostic challenges and complex clinical needs. CDGs are often considered and actively investigated where there is a history of global developmental delay and neurological symptoms, with or without additional features. CDGs are investigated for as part of a wider screen for IEM in children and may be conducted in parallel with genetic testing, such as a microarray analysis. While most CDG subtypes are extremely rare, they are cumulative a growing number of IEM with over 150 CDG subtypes described in the literature thus far [1]. There are also other, more common diseases, including, e.g., chronic inflammatory diseases, cancers, Alzheimer's disease or diabetes mellitus associated with abnormal protein glycosylation [2].
2. Case Report
Our patient was the second child of non-consanguineous healthy Irish parents. She was born post term in good condition, following an uncomplicated pregnancy and labour. The baby initially breastfed well and examined normally. On day three of life concerns were raised regarding seizure activity with eye rolling, lip and chin quivering with generalised body stiffening lasting less than one minute, followed by floppiness and poor feeding. A bedside blood glucose test showed hypoglycaemia (0.6mmol/L). This was treated with an intravenous bolus of 10% dextrose and a laboratory blood glucose level was 2.8 mmol/L (ref. 3-5 mmol/L). She was admitted to the special care baby unit where a loss of 9.2% of birth weight was noted along with mild hypernatraemic dehydration. She was screened and treated for possible sepsis until cultures came back negative. The neonatal course was otherwise uneventful with no further seizures or hypoglycaemia.
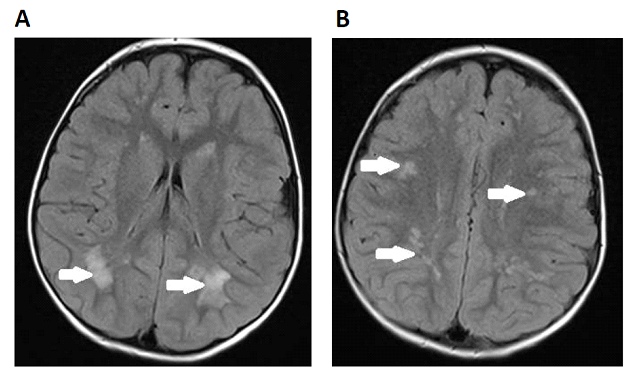
She remained in good general health with a normal diet and steady growth though a pattern of developmental delay evolved, including delayed walking (at 2 yrs. 10 months of age) and speech delay (babbling at 3 yrs. of age). An MRI brain at age 2 years 6 months showed extensive signal abnormality in the deep white matter with numerous focal areas of high T2 and FLAIR signal abnormality in the corona radiata and white matter adjacent to the lateral ventricles (Figure 1). There was no volume loss or calcification present. MR spectroscopy was normal.
Figure 1: Patient MRI brain at age 2 yrs 6 months. Axial FLAIR image taken at the level of the lateral ventricles showing extensive abnormal signal in the white matter adjacent to the posterior horns of the lateral ventricles (A) and abnormal signal in the deep white matter of the corona radiata (B)
There was no history of developmental regression. She experienced sensory and behavioural difficulties. At five years of age, she was diagnosed with moderate learning disability, and placement in a special needs school was going well. The girl could run steadily and understood some commands but had few clear words. Her tone appeared slightly reduced with normal reflexes and she was hypermobile. She also tended to invert her feet and required orthoses. Clinical examination was otherwise unremarkable, and there was no facial dysmorphism. Her weight was tracking along the 9th centile and her height was between 2nd and 9th centile; however, her head circumference was along the 75-90th. She experienced one further generalised seizure at 5 years of age and, following a seizure-free period, she developed further episodes out of sleep at 7 years of age which were complex partial, secondarily generalized seizures. She was commenced on an anticonvulsant (valproate) with good clinical response.
As her neuro-imaging findings were not specific e.g. for neonatal hypoglycaemia or hypoxia, she was further investigated to establish their underlying aetiology. Extensive investigations included glucose/lactate profiling, renal, liver and bone profiles, ammonia, thyroid function, plasma amino acids, homocysteine, acylcarnitines and urine organic acids which were all normal. Very long chain fatty acids, plasma and white cell lysosomal enzymes, urinary purines and pyrimidines, mucopolysaccharides, oligosaccharides and sialic acid were also normal. Of note, creatine kinase (CK) was persistently slightly elevated at 174 – 271 U/l (ref. 20-155 U/L), without seizure activity at the time. Serum transferrin isoform analyses were tested at five years of age. This revealed a slightly abnormal transferrin N-glycosylation pattern detected by mass spectrometry with increase loss of one sialic acid residue (8.3%, reference range <6%). This finding was not indicative of a diagnosis of CDG type I; however, CDG type II or variant forms could not be excluded. APOCIII O-glycosylation was normal. This pattern was confirmed in two further serum samples taken over the course of two years.
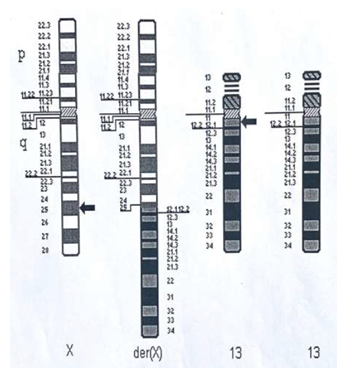
Genetic testing was conducted in parallel with extensive biochemical and metabolic investigations for this girl. Microarray-based Comparative Genomic Hybridization demonstrated a 29.922 Mb terminal deletion from the long arm of the X chromosome, Xq25qter, and a large 93.308 Mb duplication of terminal material from the long arm of chromosome 13, 13q12.11q3ter. Karyotyping confirmed an unbalanced translocation with monosomy Xq25-qter and partial trisomy 13q12.11-qter and confirmed that this was the result of an unbalanced X autosomal translocation (Figure 2). The derivative X chromosome, in addition to a Xq25-qter deletion encompasses a large duplicated segment of chromosome 13. Parental karyotypes were normal. Further X chromosome inactivation studies on metaphase spreads derived from the girl’s blood cells revealed inactivation of the derivative X chromosome in 35/61 (57.4%) cells examined. Molecular X inactivation studies using Androgen receptor and ZMYM3 primer sets both revealed unilateral X inactivation at 100% on testing DNA derived from lymphocytes.
Taken together, it was concluded that there was a connection between these findings with a possibly dual pathology with a (primary) genetic diagnosis and a (secondary) glycosylation abnormality involving numerous candidate genes.
Karyotype: der(X)t(X;13)(q25;q12.11) dn
Figure 2: Patient karyotype indicating affected areas of translocation between the X chromosome and chromosome 13. These imbalances are consistent with the presence of a derivative X chromosome from a translocation between the X chromosome and chromosome 13, including a terminal deletion of 29.922Mb from the long arm of the X chromosome from band q25 to qter and a duplication of 93.308 Mb from the long arm of chromosome 13 from band q12.11 to qter.
3. Discussion
CDGs are a genetically and phenotypically heterogeneous and expanding group of disorders which encompass a spectrum of glycosylation defects of various proteins and lipids. In principle, these are multisystem conditions which often involve a neurological presentation or developmental brain disorder. Among the most common findings on MRI brain for the various subtypes are cerebral atrophy, delayed myelination or multifocal patchy white matter hyperintensities [3], the latter being prominent in our case.
Hypoglycosylation may lead to altered activity of many glycoproteins, including plasma proteins, hormones, membrane proteins and enzymes. Mostly CDGs are recessively inherited though autosomal dominant and X-linked inheritance has been reported [4,5]. There are four types of CDG involving either: (I) N-linked glycosylation; (II) O-linked glycosylation; (III) combined N- and O-linked/multiple glycosylation and (IV) lipid and glycosylphosphatidylinositol (GPI) anchor biosynthesis defects. Type I (N-glycosylation pathway) disorders being the most extensively studied and CDG-Ia (phosphomannomutase [PMM] 2-CDG) is the commonest form with more than 700 cases reported globally. Some patients with a primary monogenic CDG may demonstrate biochemical abnormalities such as altered liver function tests, coagulopathy, hypoglycaemia and occasionally raised creatine kinase, as in our patient. A possible explanation for her persistently slightly elevated CK could be that her complex genotype may affect genes relevant to sarcolemmal/transmembrane glycoproteins on chromosome 13 and or genes encoding for dystrophin–related proteins on chromosome X. Furthermore, a loss of carbohydrates may also affect creatine kinase isoenzyme metabolism, as it is a glycoprotein [6].
Identification of CDGs requires a high index of clinical suspicion and diagnostic testing involves e.g. assessing N-glycosylation by evaluation of transferrin glycosylation with isoelectric focussing and mass-spectrometry whereas APOC III isoelectric focussing testing can be added for some type II CDGs [2,4]. Confounding factors leading to false positive results include alcohol abuse, acute infection or severe liver disease and repeated testing may be required as glycosylation patterns of transferrin can intermittently normalise. We and others have described that Classical Galactosaemia, an inborn disorder of galactose metabolism caused by galactose-1-phosphate uridyl transferase deficiency, can also cause N-glycosylation abnormalities [7]. Our patient had been tested for Classical Galactosaemia via National Newborn Screening Programme with normal results.
Importantly, it has been recognised that glycosylation studies can be close to normal in certain CDGs and the diagnosis could be missed if confirmatory genetic studies are omitted [5]. Therefore, more specific biomarkers are needed for fast, accurate CDG diagnosis [8].
We suggest that other genetic variants, such as the de novo translocation presented here, alongside epigenetic defects, are expected to expand the clinical and genetic heterogeneity of the CDG spectrum. Although the translocation breakpoints do not appear to disrupt known CDG genes directly in our case, they may have revealed a phenotype if a regulatory region of a known CDG gene is disrupted. There are several genes relevant to glycosylation pathways on these chromosomes including, e.g., Xq21.1 (MAGT1), Xq28 (SSR4), 13q14.3 (ALG 1), 13.q12.3 (B3GALTL) or 13q13.3 (COG6). Most CDGs are recessive or X-linked and result from haploinsufficiency. Confirmatory genetic studies are required to establish a diagnosis as biochemical findings may be subtle [5].
Our patient presented with a moderate phenotype overall, considering the degree of imbalance. In an unbalanced X-autosomal karyotype of this size, the X chromosome which is inactivated tends to be the abnormal chromosome, assuming the abnormal chromosome contains the X-inactivation centre (XIC) which maps to Xq13.2. In our case, the XIC was intact and it seems the abnormal chromosome is preferentially inactivated. Whilst the derivative X was shown to be switched off in metaphases derived from blood lymphocytes in 35/61 (57.4%) the DNA X inactivation studies on DNA extracted from lymphocytes revealed complete skewing. Both tests were done on blood lymphocytes and we do not know what the results would be in other tissues. Generally, in patients with unbalanced translocations, disruption of a gene or a regulator region of a gene may cause a phenotype even in a heterozygous state if the normal allele was not enough to compensate. There remains the possibility that the CDG like phenotype in this girl has arisen because of disturbance of an X linked CDG gene or, less likely, disturbance of a CDG gene on chromosome 13.
Overall, we feel that the abnormal transferrin isoform pattern is likely explained by the novel genetic pathogenic variant, with some possible epigenetic process. We conclude the novel genetic abnormality identified is responsible for this girl’s clinical presentation, abnormal serum transferrin glycosylation as well as her neuroimaging findings, reflecting her underlying neuro-genetic disorder.
Declarations
Consent
Written parental consent was obtained for this publication relating to a paediatric patient.
Ethics approval and consent to participate
Approval for use of unidentified patient data was obtained from the Children’s Health Ireland at Temple Street Institutional Review Board.
Availability of data and materials
Not applicable.
Competing Interests
All authors declare that they have no conflicts of interest.
Funding
The authors are very grateful to the Temple Street Foundation/Children’s Health Foundation (CHF), Dublin for financial assistance of their research (RPAC 19.02 to IK).
Author Contributions
CH- Co-writing, data curation; EC-Validation; PC- Data collection; CB- Investigation; BL – Investigation; DJL – Methodology; SAL- Investigation; PF- Methodology; IK- Conceptualisation, co-writing.
All listed authors have received and approved the manuscript prior to submission.
Acknowledgment
We would like to express our gratitude to the patient and her family. We thank all physicians and other health care professionals involved in patient care and diagnostic workup. Special thanks to the Temple Street Foundation/Children’s Health Foundation Ireland for financial assistance of our studies.
References
- Verheijen J, Tahata S, Kozicz T, et al. Therapeutic approaches in Congenital Disorders of Glycosylation (CDG) involving N-linked glycosylation: an update. Genet Med 22 (2020): 268-279.
- Van Scherpenzeel M, Willems E, Lefeber DJ. Clinical diagnostics and therapy monitoring in the congenital disorders of glycosylation. Glycoconj J 33 (2016): 345-358.
- Vals MA, Ashikov A, Ilves P, et al. EPGEN Study, Ng BG, Freeze HH, Lefeber DJ, Õunap K2. Clinical, neuroradiological, and biochemical features of SLC35A2-CDG patients. J Inherit Metab Dis 42 (2019): 553-564.
- Chang IJ, He M, Lam CT. Congenital disorders of glycosylation. Annals of Translational Medicine 6 (2018): 477.
- Galama WH, Verhaagen- van der Akker SLJ, Lefeber DJ, Feenstra Ilse, Verrips Aad. ALG13-CDG with infantile spasms in a male patient due to a de novo ALG13 gene mutation. JIMD reports (2017).
- McBride JH, Rodgerson DO, Hilborne LH. Human, rabbit, bovine, and porcine creatine kinase isoenzymes are glycoproteins. J Clin Lab Anal 4 (1990): 196-198.
- Maratha A, Colhoun HO, Knerr I, et al. Classical Galactosaemia and CDG, the N-Glycosylation Interface. A Review. JIMD Rep 34 (2017): 33-42.
- Péanne R, de Lonlay P, Foulquier F, et al. Congenital disorders of glycosylation (CDG): Quo vadis? Eur J Med Genet 61 (2018): 643-663.


 Impact Factor: * 5.3
Impact Factor: * 5.3 Acceptance Rate: 75.63%
Acceptance Rate: 75.63%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 
