Nivolumab PD-1 Blockade as Bridge to Allogeneic Stem Cell Transplant (HSCT) in Gray-Zone Lymphoma: Case Report
Article Information
Maura Nicolosi1*, Chiara Maria Dellacasa2, Mattia Novo1, Alessandro Busca1, Roberto Freilone1, Barbara Botto1
1Divisions of Hematology, Città della Salute e della Scienza, Torino, Italy
2Bone Marrow Transplant Center, University- Hospital Città della Salute e della Scienza, Torino, Italy
*Corresponding Author;Maura Nicolosi, Division of Hematology, Città della Salute e della Scienza, Corso Bramante 88, Turin, 10125, Italy.
Received: April 17, 2023;Accepted: May 25, 2023; Published: June 21, 2023
Citation: Maura Nicolosi, Chiara Maria Dellacasa, Mattia Novo, Alessandro Busca, Roberto Freilone, Barbara Botto. Nivolumab PD-1 Blockade as Bridge to Allogeneic Stem Cell Transplant (HSCT) in Gray-Zone Lymphoma: Case Report. Archives of Clinical and Medical Case Reports. 7 (2023): 287-289.
View / Download Pdf Share at FacebookAbstract
Mediastinal Gray-zone lymphoma (GZL) is a rare entity of lymphoma, with a typical overlapping in terms of biologic and morphologic features between classic Hodgkin lymphoma (cHL) and primary mediastinal large B-cell lymphoma (PMBCL). The therapeutic approaches for these patients remain controversial, and most standard approaches include a combination of chemotherapy, immunotherapy, and, recently, radiation. Unfortunately, overall survival (OS) and progression free survival (PFS) remain dreary. This case report will discuss an adult patient with mediastinal GZL treated as first line with chemotherapy approaches; after an initial lack of response, we decided for a strategy with the anti PD-1 blockade Nivolumab as bridge to the allogeneic hematopoietic stem cell transplant (HSCT). The patient obtained a complete response before the HSCT maintaining this result after the HSCT with temporary grade I and II GVHD, suggesting the important role of the checkpoint inhibitor in this setting of patients. Clinical trials, with larger groups of patients, and longer follow-up are needed to better define safety and prognostic impact of anti PD1 and subsequent allogeneic HCT for the treatment of GZL.
Keywords
Grey- Zone lymphoma; Anti PD-1; Nivolumab; Allogeneic Stem Cell ransplantation
Article Details
Introduction
According to the 2016 WHO classification revision [1] , Gray-zone lymphoma (GZL), is included into unclassifiable B cell-lymphoma; it shares intermediate clinical, morphologic and immunohistochemical (IHC) features between diffuse large B cell lymphoma (DLBCL), in particular primary mediastinal lymphoma (PMBL), and classic nodular sclerosis Hodgkin lymphoma (cHL) [2]. Immunophenotype (IF) analysis shows typical DLBCL’s cluster of differentiation (CD), as CD20, CD79a, PAX5, and also CD30 and CD15, typically expressed in cHL [3]. Due to these overlapping features, no clear diagnostic criteria are available and there is heterogeneity in the treatment strategies. Recently, genetic information about 9p24.1 amplification, which occurs in mediastinal and Hodgkin lymphoma, highlights a possible role of the programmed death 1 (PD-1) pathway as a target of GZL treatment [4]. Based on these observations, a sequential treatment with anti- PD1 and subsequent allogeneic stem cell transplant (HSCT) is considered an appealing approach for patients with GZL. However, there is concern regarding a possible effect of anti PD-1 as a trigger of GVHD. Herein, we report our experience about a single case of refractory GZL treated with anti- PD1 agent and subsequent allogeneic hematopoietic stem cell transplantation (HSCT).
Case Presentation
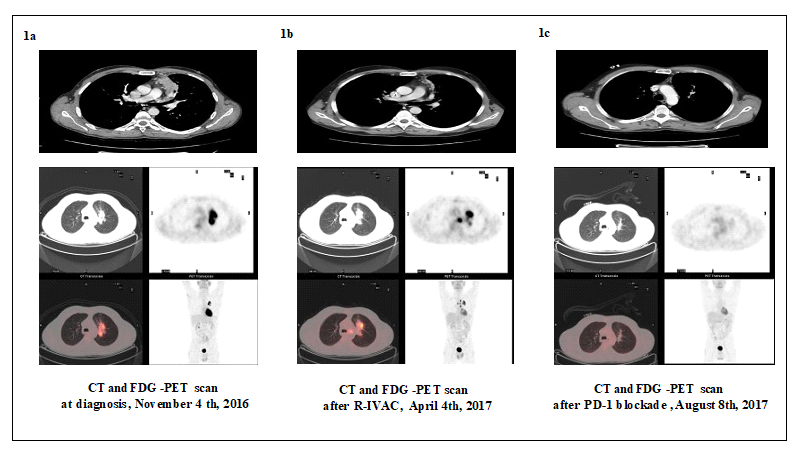
The patient was a 53-years old male who presented to our institution in May 2016, with dyspnea and thoracic pain. He underwent a total computed tomography (CT) scan (Figure 1a), showing a parenchymal lung mass of 3 x 7 cm. A thoracic biopsy specimen was obtained. Cytological examination showed B cell-lymphoma with intermediate characteristics between DLBCL and cHL, which according to WHO 2016 was classified as Gray zone lymphoma (stage IVA sec. 2014 Cheason et al criteria [5]). The immunophenotypic and IHC spectrum showed a low CD30+ expression, CD5+, CD20+, Pax5+, CD45+, Cd3+, ALK-. In addition, Epstein Barr Virus (EBV) evaluation was negative. All laboratory values, including lactate dehydrogenase (LDH), were normal. 18Fluorodeoxyglucose (FDG) Positron Emission Tomography/computed tomography (FDG-PET/ CT) confirmed the hypermetabolic uptake on the anterior and superior mediastinal zone (SUV max 20 and SUV max 14 respectively). Cerebrospinal fluid examination did not show central nervous system (CNS) involvement.
The patient received five cycles of dose-adjusted etoposide (VP-16), doxorubicin (ADM), cyclophosphamide (CTX) with vincristine (VCR) and prednisone plus rituximab (DA- EPOCH-R). The treatment was well tolerated with grade III upper extremity paresthesia as the only adverse event. Unfortunately, at the end of treatment, CT scan and FDG-PET/ CT showed no response (Figure 1b). The patient subsequently received three cycles of R-CODOX chemotherapy including
Rituximab, CTX, ADM, VCR, Cytarabine (ARA-C), with evidence of disease progression at the end of the treatment. On the basis of published reports, salvage therapy with anti PD1-Nivolumab as a bridge to allogeneic HSCT was started. In the meantime, a fully HLA mathed sibling donor had been identified. After three cycles of Nivolumab 3 mg/mq every two weeks, the CT scan and PET/TC showed a complete remission (CR) (Figure 1c). The patient received a total of seven cycles of Nivolumab 3 mg/mq without any side effect. Two months after the last Nivolumab administration, on September 14th, 2017, the patient received peripheral stem cell transplantation from matched sibling donor.
Figure 1a represents the scan results at the time of diagnosis.
As showed in Figure 1b the patient had a progression disease after R-IVAC treatment.
In Figure 1c the patient had a complete metabolic response after PD-1 blockade treatment.
Myeloablative conditioning consisted on TBF regimen (Thiotepa, Busulfan and fludarabine). Graft-versus-host disease prophylaxis included Cyclosporine and short course Methotrexate.
Neutrophil engraftment occurred on day + 11 post- transplant and during the aplastic phase the patient developed hyperthermia and pneumonia without microbiological isolates in the bronchoalveolar lavage. On day + 27 acute grade II cutaneous GVHD occurred, successfully treated
with prednisone 0,5 mg/kg. One month after transplantation, bone marrow chimerism was full donor, IF and IHC was negative for disease recurrence. The CT-scan and FDG-PET/ CT performed three months after HSCT showed persistent complete lymphoma remission. Six months after HSCT, the patient presented with National Institute of Health (NIH) grade II chronic GVHD involving skin, eyes, mouth, liver gut and genitalia. He was treated with cyclosporine and prednisone 1 mg/kg with progressive symptom improvement. At present (sixty-six months post HSCT) the patient is alive, in continuous complete remission, with persistent grade I-II ocular and oral chronic GVHD, still requiring immunosuppression.
Discussion and Conclusion
Mediastinal gray zone lymphoma (GZL) is a rare aggressive lymphoma. The optimal treatment approach in this setting still represents an unmet clinical need. Given to the identification of 9p24.1 amplification in a large proportion of patients with GZL, anti PD-1 agents have been investigated as a treatment option in these patients and results of preclinical studies suggest that Nivolumab may be effective in heavily pretreated patients with mediastinal GZL [4, 6]. Moreover, Merryman et al. described the preliminary results of HSCT in 39 patients affected by different types of lymphoma, who received a PD-1 inhibitor as a salvage therapy prior to HSCT. One -year overall survival (OS) and progression free survival (PFS) were 89 and 76% respectively [7]. By contrast, Rashidi et all., in his meta-analysis of patients affected by HL treated with HSCT, showed as primary endpoints at 6-month, 1-year, 2-year and 3-year a relapse-free survival (RFS) of 77%, 50%, 37% and 31%, respectively. OS was 83%, 68%, 58% and 50%, respectively at 6-month, 1-year, 2-year and 3-year. This study showed that more than one-third of patients eventually experiences relapse after HSCT with a poor prognosis [8]. Recently, in a retrospective cohort of 209 HL patients, who underwent to HSCT after PD1 blockade, the PFS and OS were 69% and 82%, respectively [9]. In addition, a longer interval from PD-1 to allo-HCT was associated with less frequent severe acute GVHD [9]. Our report suggests that in heavily pretreated refractory GZL, the PD-1 blockade agents may represent a feasible and safe approach in order to obtain a rapid response, also as a bridge to allo-HCT. A possible role of anti-PD1 agents in GVHD incidence and severity has to be taken into consideration. Clinical trials, with larger cohorts of patients, and longer follow-up are needed to better define safety and prognostic impact of anti PD1 and HSCT for the treatment of GZL.
Conflict of Interest
None of the authors have any conflict of interest to report.
References
- Swerdlow SH, et The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood 127 (2016): 2375-2390.
- Traverse-Glehen A, et Mediastinal gray zone lymphoma: the missing link between classic Hodgkin's lymphoma and mediastinal large B-cell lymphoma. Am J Surg Pathol 29 (2005): 1411-1421.
- Gualco G, Natkunam Y, Bacchi The spectrum of B-cell lymphoma, unclassifiable, with features intermediate between diffuse large B-cell lymphoma and classical Hodgkin lymphoma: a description of 10 cases. Mod Pathol 25 (2012): 661-674.
- Ansell SM, et PD-1 blockade with nivolumab in relapsed or refractory Hodgkin's lymphoma. N Engl J Med 372 (2015): 311-319.
- Cheson BD, et Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non- Hodgkin lymphoma: the Lugano classification. J Clin Oncol 32 (2014): 3059-3068.
- Melani C, et PD-1 Blockade in Mediastinal Gray-Zone Lymphoma. N Engl J Med 377 (2017): 89-91.
- Merryman RW, et al. Safety and efficacy of allogeneic hematopoietic stem cell transplant after PD-1 blockade in relapsed/refractory lymphoma. Blood 129 (2017): 1380-
- Rashidi A, et Allogeneic hematopoietic cell transplantation in morphologic leukemia-free aplastic state. Am J Hematol 92 (2017): E549-E552.
- Merryman RW, et Allogeneic transplantation after PD-1 blockade for classic Hodgkin lymphoma. Leukemia 35 (2021): 2672-2683.


 Impact Factor: * 3.1
Impact Factor: * 3.1 CiteScore: 2.9
CiteScore: 2.9  Acceptance Rate: 11.01%
Acceptance Rate: 11.01%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 
