Oral Lichenoid Lesions May Regress after Amalgama Removal: A Case Report Becomes a Proof of the Concept
Bettoni E, Bellucci G, Damiani G, Crescentini M*
Department of Biomedical, Surgical and Dental Sciences, School of Dentistry, UOC Maxillo-facial surgery and dentistry Fondazione IRCCS Cà Granda, Ospedale Maggiore Policlinico, University of Milan, Italy
*Corresponding Author: Michele Luigi Crescentini, Department of Biomedical, Surgical and Dental Sciences, School of Dentistry, UOC Maxillo-facial surgery and dentistry Fondazione IRCCS Cà Granda, Ospedale Maggiore Policlinico, University of Milan, Italy
Received: 27 June 2023; Accepted: 13 July 2023; Published: 03 August 2023
Article Information
Citation: Bettoni E, Bellucci G, Damiani G, Crescentini M. Oral Lichenoid Lesions May Regress after Amalgama Removal: A Case Report Becomes a Proof of the Concept. Archives of Clinical and Medical Case Reports. 7 (2023): 338-344.
View / Download Pdf Share at FacebookAbstract
The aim of this report is to present a clinical case of oral lichenoid lesions (OLL) associated with amalgam and various metal restorations, including palladium, mercury, and gold. The patient presented with symptoms and lesions consistent with OLL, and the diagnosis was confirmed through a synthesis of the patient's medical history, clinical examination, histopathological analysis, and direct immunofluorescence. Oral lichenoid lesions are a group of disorders characterized by lesions that resemble oral lichen planus but are caused by a hypersensitivity reaction to certain substances, such as dental materials. In this case, the amalgam and metal restorations were suspected to be the triggering factors for the lichenoid lesions. The histopathological analysis revealed findings consistent with OLL, further supporting the diagnosis. Additionally, direct immunofluorescence testing provided additional evidence for the presence of OLL. The combination of these diagnostic approaches allowed for a comprehensive understanding of the patient's condition. As part of the treatment plan, the metal restorations were replaced, and this intervention led to significant improvements in the patient's clinical condition. The lichenoid lesions on the tongue and floor of the oral cavity showed regression and almost complete remission, exhibiting a reticular appearance. This outcome indicated a positive response to the removal of the suspected triggering factors. In conclusion, this case highlights the association between oral lichenoid lesions and metal restorations, particularly amalgam, palladium, mercury, and gold. The diagnosis was established through a comprehensive evaluation of the patient's medical history, clinical examination, histopathological analysis, and direct immunofluorescence testing. The removal of the metal restorations resulted in notable improvements in the patient's condition, with regression and near-complete remission of the lichenoid lesions.
Keywords
<p>Amalgam hypersensitivity; Metals; Oral lichen planus; Lichenoid reaction; Allergic reaction</p>
Article Details
Abbreviations
OLL = oral lichenoid lesion; OLR = oral lichenoid reaction; OLP = oral lichenoid planus; SCC = squamous cell carcinoma; NSAIDs = Non-steroidal anti-inflammatory drugs; ACEI = angiotensin-converting enzyme inhibitors
1. Introduction
Many dental materials and medications contain substances that can induce hypersensitivity reactions of the oral mucosa or skin [1-4]. Saliva cleansing action and the vascularization of the oral mucosa reduce the chances of irritant and allergic reactions compared to the skin [5, 6]. However, when tissues are repeatedly exposed to a potential allergen, the possibility of sensitization increases. Metal amalgams are alloy mixtures containing mercury, silver, tin, copper, and sometimes zinc. Corrosion of amalgam fillings releases small amounts of metal ions. Some of these ions can cause allergic reactions [7-9]. Reports describing allergic reactions and highlighting a causal relationship between oral lesions and amalgam and metals such as palladium and gold are available in the literature [2, 6, 10-15]. Symptoms are typically classified as delayed hypersensitivity reactions (type IV) and have been defined by Finne et al [16] as oral lichenoid reaction (OLR). The clinical presentation of OLR is similar to oral lichen planus (OLP), it may be reticular, in the form of plaques, atrophic and erosive, or a combination of these [17-20]. However, OLR has a defined etiological factor, while OLP is generally idiopathic [19, 20]. This case report presents a patient with bilateral OLR or diffuse lichenoid mucositis in the oral cavity, who also has various amalgam restorations and prosthetic elements with restorations made of various metallic materials (gold, mercury, and palladium). This clinical case was prepared following the CARE guidelines [21, 22]. As previously mentioned, there are similar cases in the literature but the sources are still limited. For this reason, we believe that the considerations arising from this case report are useful in improving the ability of clinicians to detect and treat oral lichenoid lesions, especially if associated with the presence of metal restorations.
2. Case Report
A 61-year-old Italian was referred for oral whitish lesions adherent to the tongue and oral mucosa occurred in the previous 3 months. On loco-regional clinical examination, the mucous membranes lining the oral cavity were moderately hyperemic, normo-irritated, and normo-chromatic, conditions compatible with the patient's age. Salivation was clinically normal. On the dorsal surface of the tongue, there were two slightly elevated, leukoplakic lesions, measuring approximately 10 mm x 10 mm and 15 mm x 10 mm, respectively. The lesions were asymptomatic upon palpation, and the patient reported no pain or discomfort but she experiences a sensation of dryness localized to the area where the lesions are present. The patient doesn’t have any neurological pathology and doesn’t take any drugs that could cause a change in salivation or a decrease in it. The presence of Sjogren's syndrome was also excluded, as no anti-Sjogren's antibodies were detected [23]. Additionally, the patient reports increased sensitivity to hot foods or drinks.
In the patient's medical history, she reports having had breast cancer 12 years ago, which was completely resolved through radiotherapeutic treatments, three years of Enantone, and seven years of Tamoxifen. In this kind of patients the main problem is the strong modification of eating habits and consequently the alteration of the oral microbiota, salivary pH and salivary secretion. This condition is aggravated by taking drugs that worsen the patient's clinical situation. Furthermore, no other medical conditions or allergies were reported, including those related to drugs and anesthetics. Patch test is crucial in the diagnosis and recognition of causative allergens because it reveals contact allergies, and is still superior in differentiating allergic and irritant contact reactions [24]. Although a negative patch test can exclude an allergic hypersensitivity reaction, it cannot exclude the possibility of idiosyncrasy. The patch test to check for allergic reactions to metals was denied, so it is suspected to be an idiosyncrasy reaction. The patient also reports being a non-smoker. Following some questions, it was possible to explore some aspects of the patient's lifestyle, and no particular stressors were identified that could have a negative correlation with the patient's clinical situation.
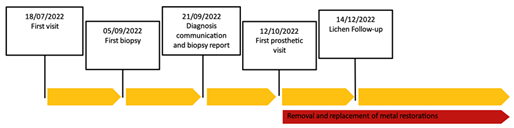
Figure 1: Time line.
Therefore, an incisional biopsy (Figure 2) was performed on the dorsal surface of the tongue, using a mucosal punch with a diameter of 0.4 cm. The histopathological diagnosis describes the tissue sample from the tongue mucosa as having a lichenoid lymphocytic infiltrate with exocytosis and cytoid bodies. No dysplasia was observed, and the finding is compatible with oral lichen planus. Serial sections and immunohistochemical staining with CD3, CD20, CD4, and CD8 were performed.
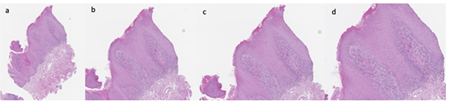
Figure 2: Biopsy: Images relating to the incisional biopsy, carried out in correspondence with the lesions present on the back of the patient's tongue. a) sampling overview, b) detail 1, c) detail 2, d) detail 3.
Based on current recommendations and specific guidelines, the following therapeutic approach is recommended:
- Use Biotene® or Bioxtra® Gel as toothpaste with a soft-bristled brush for oral hygiene after main meals.
- Use Biotene® or Bioxtra® mouthwash for rinsing 3-4 times daily after oral hygiene.
- Use baking soda, 1 teaspoon in a small amount of cold water, for a 1-minute rinse 2-3 times a day, separately from the use of mouthwash.
Oral lichen planus (or lichenoid stomatitis) is a benign inflammatory dermatological condition that can affect the skin, oral mucosa (in 50% of cases), and genital mucosa. This condition is supported by an autoimmune reaction mediated by cytotoxic T lymphocytes directed against cells of the oral mucosa, for various reasons, including the presence of metal restorations; the presence of mercury-containing amalgam can induce an abnormal immune response in the oral mucosa [17, 19, 25]. Importantly, the occurrence of lichen planus is multifactorial and can be influenced by a combination of several factors [26]. For example the genetic predisposition, associations have been identified between certain types of genes and the onset of lichen planus. Genetic predisposition can affect the immune response and susceptibility to disease [27]. In addition, some viral infections, such as hepatitis C, have been associated with the onset of lichen planus [28]. Stress and psychological factors can contribute to the onset of lichen planus or can make symptoms worse. Stress is thought to affect the immune response and inflammation [29]. In addition, some drugs, such as some antimalarials, antihypertensive drugs, nonsteroidal anti-inflammatory drugs (NSAIDs), and anticancer therapies (for example, methotrexate, doxorubicin, and anti-PD-1 immune system inhibitors) have also been associated with the onset of lichen planus [30]. The definitive data on the mechanisms inducing potential triggers and the extent of the target antigens are currently unknown, although the resulting immune response dysregulation, although never demonstrated, tends to support the hypothesis of a chronic inflammatory reaction with an autoimmune pathogenetic basis. Several studies have associated it with a delayed hypersensitivity reaction represented by a contact reaction. In general, some substances do not have an antigenic nature, unlike certain metals, but by penetrating the skin and mucous membranes, they can bind to specific surface proteins, thus acquiring an allergenic character [10, 13, 15]. These allergens are captured by Langerhans cells that can migrate through the epithelium and various tissues to the reference lymph nodes, where they will adequately present the processed antigen to CD4+ helper T lymphocytes. This delayed contact form is generally aligned with lichenoid mucositis, a generic term used to describe a mucositis, a chronic inflammation of the mucosa, OLP-like, in response to the presence of metal restorations, such as dental amalgams, and/or reactions to certain drugs. In the light of these considerations present in the literature, it has been suggested to the patient, before resorting to systemic therapies, to attempt to resolve the problem by removing all metal- containing restorations present inside the oral cavity. The following month after the diagnosis, the patient was first visited in the prosthodontic department. During the clinical examination and analysis of the orthopantomography (Figure 3), several elements rehabilitated through direct restorations made with metal alloys and indirect restorations (such as single and multiple crowns on two abutments) also made of metal alloys were identified. In particular, metal alloy crowns were found in positions 16 and 17, amalgam in position 25 and 26, a bridge with a metal substructure in position 34-37 (with difficulty in replacement as element 35 was not adequately treated endodontically), and a gold ceramic bridge in position 45-47 (Pontic)-45.
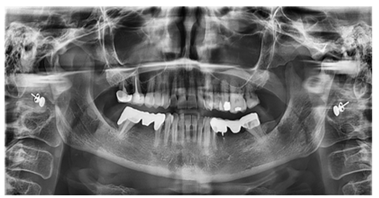
Figure 3: Orthopantomography, performed on 09/26/2022.
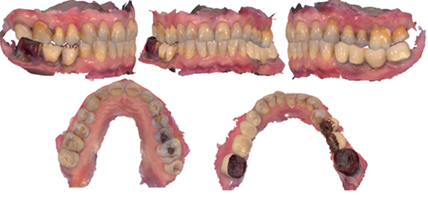
Figure 4: Scanning using a 3Shape intraoral digital scanner and processing using dedicated software.
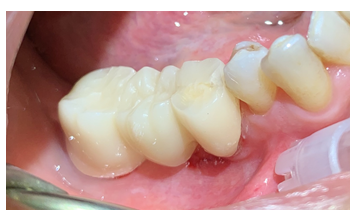
Figure 5: Multiple indirect restoration on elements 47-46 (Pontic)-45 in Zirconia. Natural abutment corresponding to element 45 and 47, pontic element corresponding to 46.
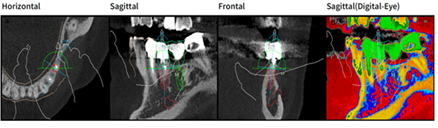
Figure 6: Design with Megagen R2 Gate software, in relief (red color) implant element 35 in transversal (a) sagittal; (b) frontal; (c) and sagittal vision highlighting bone density (d).
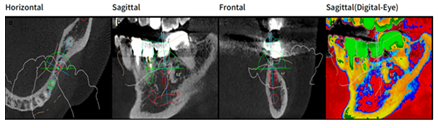
Figure 7: Design with Megagen R2Gate software, in relief (red color) implant element 36 in transversal (a) sagittal; (b)frontal; (c) and sagittal vision highlighting bone density (d).
As can be seen from the images, the entire analysis, design, and execution were managed through the use of digital scanners and dedicated software (Figure 4). Moreover, during the visit, periodontal problems or issues related to an altered bacterial flora in the oral cavity were excluded through an examination performed with a periodontal probe (PCPUNC15, Hu-Friedy®, Chicago, IL, USA). The main issue to be resolved remains the lichenoid lesion, as previously diagnosed. Once the therapeutic plan was explained to the patient and their consent was obtained, the multiple indirect restoration on elements 47-46 (Pontic)-45 was removed and replaced with a multiple indirect restoration in zirconia, partially maintaining, reconstructing the abutment of element 47, and re-preparing element 45. (Figure 5) The direct restoration on element 26 was removed and replaced with composite material. The direct restoration on element 25 was removed, and the abutment was reconstructed using composite resin material, and an indirect restoration was made using a zirconia crown. Through the processing and matching of the STL (scanning) and DICOM (CBCT) files, it was possible to create a prosthodontically guided surgical plan (Figures 6, 7, 8).
Following this planning, a dental support mask (Figure 9) was created, which was essential for carrying out a surgically and prosthodontically guided intervention, respecting the most modern techniques of implant placement. On 03/08/2023, under plexus and trunk anesthesia, 2 Megagen® Anyone implants were inserted, using a R2gate Megagen® guided surgery template, at positions 35 (4 x 8.5 mm implant) and 36 (4 x 8.5 mm implant). A submerged healing option was chosen (Figures 10, 11).
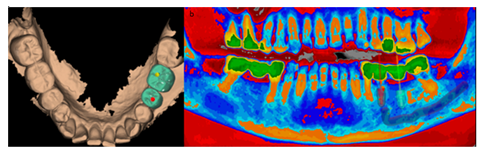
Figure 8a: Design with Megagen R2Gate software. Occlusal vision with prosthetic design, as the study with the technology used, allows positioning the implants in a prosthetically guided way, visualizing the prosthetic emergency at the same time as the endoxy position of the implants.
Figure 8b: Panoramic frontal view highlighting bone density.
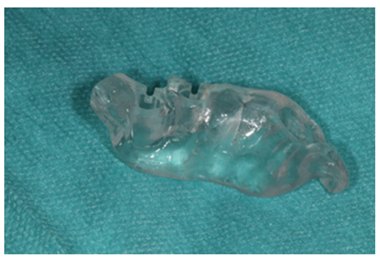
Figure 9: Dental mesh for guided surgery.
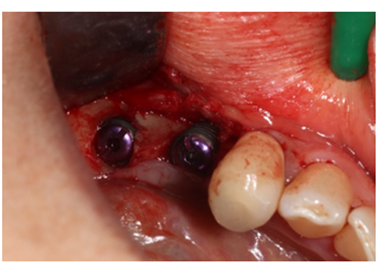
Figure 10: Insertion of implant fixture zone 35 and 36, with insertion of cap screws.
During the treatment, it was possible to observe an almost complete regression of the lichenoid lesions, initially evidenced by the report issued by the Oral Pathology department, which described the mucous membranes as normally represented, normochromic, normotrophic, and normoreflective, with very few lesions present on the back of the tongue. Overall, at 3 months from the diagnosis and about 2 months from the start of the prosthodontic interventions, the patient showed a visible improvement.
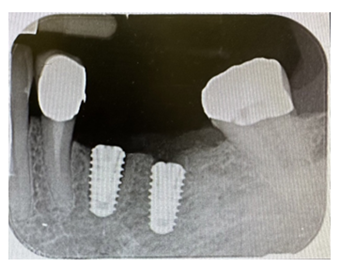
Figure 11: Intraoral radiographic control, performed with a Rinn centerer.
3. Discussion
It has been found that hypersensitivity reactions are associated with over 130 dental materials, including dental amalgam. The information network of German dermatology departments ("Informationsverbund Dermatologischer Kliniken," IVDK) analyzed 756 patients with oral mucosal allergy to dental materials. Positive reactions were most frequently observed towards nickel sulfate, palladium chloride, sodium thiosulfate gold, followed by benzoyl peroxide and amalgam [31]. The incidence of type IV hypersensitivity allergy due to dental amalgam is about 3-5% of the population and 37-78% among those who are also allergic to mercury and/or silver [31, 32]. It is generally accepted that poorly polished, adapted, and old amalgam restorations often cause oral mucosa irritations. Therefore, it is an important duty of dental healthcare providers to carefully inspect not only changes in the oral mucosa but also all defective restorations and all possible causes of mucosal irritation. Diagnostic criteria for oral lichenoid lesions (OLL) include clinical and histopathological features, as well as improvement or resolution of the lesion after removal of the suspected causal material, to confirm the diagnostic hypothesis. It is indeed difficult to distinguish OLL from oral lichen planus (OLP) [33]. Many researchers have demonstrated unique features of OLL, including a higher number of eosinophils, mast cells, and capillaries, increased COX-2 expression, loss of heterozygosity in chromosomes 9p (D9S157, D9S162, D9S171), 11q (D11S1369), and 17p (TP53, AFM238WF2), and weaker immunopositivity of metallothionein in the basal and parabasal layers compared to OLP [34]. If there are no suspected causal dental materials intraorally, the history of drug use is important. Several medications can cause bilateral OLL, such as NSAIDs, angiotensin-converting enzyme inhibitors (ACEI), dapsone, diuretics, oral hypoglycemic agents, gold salts, and penicillamine [31]. Similarly to our case, many authors have demonstrated remission of OLL after removal of amalgam filling material (range: 2 days-2 years) [35-37]. The replacement of suspected causal material can be a diagnostic and therapeutic approach to the lesion simultaneously without the need for biopsy or drug treatment. On the other hand, it should be noted that the removal of suspected dental materials "does not" guarantee improvement of OLL symptoms [31]. In a Spanish study, 71.4% (i.e., 5 out of 7) of patients with OLL due to amalgam fillings did not have direct contact between the restored amalgam tooth and the lesion. The average age of the restoration was 27 years (range: 20-40). The older the filling material, the greater the risk of corrosive damage to the restoration surface. Metallic ions and corrosive products can create a toxic and irritating reaction, leading to OLL (the so-called "toxic-corrosive phenomenon") [38]. A recent systematic review also analyzed the malignant transformation of 19,676 OLP and 419 OLL reported from 57 studies. The onset of oral squamous cell carcinoma (OSCC) in OLP was much lower than in OLL (280 OLP patients (1.4%) vs. 13 OLL patients (3.1%): P=0.0045). The average time from OLP/OLL diagnosis to malignant transformation was 62 months (range: 58.5 + 8.1), and multiple pathways were mentioned, e.g., nitric oxide (NO)-O2 pathway, COX-2 activity within infiltrating inflammatory cells, deficient function of the apoptotic protein p53, and hyperactivation of the Akt/Protein kinase-B cell survival pathway. Smoking, alcoholism, and HCV infection were found to be potential triggering factors of the lesions [39]. This underscores the danger of such lesions and highlights the need for long-term follow-up (5 years) for patients.
4. Conclusion
OLL is often associated with partial or complete contact with restorations made of metals and amalgams. Although a biopsy is the gold standard for establishing a definitive diagnosis and exclude the presence of squamous cell carcinoma (SCC). The treating physician/dentist should recognize such lesions and be able to formulate a diagnostic hypothesis based on the relevant scientific literature. A trial treatment by removing the suspected causal material on the tooth or teeth adjacent to the lesion is an alternative and can help diagnose OLL, distinguishing them from OLP. The patient in question showed a marked improvement within a few months, supporting the hypothesis that it was OLL related to a metal reaction. As emphasized, however, it cannot be excluded that these lesions may have malignant progression. This is a risk that the patient should be informed about.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
References
- Athavale PN, Shum KW, Yeoman CM, et al. Oral lichenoid lesions and contact allergy to dental mercury and gold. Contact Dermatitis 49 (2003): 264-265.
- Laeijendecker R, Dekker SK, Burger PM, et al. Oral Lichen Planus and Allergy to Dental Amalgam Restorations. Arch Dermatol 140 (2004).
- Udoye C, Aguwa E. Amalgam Safety and Dentists’ Attitude: A Survey among a Subpopulation of Nigerian Dentists. Operative Dentistry 33 (2008): 467-471.
- Lind PO, Hurlen B, Lyberg T, et al. Amalgam-related oral lichenoid reaction. Eur J Oral Sci 94 (1986): 448-451.
- De Rossi SS, Greenberg MS. Intraoral Contact Allergy: A Literature Review and Case Reports. The Journal of the American Dental Association 129 (1998): 1435-1441.
- Dunsche A, Frank MP, Luttges J, et al. Lichenoid reactions of murine mucosa associated with amalgam. Br J Dermatol 148 (2003): 741-748.
- Eley BM. The future of dental amalgam: a review of the literature. Part 6: Possible harmful effects of mercury from dental amalgam. Br Dent J 182 (1997): 455-459.
- Bratel J, Haraldson T, Meding B, et al. Potential side effects of dental amalgam restorations.: (I). An oral and medical investigation. Eur J Oral Sci 105 (1997): 234-243.
- Skoglund A, Egelrud T. Hypersensitivity reactions to dental materials in patients with lichenoid oral mucosal lesions and in patients with burning mouth syndrome. Eur J Oral Sci 99 (1991): 320-328.
- Laine J, Kalimo K, Forssell H, et al. Resolution of oral lichenoid lesions after replacement of amalgam restorations in patients allergic to mercury compounds. Br J Dermatol 126 (1992): 10-15.
- Aggarwal V, Jain A, Kabi D. Oral lichenoid reaction associated with tin component of amalgam restorations: a case report. Am J Dermatopathol 32 (2010): 46-48.
- Klaisiri A, Iamaroon A, Neff A, et al. Oral lichenoid lesion related to dental amalgam: A case report. Journal of Stomatology, Oral and Maxillofacial Surgery 120 (2019): 591-594.
- Issa Y, Duxbury AJ, Macfarlane TV, et al. Oral lichenoid lesions related to dental restorative materials. Br Dent J 198 (2005): 361-366.
- Pang BK, Freeman S. Oral lichenoid lesions caused by allergy to mercury in amalgam fillings. Contact Dermatitis 33 (1995): 423-427.
- Tsushima F, Sakurai J, Shimizu R, et al. Oral lichenoid contact lesions related to dental metal allergy may resolve after allergen removal. Journal of Dental Sciences 17 (2022): 1300-1306.
- Finne K, Göransson K, Winckler L. Oral lichen planus and contact allergy to mercury. International Journal of Oral Surgery 11 (1982): 236-239.
- Dunsche A, Kastel I, Terheyden H, et al. Oral lichenoid reactions associated with amalgam: improvement after amalgam removal. Br J Dermatol 148 (2003): 70-76.
- Thorn JJ, Holmstrup P, Rindum J, et al. Course of various clinical forms of oral lichen planus. A prospective follow-up study of 611 patients. J Oral Pathol Med 17 (1988): 213-218.
- Hamour AF, Klieb H, Eskander A. Oral lichen planus. CMAJ 192 (2020): E892.
- Alrashdan MS, Cirillo N, McCullough M. Oral lichen planus: a literature review and update. Arch Dermatol Res 308 (2016): 539-551.
- Gagnier JJ, Kienle G, Altman DG, et al. The CARE guidelines: consensus-based clinical case reporting guideline development. Case Reports 2013 (2013): bcr2013201554-bcr2013201554.
- Riley DS, Barber MS, Kienle GS, et al. CARE guidelines for case reports: explanation and elaboration document. Journal of Clinical Epidemiology 89 (2017): 218-235.
- Thorne I, Sutcliffe N. Sjögren’s syndrome. Br J Hosp Med (Lond) 78 (2017): 438-442.
- Lugovic-Mihic L, Ilic I, Budimir J, et al. Common Allergies and Allergens in Oral and Perioral Diseases. Acta Clin Croat 59 (2020): 318-328.
- McParland H, Warnakulasuriya S. Oral Lichenoid Contact Lesions to Mercury and Dental Amalgam-A Review. J Biomed Biotechnol 2012 (2012): 589569.
- Payeras MR, Cherubini K, Figueiredo MA, et al. Oral lichen planus: focus on etiopathogenesis. Arch Oral Biol 58 (2013): 1057-1069.
- Carrozzo M, Thorpe R. Oral lichen planus: a review. Minerva Stomatol 58 (2009): 519-537.
- Alaizari NA, Al-Maweri SA, Al-Shamiri HM, et al. Hepatitis C virus infections in oral lichen planus: a systematic review and meta-analysis. Aust Dent J 61 (2016): 282-287.
- De Porras-Carrique T, González-Moles MÁ, Warnakulasuriya S, et al. Depression, anxiety, and stress in oral lichen planus: a systematic review and meta-analysis. Clin Oral Investig 26 (2022): 1391-1408.
- Wat M, Mollanazar NK, Ellebrecht CT, et al. Lichen-planus-pemphigoides-like reaction to PD-1 checkpoint blockade. J Cutan Pathol 49 (2022): 978-987.
- Raap U, Stiesch M, Kapp A. Contact allergy to dental materials. J Dtsch Dermatol Ges 10 (2012): 391-396.
- Bains VK, Loomba K, Loomba A, et al. Mercury sensitisation: review, relevance and a clinical report. Br Dent J 205 (2008): 373-378.
- van der Meij EH, van der Waal I. Lack of clinicopathologic correlation in the diagnosis of oral lichen planus based on the presently available diagnostic criteria and suggestions for modifications. J Oral Pathol Med 32 (2003): 507-512.
- Mendes GG, Servato JPS, Borges FC, et al. Differential metallothionein expression in oral lichen planus and amalgam-associated oral lichenoid lesions. Med Oral Patol Oral Cir Bucal 23 (2018): e262-e268.
- Al-Hashimi I, Schifter M, Lockhart PB, et al. Oral lichen planus and oral lichenoid lesions: diagnostic and therapeutic considerations. Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology, and Endodontology 103 (2007): S25.e1-S25.e12.
- Lopes de Oliveira LM, Batista LHC, Neto AP dos S, et al. Oral Lichenoid Lesion Manifesting as Desquamative Gingivitis: Unlikely Association? Case Report. Open Dent J 12 (2018): 679-686.
- Mallo Pérez L, Díaz Donado C. Intraoral contact allergy to materials used in dental practice. A critical review. Med Oral 8 (2003): 334-347.
- Lartitegui-Sebastián MJ, Martínez-Revilla B, Saiz-Garcia C, et al. Oral lichenoid lesions associated with amalgam restorations: a prospective pilot study addressing the adult population of the Basque Country. Med Oral Patol Oral Cir Bucal 17 (2012): e545-549.
- Aghbari SMH, Abushouk AI, Attia A, et al. Malignant transformation of oral lichen planus and oral lichenoid lesions: A meta-analysis of 20095 patient data. Oral Oncology 68 (2017): 92-102.


 Impact Factor: * 5.3
Impact Factor: * 5.3 Acceptance Rate: 75.63%
Acceptance Rate: 75.63%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 
