Surgical Management of Full-Thickness Macular Hole In A Patient with Pachychoroid Spectrum Disease with Atypical Fundus Autofluorescence After Surgery
Rony Carlos Preti, MD, PhD1*, Lívia da Silva Conci, MD1, Leonardo Provetti Cunha, MD, PhD1,2, Sérgio Luís Gianotti Pimentel, MD, PhD1, Leandro Cabral Zacharias, MD, PhD1, Mario Luiz Ribeiro Monteiro, MD, PhD1
1Department of Ophthalmology, University of Sao Paulo, Sao Paulo, Brazil
2Department of Ophthalmology, Federal University of Juiz de Fora, Juiz de Fora, Minas Gerais, Brazil
*Corresponding Author: Rony Carlos Preti, Department of Ophthalmology, University of Sao Paulo, Sao Paulo, Brazil
Received: 23 March 2023; Accepted: 17 April 2023; Published: 26 April 2023
Article Information
Citation: Rony Carlos Preti, Lívia da Silva Conci, Leonardo Provetti Cunha, Sérgio Luís Gianotti Pimentel, Leandro Cabral Zacharias, Mario Luiz Ribeiro Monteiro. Surgical Management of Full-Thickness Macular Hole In A Patient with Pachychoroid Spectrum Disease with Atypical Fundus Autofluorescence After Surgery. Archives of Clinical and Medical Case Reports. 7 (2023): 166-170.
View / Download Pdf Share at FacebookAbstract
Purpose: To describe a case of atypical full-thickness macular hole (FTMH) in a patient with pachychoroid spectrum disease. The efficacy of surgical intervention is discussed.
Observations: A 64-year-old woman presented with poor vision and metamorphopsia in her left eye (OS). Swept-Source Optical Coherence Tomography (SS-OCT) revealed a grade II FTMH in association with serous macular detachment and indocyanine green angiography (ICGA) showed choroidal hyperpermeability in OS. The patient underwent pars plana vitrectomy (PPV), inner limiting membrane peel, and 20% SF6 gas fill. Macular hole closure was achieved successfully on early postoperative, with opening of FTMH in the right eye in the first posoperative week when patient in positioning. Unusual hyper-autoflurescent peripapillary spots appeared in OS early after PPV with slowly resolution, but followed by RPE mottling. Complete subretinal fluid reabsorption was observed only at 12 months of follow-up.
Conclusions and Importance: FTMH with large subretinal detachment can still be closed with standard PPV technique without fluid drainage. Atypical evolution of this case could be explained by the overlapping of two distinct eye conditions (vitreoretinal interface disease and central serous chorioretinopathy). Multimodal imaging was a useful tool for diagnostic and long-term follow-up significantly helping to understand the unusual postoperative evolution of the case.
Keywords
<p>Vitreoretinal Surgery; Fovea Centralis; Choroid Diseases; Optical Coherence Tomography</p>
Article Details
1. Introduction
Full thickness macular hole (FTMH) was first described by Knapp in 1869 in a patient with blunt ocular trauma history [1]. In general, most of FTMH are idiopathic and occur in 7th decade of life, and is bilateral in, approximately, 10% of cases [2]. Its pathogenesis has not been completely understood yet. However, it is well known that tractional forces may cause deformations and tissue disruptions at fovea [3]. Tractional forces can result from partial posterior vitreous detachment and contractile epiretinal membranes [4].
The pachychoroid spectrum diseases include choroidal features such as focal or diffuse choroidal thickening, compression of choriocapillaris layer and choroidal hyperpermeability seen at indocyanine green angiography (ICGA) [5], but it is not related to retinal tractional pathology. Central serous chorioretinopathy (CSCR) is significantly related to pachychoroid spectrum disease and it is characterized by choroidal hyperpermeability leading to foveal serous detachment of the neurosensory retina [6], that somehow could help in the development and worsening of FTMH [7].
Herein, we describe a case of FTMH superimposed on CSCR in a patient who underwent pars plana vitrectomy (PPV), with an atypical postoperative follow-up.
2. Case Report
A 63-year-old white woman presented with a history of metamorphopsia associated with central vision loss in left eye (OS) for 2 years. Her review of systems included allergic rhinitis and sinusitis, with previous nasal and systemic corticosteroids use. She was adoptive daughter and her familial history was unknown. She had undergone laser photocoagulation in the inferior peripheral retina in both eyes (OU) at another service 3 years ago for lattice degeneration. On ocular examination, her best-corrected visual acuity (BCVA) was 20/20 in right eye (OD) (+1.50 sphere diopters) and 20/30- (+3.00 sphere diopters) in OS. The anterior segment was unremarkable, no vitreous cells were noted in OU and pupillary reactions were normal. Intraocular pressure was 13/12 mmHg.
Fundus examination and color fundus photograph (DRI OCT Triton, Topcon, Inc, Tokyo, Japan) revealed an abnormal reflectivity of the macular surface in OD (Figure 1A) and a serous macular detachment in OS (Figure 2A). This serous detachment could be also observed in more detail in the fundus autofluorescence (FA) presenting with a mild hypo-autofluorescence in the macular region surrounded by a halo of hyper-autofluorescence. (Figure 2B). Ultra-wide field retinography (Daytona Ultra-widefield Retinal Imaging, Optos Inc., Marlborough, United States) revealed photocoagulation scars in inferior periphery (Figures 1C and 2C) and well-demarcated and symmetric semilunar lesions located temporal to macula in OU, representing choroidal vessel atrophy associated with corrugated retinal pigment epithelium (inset in Figure 1D and 2D). Fluorescein angiography (FA) (Spectralis, Heildelberg, Germany) was within normal limits in OD (Figure 1E) and showed late phase petaloid leakage pattern in OS (Figure 2E). Indocyanine green angiography (ICGA) (Spectralis, Heildelberg, Germany) demonstrated macular choroidal hyperpermeability in OS (Figures 1F,1G, 2F and 2G).
Swept-Source Optic Coherence Tomography (SS OCT) (DRI OCT Triton, Topcon, Inc, Tokyo, Japan), macular scanning revealed type 2 posterior vitreous detachment (PVD) associated to a mild epiretinal membrane in OD (Figure 1H). In OS, there was attachment of the posterior hyaloid to the inner retina, epiretinal membrane, grade 2 FTMH, cystoid macular edema, and extensive subretinal fluid (Figure 2H). FTMH’s minimum linear diameter was 71 µm. Central macular thickness was 295 µm in OD and 836 µm in OS. Subfoveal choroidal thickness was 250 µm in OD and 320 µm in OS, and pachyvessels were seen in OU.
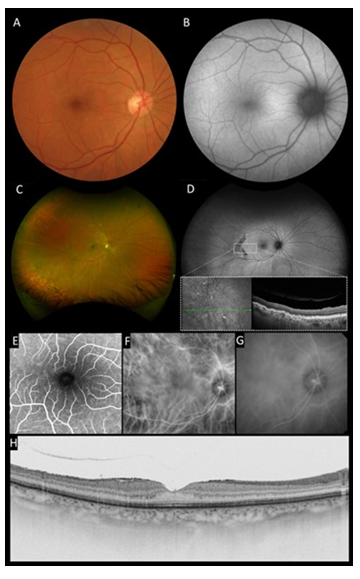
Figure 1: Multimodal imaging of the right eye: A, Color fundus photograph showed an abnormal reflectivity of the macular surface. B, Fundus autofluorescence was unremarkable. C, Ultra-wide field retinography revealed well demarcated semilunar lesions located temporal to macula and photocoagulation scars in inferior periphery. D, Ultra-wide field autofluorescence demonstrated that temporal lesions and inferior photocoagulation scars were hypoautofluorescent. Inset : SD-OCT of temporal mid-periphery lesions demonstrated corrugated RPE. E, Fluorescein angiography was unremarkable in macular region. F and G, Indocyanine green image revealed choroidal hyperpermeability at late phase. H, Cross-sectional SS-OCT image of the macula showed grade 2 posterior vitreous detachment and a grade 1 epiretinal membrane.
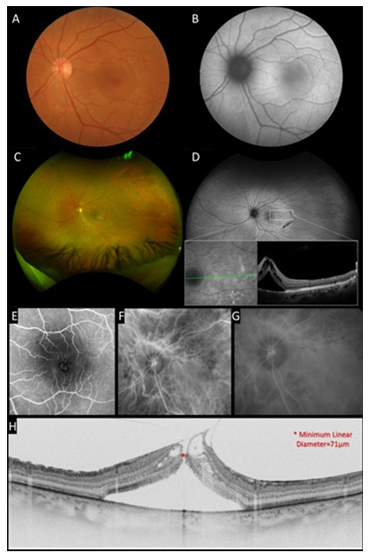
Figure 2: Multimodal imaging of the left eye. A, Color fundus photograph showed a macular serous detachment associated with an abnormal reflectivity of the macular surface. B, Fundus autofluorescence presented a mild hypoautofluorescence in macular region surrounded by a halo of hyperautofluorescence. C, Ultra-wide field retinography revealed well demarcated semilunar lesions located temporal to macula and photocoagulation scars in inferior periphery. D, Ultra-wide field autofluorescence demonstrated that temporal lesions and inferior photocoagulation scars were hypoautofluorescent. Inset : SD-OCT of temporal mid-periphery lesions demonstrated corrugated RPE. E, Fluorescein angiography demonstrated a petaloid leakage pattern in macular region. F and G Indocyanine green image revealed choroidal hyperpermeability at late phase. H, Cross-sectional SS-OCT image of the macula showed attachment of the posterior hyaloid to the inner retina, epiretinal membrane, full-thickness macular hole, cystoid macular edema, and extensive subretinal fluid.
Clinical examination and imaging results led us to diagnose stage II FTMH associated with serous macular detachment related to pachychoroid disorder in OS. She underwent PPV, inner limiting membrane peel (assisted with brilliant blue), 20% SF6 gas fill and the fluid was not aspirated through the hole (supplemental video 1). Seven days postoperative kept on face down position, the patient returned complaining of visual discomfort in OD, BCVA 20/25. On SS – OCT examination we observed Stage II MH (Figure 3A) when patient was instructed to suspend the recommended position. SS OCT of OS showed successfully closure of the FTMH (Figure 4A) and improvement of the BCVA to 20/20-, however, with persistence of macular subretinal fluid. On the 14th postoperative day, spontaneous closure of FTMH was observed in OD (Figure 3B), and an extensive peripapilar hyper-autofluorescent subretinal material was seen in OS on SS-OCT (Figure 4B), BCVA was 20/20 and 20/25, respectively. Carbonic anhydrase inhibitors were started, with very slow and gradual reduction of subretinal fluid. After six months of follow-up, OS BCVA remained 20/20- and there was still persistence of subretinal fluid and peripapillary subretinal hyperautofluorescent material (Figure 4C). Complete subfoveal fluid reabsorption was observed at 12 months of follow-up with final BCVA of 20/20 (Figure 4D). Humphrey’s visual test (30-2 SITA Standard) exhibited peripheral scotoma in OD and central inferior scotoma in OS, which were topographically correlated with posterior postoperative segment findings (Figure 5).
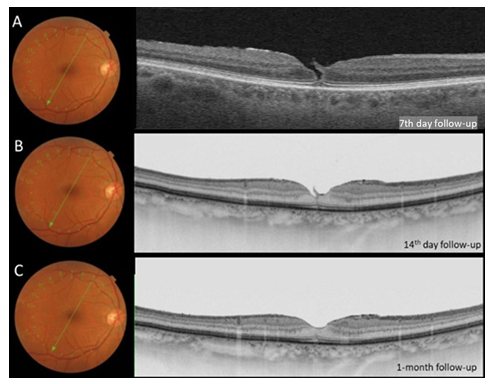
Figure 3: A, optical coherence tomography image of OD at 7th day postoperative follow-up showing grade 2 FTMH. B and C, OCT images at 14th day and 1-month follow-up, respectively, demonstrated macular hole closure and posterior vitreous detachment.
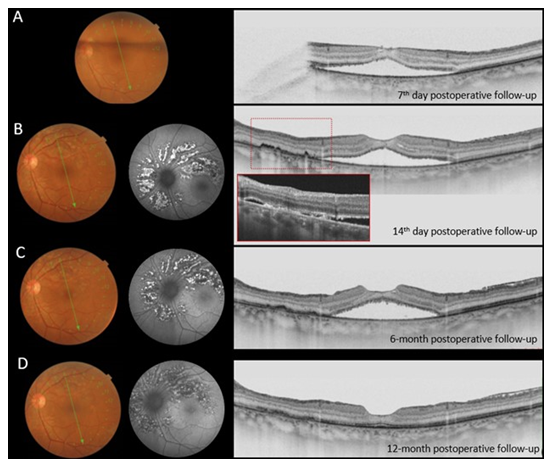
Figure 4: Long-term postoperative follow-up of OS. A, Seven days postoperative SS OCT showing FTMH closure and persistence of extensive subretinal fluid. Note the presence of subretinal hyperreflective material 14 days and six-month, of follow-up, B and C, respectivell. D, Complete subretinal fluid reabsorption was observed just at 12 months of follow-up. On autofluorescence, we can observe the characteristic aspect of peripapillary hyperautofluorescence corresponding to the subretinal lesions.
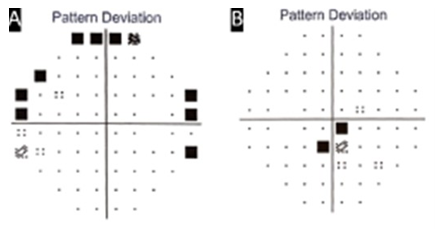
Figure 5: Computadorized perimetry of OU. A, OD exhibited peripheral scotoma. B, OS showed central scotoma, where the subretinal material was initially present.
3. Discussion
There are a few case reports of FTMH associated with CSCR. In 2012, Amde and Tewari described a case of a 63-year-old woman with previous history of CSCR that presented a FTMH [8]. The authors proposed that CSCR, and the associated RPE and choriocapillaris dysfunctions may promote the macular hole’s progression. Their suggestion was based on the theory of hydration suggested by Tornambe, that consists of hydration of the inner and outer retina by vitreous fluid, initiated by defect of inner foveal retina secondary to posterior hyaloid traction [7]. Therefore, this theory suggests that the progression of a FTMH depends on the fluid balance created by the rate of vitreous fluid entering the retina and the rate of fluid removed by the RPE cells. As the RPE is dysfunctional in disorders related to the pachychoroid syndrome, there could be a theorical facilitation for the development of a FTMH in the presence of vitreomacular traction.
In present case, the patient had some independent tractional factors that could lead to a FTMH formation, such as the adherent posterior hyaloid and the epiretinal membrane. These vitreoretinal interface disorders may have been made worse after peripheral photocoagulation or could be caused by the laser. In any case, the tractional factors were successfully removed at PPV and FTMH’s closure was achieved on early postoperative days, with improving of BCVA. However, subretinal fluid has persisted for? months following surgery, suggesting that subretinal fluid was not related to tractional factors, validating the diagnose of CSCR as the cause of persisting subretinal fluid for such long time. Previous history of corticosteroids may have influenced it as a trigger or an exacerbating factor.
Persistence of subretinal fluid following FTMH surgery is a well-known condition. Tranos et al evaluated the prognostic factors, morphological features, and functional recovery in patients with persistent subretinal fluid after successful FTMH surgery and found that it is of common occurrence (48% of incidence) [9]. In all cases, subretinal fluid gradually decreased in size, but complete resolution occurred only in 11 out of the 15 affected cases (73%) by the end of the study follow-up period (7 months). Most foveal detachments resolved 3 to 5 months after surgery. Preoperative risk factors significantly associated with persistence of subretinal fluid were stage 2 FTMH, smaller size of the closest distance between the FTMH edges, and posterior vitreous attachment to the edge of FTMH. Our patient presented all such risk factors. However, it is worth mentioning that the images presented in that article show a much smaller amount of subretinal fluid than that presented in our case.
Interestingly, in our case, OD also developed a FTMH when the patient was in face-down position, that was closed after reconsideration of the recommendation (Figure 3). We believe that when the patient was in face-down, the antero-posterior traction of vitreous to the fovea increased by the gravitational force predisposing the process of FTMH formation. This theory is supported by the fact that after resuming the standing position, the process was interrupted. Finally, we are intrigued by the hyperautofluorecent appearance of the yellowish peripapillary subretinal material present in our patient after the surgery (Figure 4) which is commonly ascribed to subretinal lipid [10]. In 2008 Ho and Yanuzzi described such finding as mixture of fluorophore compounds derived from de photoreceptor outer segments that could be in the form of one of the following or a mixture of them: shed photoreceptor outer segments along with their A2E precursor fluorophores [11], macrophages filled with photoreceptor outer segments [12]; and/or fluorophore compounds created from oxidative stress on photoreceptor outer segment membrane lipids [13]. Their publication and this case helped us to interpretate that hyperautofluorescence may not always be exclusively from lipofuscin within the RPE and can be a result of other fluorophores within the neurosensory retina or in the subretinal space. The difference from our study is that our case, at the end of one year, demonstrated colocalize hypoautofluorescence and RPE atrophy, in comparison to their cases that did not show signs of RPE damage. Perhaps the amount of material for RPE phagocytosis overloaded these cells causing damage of it.
4. Conclusions
In conclusion, FTMH with large subretinal detachment from CSCR can still be closed with standard PPV technique and the atypical presentation and evolution of this case could be explained by the overlapping of two distinct eye conditions (vitreoretinal interface disease and CSCR). Multimodal imaging was a useful tool for diagnose and clarification of unusual postoperative evolution in short and long-term follow up.
Funding
No financial support.
References
- Knapp H. Ueber Isolierte Zerreissungen der Aderhaut infolge von Traumen auf dem Augapfel. Arch Augenheilkd 1 (1869): 6-29.
- Mccannel, Colin A, et al. Population-based incidence of macular holes. Ophthalmology 116 (2009): 1366-1369.
- Scharf, Jackson M, et al. Hyperreflective stress lines and macular holes. Investigative ophthalmology & visual science 61 (2020): 50.
- Bringmann, Andreas et al. Different modes of full-thickness macular hole formation. Experimental Eye Research 202 (2021): 108393.
- Borooah, Shyamanga et al. Pachychoroid spectrum disease. Acta Ophthalmologica (2020).
- Von Graefe A. Ueber centrale recidivierende retinitis. Graefes Arch Clin Exp Ophthalmol 12 (1866): 211-215.
- Tornambe, Paul E. Macular hole genesis: the hydration theory. Retina 23 (2003): 421-424.
- Amde, Wendewessen; TEWARI, Asheesh. Full-thickness macular hole formation associated with central serous chorioretinopathy. European journal of ophthalmology 22 (2012): 1039-1041.
- Tranos, Paris G, et al. Persistent subretinal fluid after successful full-thickness macular hole surgery: prognostic factors, morphological features and implications on functional recovery. Advances in therapy 32 (2015): 705-714.
- Yannuzzi LA, Shakin JL, Fisher YL, et al. Peripheral retinal detachments and retinal epithelial atrophic tracts secondary to central serous pigment epitheliopathy. Ophthalmology 91 (1984): 1554-1572.
- Spaide RF, Noble K, Morgan A, et al. Vitelliform macular dystrophy. Ophthalmology 113 (2006): 1392-1400.
- Spaide RF, Klancnik JM. Fundus autofluorescence and central serous chorioretinopathy. Ophthalmology112 (2005): 825-833.
- Sawa M, Ober M, Spaide RF. Autofluorescence and retinal pigment epithelial atrophy after subretinal hemorrhage. Retina 26 (2006): 119-120.


 Impact Factor: * 5.3
Impact Factor: * 5.3 Acceptance Rate: 75.63%
Acceptance Rate: 75.63%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 
