The Therapeutic Potential of Metformin for Metabolic Associated Fatty Liver Disease: Bioinformatics Analysis
Timothy Brian Winslow¹, G Edward Vates2, Michael A. Nead3, Ismat Shafiq1*
1Department of Medicine, Resident Internal Medicine, University of Rochester School of Medicine and Dentistry
601 Elmwood Ave/ Box 693, Rochester, NY, USA
2Department of Neurological Surgery, University of Rochester Medical Center, 601 Elmwood Avenue, Box 607
Rochester, NY, USA
3Department of Medicine: Pulmonary Disease and Critical Care, University of Rochester School of Medicine and Dentistry 601 Elmwood Ave/ Box 692, Rochester, NY, USA
4Department of Medicine: Division of Endocrinology, Diabetes, and Metabolism, Director, UR Medicine Pituitary Program, University of Rochester School of Medicine and Dentistry, 601 Elmwood Ave/ Box 693, Rochester, NY, USA
*Corresponding Author: Ismat Shafiq, Department of Medicine: Division of Endocrinology, Diabetes, and Metabolism, Director, UR Medicine Pituitary Program, University of Rochester School of Medicine and Dentistry, 601 Elmwood Ave/ Box 693, Rochester, NY, USA
Received: 08 December 2023; Accepted: 03 January 2024; Published: 12 January 2024
Article Information
Citation: Chen-Shi Lin, Hong Ma. The Therapeutic Potential of Metformin for Metabolic Associated Fatty Liver Disease: Bioinformatics Analysis. Archives of Clinical and Medical Case Reports 8 (2024): 16-22.
View / Download Pdf Share at FacebookAbstract
Metabolic associated fatty liver disease (MAFLD) is associated with obesity and metabolic dysfunctions, but its molecular mechanisms remain elusive and effective treatments are lacking. Metformin is a potential therapeutic agent for MAFLD due to its effects on insulin resistance and fat metabolism dysregulation. This study used bioinformatics analysis to identify differentially expressed genes (DEGs) in MAFLD and explore the potential targets of metformin treatment. We selected a MAFLD dataset from the Gene Expression Omnibus (GEO) database and screened 425 DEGs, including 278 up-regulated and 147 down-regulated genes. We also identified 183 genes related to metformin treatment using the Genclip3 database and found 13 common genes with the DEGs. The Genome Ontology (GO) enrichment analysis of these genes revealed biological processes such as suppression of inflammation and promotion of glycolipid metabolism, while the Kyoto Encyclopedia of Genes and Genomes (KEGG) pathways involved diabetic complications and inflammatory signaling. The protein-protein interaction (PPI) analysis identified IL1B, IL6, IL10 and CCL2 as the core proteins. Based on the PPI network, we selected the top 9 genes as hub genes, including IL6, IL1B, IL10, CCL2, FOXO1, PIGS2, IGFBP1, GCK, and MYC. The study suggests that metformin may target multiple pathways for the treatment of MAFLD, mainly involving anti-inflammation, regulation of glycolipid metabolism, and anti-hepatic fibrosis.
Keywords
<p>Bioinformatic Analysis; Metformin; Metabolic Associated Fatty Liver Disease; Mechanism</p>
Article Details
1. Introduction
Ectopic ACTH-dependent Cushing syndrome (EACS) constitutes approximately 15-20 % of Cushing's syndrome. The key hurdle in managing EACS is the recognition of symptoms and comorbidities with hypercortisolemia, swiftly treating hypercortisolemia, and pinpointing the source. We report a case of spontaneous resolution of ACTH-dependent Cushing following medical treatment for hypercortisolemia and nocardiosis.
2. Case Presentation
66 y/o female presented to the emergency department with worsening tremors, muscle weakness, and swelling of the face and lower extremities. The symptoms started about 6 months ago. On evaluation, she had high blood pressure, hyperglycemia, and hypokalemia. Due to clinical suspicion of Cushing’s, laboratory data were obtained that showed elevated 24-hour urine cortisol of 3125 ug/24 hour (Normal <=45.0 ug/d), ACTH 140 pg/ml (normal < 65pg/ml), and a low-dose dexamethasone suppression test (DST) with non-suppressed cortisol of 62.8 ug/dl. Hypertension, hyperglycemia, and hypokalemia were medically managed with metoprolol, amlodipine, spironolactone, potassium, and insulin. Brain magnetic resonance imaging(MRI) did not reveal any pituitary abnormality.
Given the patient's presentation and negative MRI head, ectopic Cushing was suspected. Further testing with High-dose DST was suggestive of an ectopic source with a non-suppressed cortisol level of 53 ug/dl. To further localize the source, inferior petrosal sinus sampling (IPSS) was performed which confirmed the ectopic source. Chest computed tomography (CT) showed a left 4 cm cavitary mass. Abdominal CT revealed multiple hepatic cysts with the largest 6 cm in size. The Dotatate scan did not reveal any source. She had a biopsy of the left lung lesion which showed chronic inflammation and no malignant cells. Sputum cultures showed acid-fast bacilli consistent with Nocardia.
For the treatment of hypercortisolemia, she was started on metyrapone 250 mg three times daily and the dose increased to 500 mg three times daily within a week. Antimicrobial treatment with Trimoathoprim-sulfamethoxazole (TMP-SMX) was initiated. She was discharged with Metyrapone 750 mg three times daily and TMP-SMX. Dexamethasone was added as a part of the “block and replace” strategy. She was off insulin and an antihypertensive agent at the time of discharge. Three months after the initiation of metyrapone and antimicrobial treatment, CT chest and, FDG-PET were obtained. CT chest showed scarring of the left lobe with no residual cavitary lesion (Figure.1). FDG PET showed low uptake in the left lung in the location of the cystic mass. A repeat bronchoscopy was attempted which did not have any malignant cells. At this time patient has normal 24-hour urine cortisol and a decrease in ACTH level. (Table 1 illustrates the timeline of laboratory findings and metyrapone dose.) Metyrapone was tapered off at approximately nine months from diagnosis. She remained in remission for more than one year after discontinuing metyrapone.
Table 1: Timeline of lab results and medical treatment.
ACTH=adrenocorticotropin hormone
TID=three times daily
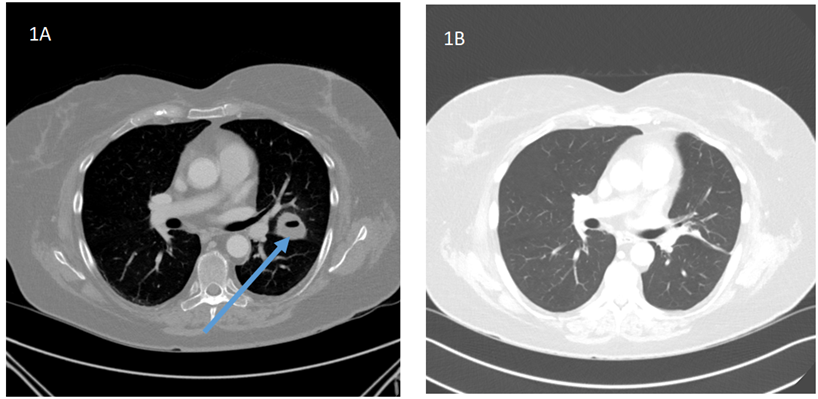
Figure 1: CT chest imaging before and after treatment.
1A: Initial CT Chest: Cavitary mass within the left upper lobe directly abutting the fissure with a central region of low attenuation/fluid measuring up to 4.0 cm (shown with arrow)
1B: CT chest 3 months after antimicrobial and steroidogenesis inhibitor: There is scarring in the left upper lung. No residual cavitary lesion.
3. Discussion
Cushing Syndrome (CS) is endogenous hypercortisolemia that can be ACTH-dependent or independent. ACTH-dependent CS can be caused by a pituitary adenoma(CD) or an ectopic source( EACS), where CD represents 85 % of CS while EACS, a rare occurrence, represents 15-20 % of CS cases[1]. Among EACS cases, approximately half originate in pulmonary carcinoid tumors and small cell cancer[2]. Distinguishing between CD from EACS poses a challenge based on clinical presentation. The variable manifestations of cushings, influenced by the onset, severity, and duration of hypercortisolemia include progressive weight gain, muscle weakness, ecchymosis, hirsutism, mood changes, hypertension, hypokalemia, and hyperglycemia [3]. CD typically exhibits an insidious, gradual onset, contrasting with EACS's more acute presentation, though significant overlap exists. Mortality and morbidity are higher in cushings due to the increased risk of fractures, cardiovascular disease, clot disorders, and opportunistic infections [3]. In the case described, the patient experienced an acute onset of worsening hypertension, hyperglycemia, and hypokalemia with an incidental Nocardia lung infection.
The severity of hypercortisolemia serves as a predictor for susceptibility to infections and heightened mortality rates [4]. Systemic infections involving Candida, Aspergillus, Nocardia, Pneumocystis Carini, Herpes, etc have been documented in cases of CS. A recent systemic review highlighted nocardiosis as an opportunistic infection in eighteen cases with CS, with the majority linked to EACS and only two instances in CD [5, 6]. Eleven out of eighteen patients (61%) faced mortality [6]. Significantly elevated ACTH levels were identified as a factor associated with an increase in mortality and risk of re-infection. Given the elevated mortality risk in CS, the guidelines from the Endocrine Society recommend considering prophylactic antibiotic treatment in CS patients with 24-hour urine cortisol five times above the normal limit [7].
The precise mechanism behind infection resulting from prolonged cortisol exposure remains incompletely understood. Despite the anti-inflammatory properties of glucocorticoids, which make them valuable in treating various medical conditions, chronic exposure to cortisol compromises the immune system. This compromise is evident in the impact on white blood cells and reduction in lymphocytes. In a study by Kronfol et al involving forty-eight cushings syndrome patients, immune markers were analyzed [8]. The findings indicated a decrease in T lymphocytes, attributed to a decline in CD-4(helper T cells) percentages, accompanied by an increase in CD-8(Suppressor and cytotoxic T-cells) percentages to the age match control. Additionally, cortisol was observed to decrease the Neutral Killer(NK) cells' activity causing increased susceptibility to viral infections[8].
The literature has documented instances of spontaneous resolution of hypercortisolemia following medical treatment in Cushing’s [9-13]. Beardwell et al reported the two cases of occult ectopic Cushings with remission after medical intervention [11]. Sharma et al described four cases of ectopic Cushing achieving remission after steroidogenesis blocker treatment like mitotane, ketoconazole, and metyrapone with treatment duration ranging from 3-10 years [9]. The exact mechanism of remission remains unclear, but is theorized that steroidogenesis inhibitors might directly affect ACTH secretion [9, 14, 15]. In a case report on ACTH-dependent thymic carcinoids, metyrapone treatment led to decreased ACTH and cortisol levels within 2 months [15]. A similar reduction in ACTH and cortisol was observed in another case report treated with ketoconazole [14]. There is speculation that higher cortisol levels are necessary for the growth of ACTH-secreting tumors, thus implying that lowering cortisol levels can result in decreased ACTH production [11, 15]. Tumor hemorrhage and or infarction has been postulated to be another cause of spontaneous remission [10]. Our patient experienced remission after 6 months of medical treatment with metyrapone and antibiotics for nocardiosis. Both the cortisol and ACTH levels declined with treatment. A comparable case by Rizwan et al highlighted the complete resolution of hypercortisolemia following nocardiosis treatment [16]. Our patient has been in remission for more than 2 years. We propose that the treatment of nocardiosis-induced infarction of the localized tumor, leading to hypercortisolemia remission.
In conclusion, we report this interesting case of EACS with spontaneous remission of CS after successful antibiotic treatment for Nocardiosis and steroidogenesis inhibitor.
References
- Hayes AR, Grossman AB. The Ectopic Adrenocorticotropic Hormone Syndrome: Rarely Easy, Always Challenging. Endocrinol Metab Clin North Am 247 (2018): 409-425.
- Ejaz S, Vassilopoulou-Sellin R, Busaidy NL, et al. Cushing syndrome secondary to ectopic adrenocorticotropic hormone secretion: the University of Texas MD Anderson Cancer Center Experience. Cancer 117 (2011): 4381-4389.
- Nieman LK. Cushing's syndrome: update on signs, symptoms, and biochemical screening. Eur J Endocrinol 173 (2015): M33-M38.
- Sarlis NJ, Chanock SJ, Nieman LK. Cortisolemic indices predict severe infections in Cushing syndrome due to ectopic production of adrenocorticotropin. J Clin Endocrinol Metab 85 (2000): 42-47.
- Mylonas CC, Gomatou G, Asimakopoulou A, et al. Pulmonary nocardiosis associated with Cushing's disease: a case report. Monaldi Arch Chest Dis 89 (2019).
- Zhang D, Jiang Y, Lu L, et al. Cushing's Syndrome With Nocardiosis: A Case Report and a Systematic Review of the Literature. Front Endocrinol (Lausanne) 12 (2021): 640998.
- Nieman LK, Biller BM, Findling JW, et al. Treatment of Cushing's Syndrome: An Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metabolism 100 (2015): 2807-2831.
- Kronfol Z, Starkman M, Schteingart DE, et al. Immune regulation in Cushing's syndrome: relationship to hypothalamic-pituitary-adrenal axis hormones. Psychoneuroendocrinol 21 (1996): 599-608.
- Sharma ST, Nieman LK. Prolonged remission after long-term treatment with steroidogenesis inhibitors in Cushing's syndrome caused by ectopic ACTH secretion. Eur J Endocrinol 166 (2012): 531-536.
- Loh KC, Gupta R, Shlossberg AH. Spontaneous remission of ectopic Cushing's syndrome due to pheochromocytoma: a case report. Eur J Endocrinol 135 (1996): 440-443.
- Beardwell CG, Adamson AR, Shalet SM. Prolonged remission in florid Cushing's syndrome following metyrapone treatment. Clin Endocrinol (Oxf) 14 (1981): 485-492.
- Iwayama H, Hirase S, Nomura Y, et al. Spontaneous adrenocorticotropic hormone (ACTH) normalization due to tumor regression induced by metyrapone in a patient with ectopic ACTH syndrome: case report and literature review. BMC Endocr Disord 18 (2018): 19.
- Popa Ilie IR, Herdean AM, Herdean AI, et al. Spontaneous remission of Cushing's disease: A systematic review. Ann Endocrinol (Paris) 82 (2021): 613-621.
- Steen RE, Kapelrud H, Haug E, et al. In vivo and in vitro inhibition by ketoconazole of ACTH secretion from a human thymic carcinoid tumor. Acta Endocrinol (Copenh) 125 (1991): 331-334.
- Mizoguchi Y, Kajiume T, Miyagawa S, et al. Steroid-dependent ACTH-produced thymic carcinoid: regulation of POMC gene expression by cortisol via methylation of its promoter region. Horm Res 67 (2007): 257-262.
- Rizwan A, Sarfaraz A, Jabbar A, et al. Case report: Nocardia infection associated with ectopic cushings. BMC Endocr Disord 14 (2014): 51.


 Impact Factor: * 5.3
Impact Factor: * 5.3 Acceptance Rate: 75.63%
Acceptance Rate: 75.63%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 
