Gastrointestinal Tuberculosis: An overview
Uma Debi MD*, Simran MD, Lokesh Singh MD, Sathya Sagar MD, Vishal Sharma MD, DM, Vikas Bhatia MD,DNB,DM, Muniraju Maralakunte MD, Anindita Sinha MD, Gita Devi MD, Kaushal Kishor Prasad MD
Department of Radio diagnosis, PGIMER Chandigarh, India
*Corresponding Author: Uma Debi MD, Department of Radio diagnosis, PGIMER Chandigarh, India
Received: 16 June 2020; Accepted: 20 July 2020; Published: 10 September 2020
Article Information
Citation: Uma Debi, Simran, Lokesh Singh, Sathya Sagar, Vishal Sharma, Vikas Bhatia, Muniraju Maralakunte, Anindita Sinha, Gita Devi, Kaushal Kishor Prasad. Gastrointestinal Tuberculosis: An overview. Archives of Clinical and Medical Case Reports 4 (2020): 820-835.
View / Download Pdf Share at FacebookAbstract
Tuberculosis has been one of the most common communicable diseases not only in India, but also world over. Abdomen is the most common extra-pulmonary site of the disease. It has been standard practice in the Indian subcontinent to consider tuberculosis as one of the differential diagnosis in most clinical scenarios, due to its high incidence and non-specificity of symptoms. It is imperative to be competent with a disease so prevalent. Radiological diagnosis goes a long way in proving or disproving a clinical diagnosis. One must always keep tuberculosis as a possible diagnosis and be familiar with various presentations of the disease.
Keywords
Tuberculosis; Gastrointestinal; CT; Lymphadenopathy; Extra pulmonary; Barium meal
Tuberculosis articles, Gastrointestinal articles, CT articles, Lymphadenopathy articles, Extra pulmonary articles, Barium meal articles
Tuberculosis articles Tuberculosis Research articles Tuberculosis review articles Tuberculosis PubMed articles Tuberculosis PubMed Central articles Tuberculosis 2023 articles Tuberculosis 2024 articles Tuberculosis Scopus articles Tuberculosis impact factor journals Tuberculosis Scopus journals Tuberculosis PubMed journals Tuberculosis medical journals Tuberculosis free journals Tuberculosis best journals Tuberculosis top journals Tuberculosis free medical journals Tuberculosis famous journals Tuberculosis Google Scholar indexed journals Gastrointestinal articles Gastrointestinal Research articles Gastrointestinal review articles Gastrointestinal PubMed articles Gastrointestinal PubMed Central articles Gastrointestinal 2023 articles Gastrointestinal 2024 articles Gastrointestinal Scopus articles Gastrointestinal impact factor journals Gastrointestinal Scopus journals Gastrointestinal PubMed journals Gastrointestinal medical journals Gastrointestinal free journals Gastrointestinal best journals Gastrointestinal top journals Gastrointestinal free medical journals Gastrointestinal famous journals Gastrointestinal Google Scholar indexed journals CT articles CT Research articles CT review articles CT PubMed articles CT PubMed Central articles CT 2023 articles CT 2024 articles CT Scopus articles CT impact factor journals CT Scopus journals CT PubMed journals CT medical journals CT free journals CT best journals CT top journals CT free medical journals CT famous journals CT Google Scholar indexed journals Lymphadenopathy articles Lymphadenopathy Research articles Lymphadenopathy review articles Lymphadenopathy PubMed articles Lymphadenopathy PubMed Central articles Lymphadenopathy 2023 articles Lymphadenopathy 2024 articles Lymphadenopathy Scopus articles Lymphadenopathy impact factor journals Lymphadenopathy Scopus journals Lymphadenopathy PubMed journals Lymphadenopathy medical journals Lymphadenopathy free journals Lymphadenopathy best journals Lymphadenopathy top journals Lymphadenopathy free medical journals Lymphadenopathy famous journals Lymphadenopathy Google Scholar indexed journals Extra pulmonary articles Extra pulmonary Research articles Extra pulmonary review articles Extra pulmonary PubMed articles Extra pulmonary PubMed Central articles Extra pulmonary 2023 articles Extra pulmonary 2024 articles Extra pulmonary Scopus articles Extra pulmonary impact factor journals Extra pulmonary Scopus journals Extra pulmonary PubMed journals Extra pulmonary medical journals Extra pulmonary free journals Extra pulmonary best journals Extra pulmonary top journals Extra pulmonary free medical journals Extra pulmonary famous journals Extra pulmonary Google Scholar indexed journals treatment articles treatment Research articles treatment review articles treatment PubMed articles treatment PubMed Central articles treatment 2023 articles treatment 2024 articles treatment Scopus articles treatment impact factor journals treatment Scopus journals treatment PubMed journals treatment medical journals treatment free journals treatment best journals treatment top journals treatment free medical journals treatment famous journals treatment Google Scholar indexed journals Barium meal articles Barium meal Research articles Barium meal review articles Barium meal PubMed articles Barium meal PubMed Central articles Barium meal 2023 articles Barium meal 2024 articles Barium meal Scopus articles Barium meal impact factor journals Barium meal Scopus journals Barium meal PubMed journals Barium meal medical journals Barium meal free journals Barium meal best journals Barium meal top journals Barium meal free medical journals Barium meal famous journals Barium meal Google Scholar indexed journals surgery articles surgery Research articles surgery review articles surgery PubMed articles surgery PubMed Central articles surgery 2023 articles surgery 2024 articles surgery Scopus articles surgery impact factor journals surgery Scopus journals surgery PubMed journals surgery medical journals surgery free journals surgery best journals surgery top journals surgery free medical journals surgery famous journals surgery Google Scholar indexed journals radiograph articles radiograph Research articles radiograph review articles radiograph PubMed articles radiograph PubMed Central articles radiograph 2023 articles radiograph 2024 articles radiograph Scopus articles radiograph impact factor journals radiograph Scopus journals radiograph PubMed journals radiograph medical journals radiograph free journals radiograph best journals radiograph top journals radiograph free medical journals radiograph famous journals radiograph Google Scholar indexed journals Electrocardiography articles Electrocardiography Research articles Electrocardiography review articles Electrocardiography PubMed articles Electrocardiography PubMed Central articles Electrocardiography 2023 articles Electrocardiography 2024 articles Electrocardiography Scopus articles Electrocardiography impact factor journals Electrocardiography Scopus journals Electrocardiography PubMed journals Electrocardiography medical journals Electrocardiography free journals Electrocardiography best journals Electrocardiography top journals Electrocardiography free medical journals Electrocardiography famous journals Electrocardiography Google Scholar indexed journals
Article Details
1. Introduction
In the latter half of 19th century, Robert Koch, credited with identifying mycobacterium as the causative factor for tuberculosis, pointed out that 1/7th of humanity died of this disease [1]. Things have improved remarkably over the following 130 years, but tuberculosis continues to haunt mankind with its penchant for bringing misery to its bearer along with pervasiveness seldom seen spread over such a long period in the history of humanity. Tuberculosis (TB) has been one of the most common communicable diseases not only in India, but also the rest of the world. In 2013 itself, nearly 9.0 million people developed the disease worldwide with nearly 1/6th succumbing to the disease. India accounted for 24% of the total number of cases with an expenditure of more than 250 million USD on the various national programs in 2013 [2]. Forty percent of the Indian population is estimated to harbor the tuberculosis bacillus according to RNTCP, with an annual risk of infection being 1.5%. In India, the burden of the disease can be identified by the fact that, 17.6% deaths from communicable diseases were attributable to tuberculosis alone. The estimated incidence of TB in India is 27 lakh [3]. Thus, the burden of the disease can be fathomed in terms of loss of human life as well as the economical strain it causes on an already overburdened economy.
The abdomen is the most common extra-pulmonary site of the disease and the disease has often been classified depending upon the organ(s) being involved, into mainly tubercular lymphadenopathy, peritoneal tuberculosis, gastrointestinal (GI) tuberculosis and other visceral tuberculosis [4, 5]. It has been standard practice in the Indian subcontinent to consider tuberculosis as one of the differential diagnosis in most clinical scenarios, due to its high incidence, as well as the relative non-specificity of symptoms [5].
Gastrointestinal tuberculosis (GITB) is being classified depending upon the site being involved. The most common site of gastrointestinal tuberculosis is the ileum and ileo-cecal junction [4]. Presentation of the disease is as varied as the sites it involves, the list of differential diagnosis as diverse and options for investigation, manifold with each having its own limitations. Thus, it is imperative to be competent with a disease so prevalent, yet deceptive.
2. Esophageal TB
Tuberculosis involving the esophagus is rare, with esophagus being the least common site involved in the GI Tract having an incidence of 0.14% [6]. An Upright posture, stratified squamous epithelium, along with coordinated peristalsis, mucus, saliva and an intact lower esophageal sphincter have been several reasons for infrequency of esophagus amongst sites for GITB [7]. Primary tuberculosis of the esophagus is a rare entity caused by the ingestion of infected sputum. Most of the patients have an underlying defect in the mucosa like Barret’s esophagus, mucosal tear, strictures or carcinoma. It is highly doubtful that normal esophagus is infected by the ingestion of infected sputum [8]. Tubercular involvement in esophagus is generally secondary as a result of contiguous spread from the mediastinal lymph node or carious lesions in the spine. Lymphatic and hematogenous spread from a primary site is also seen. The most common site of involvement is the middle one third near the bifurcation of trachea because of the close proximity to the mediastinal lymph nodes [9] (Figure 1) The lymph nodes can cause mass effect and compression of the esophagus with lymphadenitis resulting in diverticula formation causing distortion and tenting of the esophagus. The caseous mediastinal lymph nodes can erode into the lumen of the esophagus resulting in fistula formation (Figure 2).
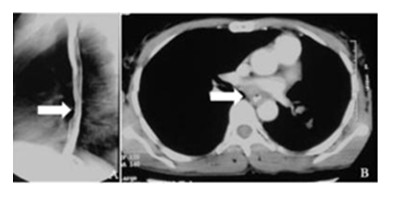
Figure 1: A. Barium swallow lateral spot showing reduced distensibility in the mid-esophagus with smooth luminal narrowing. B. The corresponding CECT image shows asymmetric circumferential mural thickening involving the esophagus. Endoscopic biopsy taken from the thickening was positive for acid fast bacilli.
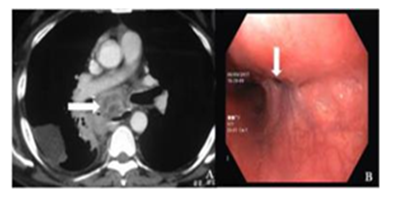
Figure 2: A. CECT showing multiple necrotic lymph nodes in the mediastinum in the peri-esophageal location. Few lymph nodes are also seen in the right hila. A patch of consolidation is seen in right lower lobe. Endoscopy in the same patient showed multiple esophageal fistulae.
The most frequent symptom is dysphagia seen in 90 % of cases followed by odynophagia and restrosternal pain [7, 10]. Constitutional symptoms like fever, malaise, weight loss often accompanies these symptoms. Post prandial coughing in a known patient of pulmonary tuberculosis should be evaluated with a high index of suspicion for the formation of a trachea-esopahgeal or broncho-esophageal fistula as a result of rupture of the necrotic mediastinal lymph nodes. There can be rare presentation such as hematemesis from an esophageal ulcer or the dreadful complication of aorto-esopahgeal fistula resulting in catastrophic haemorrhage [11]. The diagnosis of esophageal TB is difficult and requires a very high index of suspicion especially in a population where tuberculosis is endemic. Esophageal TB should be considered in known patients of pulmonary or systemic tuberculosis who develop dysphagia or odynophagia [6].
Esophageal tuberculosis usually occurs in three forms; ulcerative, hypertrophic and granular [12]. Barium swallow depicts external indentation, kinking, mucosal ulcerations, strictures, traction diverticulae, trachea-esophageal or broncho-esophageal fistulae and pseudomasses [13]. Chest CT will demonstrate presence of bulky necrotic mediastinal lymphadenopathy along with pulmonary findings of tuberculosis. CT is helpful in depiction of various complications like trachea –esophageal or broncho-esophageal fistulae. Endoscopy in the current investigation of choice as it allows a tissue diagnosis. The tubercular granulomas are located deep in the submucosal layer so multiple and deep biopsies should be obtained for optimum diagnosis [14]. The endoscopic findings are often non-specific and include ulceration with or without undermined edges, carcinoma like pseudo masses, openings of sinuses or fistulae. The definitive diagnosis rests on the demonstration of acid-fast bacilli in the biopsy material or the presence of typical caseating epitheloid granulomas. Esophageal TB should be considered as a differential diagnosis of an ulcerophypertrophic mass showing non-specific acute on chronic inflammation on repeated biopsies [15].
Findings on endoscopic ultrasound are not specific and depict the presence of heterogenous or homogenous masses in the esophageal wall, mediastinal lymphadenitis, interruption of the adventitia, and incrassation [16]. Fine needle aspiration samples can be obtained from the periesophageal mediastinal lymph nodes using endoscopic ultrasound. Patients show excellent response to the standard antitubercular treatment with complete resolution of the disease. Even in patients with complications like fistulae and sinus formation, medical treatment is all that is required. Surgery is indicated for patients who do not respond to anti-tubercular treatment and in multidrug resistant tuberculosis.
3. Stomach TB
Tuberculosis affecting the stomach is a rare entity even in a country like India where the disease is so rampant; comprising 0.2 to 1% of patients with GITB [17]. The acidic environment, scarcity of lymphoid follicles, active gastric motility and the mucosa act as protective factors. Stomach is generally involved by the ingestion of infected sputum in patients of pulmonary tuberculosis. Primary tuberculosis of the stomach without involving any other organ in the body is rare. Lymphatic and hematogenous routes are other modes of spread. Gastric TB has non-specific symptoms and is a great mimicker. It can mimic peptic ulcer disease, malignancy, gastric outlet obstruction, pyrexia of unknown origin and rarely stomach perforation [18]. Therefore, its early diagnosis is a big challenge in order to reduce morbidity. There are six types of gastric tuberculosis pathologically – 1. Tubercular ulcers 2. Miliary tubercles 3. Hypertrophic 4. Tuberculous pyloric stenosis 5. Solitary tuberculoma 6. Tubercular lymphadenitis [19].
Tubercular ulcers are the most common type mainly affecting the lesser curvature and the pylorus (Figure 3) [20]. These ulcers can be irregular with or without undermined edges. Deep ulcerations can extend into the surrounding tissues giving rise to adhesions and fistula formation. Healing of the ulcers occurs with marked fibrosis and scarring resulting in narrowing of the pylorus, presenting as gastric outlet obstruction. Tubercle formation in the submucosa produces elevated polypoidal mass like lesions which later shows ulceration of the overlying mucosa as a result of obliterative endarteritis, thus mimicking a carcinoma. There can also be diffuse infiltration of the submucosa similar to linitis plastica.
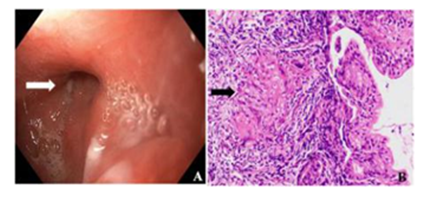
Figure 3: Endoscopy showing distorted pylorus with ulceration in antrum. Non-caseating granuloma [black arrow] seen in a patient with granulomatous gastritis (H&E X 200)-gastrointestinal tuberculosis.
There are no specific radiographic findings which favour a diagnosis of tuberculosis over malignancy. However, tuberculosis should be suspected in patients with a non-healing peptic ulcer disease in the pyloroduodenal segment despite adequate medication; especially in an endemic population [19]. Tuberculosis should also be kept in the differential diagnosis of an atypical ulcer either benign or malignant in young patients [19]. The differential diagnosis includes carcinoma, peptic ulcer, crohn’s disease, lymphoma, infections like syphilis, and exposure to beryllium and silicates [21].
Definitive diagnosis requires the demonstration of acid-fast bacilli or typical caseating epithelioid granulomas on biopsy. Staining for acid fast bacilli is usually negative and diagnosis is established by culture or PCR. PCR of the biopsy specimen is a faster means of diagnosis having a specificity of 100 % and sensitivity of 27-75% [22]. The rapid clinical response to ATT also supports the diagnosis. A standard course of anti-tubercular treatment is sufficient to cure most of the patients with surgery being indicated only in cases of complications.
4. Duodenal TB
Duodenal involvement by tuberculosis is again a rare entity constituting only 2% of all GITB [23]. The presenting features can be divided into two groups. The first group constitutes patients presenting with dyspeptic symptoms like epigastric pain, discomfort and nausea. The second group consists of the obstructive symptoms like frequently vomiting after meals and abdominal pain [24]. Pain (56.5%) and vomiting (60.8%) are common symptoms of duodenal TB which may be associated with fever, weight loss and a palpable epigastric mass (33%) [25]. The third part of duodenum is the most common site of involvement. The duodenal involvement can be intrinsic or extrinsic or both [24]. The extrinsic form consists of bulky lymphadenopathy in the C loop of duodenum resulting in extrinsic compression and symptoms of obstruction (Figure 4).
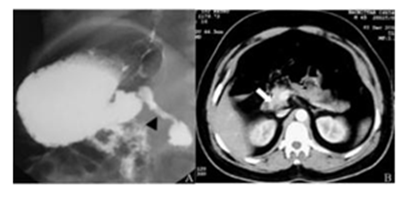
Figure 4: A. Barium meal lateral spot showing narrowing involving the second part of duodenum with mucosal irregularity and ulceration, in a proven case of tuberculosis. There is evidence of gastric outlet obstruction with delayed emptying. B. The corresponding Contrast Enhanced CT (CECT) abdomen shows mural thickening involving D2 of duodenum with luminal compromise.
The intrinsic from can be ulcerative, hypertrophic or ulcero-hypertrophic and mimics peptic ulcers or malignancy. It can further be complicated by the formation of sinuses or fistulae. There can be fistula formation between the duodenum and the bile duct or the renal pelvis [26, 27]. Intrinsic strictures can also from as a result of extensive fibrosis during the healing stage. A rare presentation includes segmental portal hypertension with bleeding gastric varices as a result of obstruction of the splenic vein by the perihilar lymph nodes [25]. Massive haemorrhage from an aorto-duodenal fistula has also been reported [28].
Chest X ray shows e/o pulmonary tuberculosis in 20 % of cases [29]. Barium meal findings are non-specific and constitutes extrinsic compression, luminal narrowing, mucosal irregularities, ulcerations, sinuses and fistula formation. However, it helps to delineate the mucosal lesions and determine their site and extent. Computed tomography depicts mural thickening, luminal narrowing adjoining lymphadenopathy. The differential diagnoses include peptic ulcer disease, malignancy, carcinoma head of pancreas and lymphoma. Around 10 % of gastric tuberculosis is associated with duodenal tuberculosis. The simultaneous involvement of the pylorus and the duodenum is seen in many cases [19].
The management of duodenal tuberculosis is primarily medical. Even in patients with strictures, balloon dilatation along with the standard ATT suffices [30]. Surgery is indicated in patients with complications like obstruction, bleeding or fistula formation.
5. Ileocaecal TB
Ileocecal junction is the most common site involved by tuberculosis in the gastrointestinal tract, involved in approximately 64% cases of GITB [31]. This high propensity to be involved can be explained by the physiological stasis that takes place at the ileocecal valve, the abundance of lymphoid tissue, increased absorption of fluid and electrolytes, minimal digestive activity and closer contact of the bacilli with the mucosa [32]. The clinical presentation can be protean consisting of various symptoms. The patients present with abdominal pain, borborygmi, altered bowel habits and episodes of diarrhoea alternating with constipation. Constitutional symptoms like fever, evening rise of temperature, weight loss, loss of appetite and malaise are also seen. The patients can also present as an acute abdomen as a result of perforation peritonitis. TB is the second commonest cause of perforation after typhoid in India responsible for 5%-9% of all small bowel perforations [31]. Acute and subacute intestinal obstruction can occur as a result of stricture formation, mural thickening and adhesions [33].
The initial pathological process consists of formation of epitheloid tubercles in the submucosa. In about 2 to 4 weeks, these tubercles coalesce with one another and there occurs ulcerations of the overlying mucosa. The ulcers are shallow and are transversely oriented at right angles to the long axis of the bowel in the direction of lymphoid follicles. They rarely extend beyond the muscularis layer. The bacilli then gain access to the lymphatic system and reach the regional lymph nodes resulting in their enlargement. Mesenteric lymphadenopathy is almost always seen with the intestinal pathology. Obliterative endarteritis occurs resulting in ischemia which is responsible for stricture formation. Obliterative endarteritis is also the reason that massive bleeding is rarely seen in association with GITB. Later in the course of the disease, healing of these ulcers result in short segment annular strictures.
There are three morphological types of intestinal tuberculosis, namely, ulcerative, hypertrophic and ulcero-hypertrophic. There is no clear-cut separation between them and they often overlap. There are four criteria for the diagnosis of TB. These are clinical manifestations of TB, imaging findings suggestive of abdominal TB, histopathological or microbiological evidence of TB and/or therapeutic response to treatment [34]. Radiological investigations mainly include abdominal radiograph, Barium meal follow through, Barium enema, Ultrasound and Computed Tomography. Abdominal X-ray (erect and supine) demonstrates the presence of enteroliths, small bowel obstruction characterised by multiple dilated bowel loops in the centre of the abdomen and multiple air fluid levels. It can also depict hepatosplenomegaly, calcified mesenteric lymphadenopathy and calcified granulomas. A concurrent chest radiograph may depict findings suggestive of old or active tubercular infection in 22 – 80 % of cases [35, 36].
Findings on Barium meal follow through which are highly suggestive of tuberculosis are deformed ileocaecal valve with dilated ileum, contracted caecum with abnormal ileocaecal valve or terminal ileum, or stricture of ascending colon with shortening or involvement of ileocaecal region. Findings suggestive of TB consists of contracted caecum, ulceration or narrowing of terminal ileum, stricture of ascending colon or multiple sites of narrowing and dilatation (Figure 5).
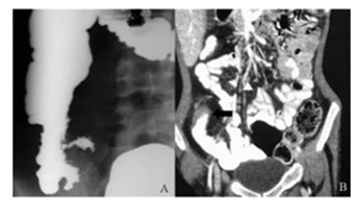
Figure 5: (A) Barium meal follow through image show reduced reduce distensibility of IC junction, adjacent terminal ileum,caecum and ascending colon with multiple ulcerations; (B) CECT abdomen coronal view show circumferential mural thickening of terminal ileum (black arrow) with increased locoregional vascularity and sub centimetric lymphadenopathy in ileocolic mesentry (arrowhead).
Non-specific changes include features of adhesions, dilatation and mucosal thickening of small bowel loops [37]. Barium meal follow through can depict accelerated intestinal transit and hypersegmentation of barium column (chicken intestine). Flocculation, precipitation and dilution of barium can be seen. Hour glass stenosis having smooth but stiff contours with multiple small segment strictures can also be seen (Figure 6). The bowel loops appear fixed and matted and can also appear widely separated as a result of enlarged lymph nodes.
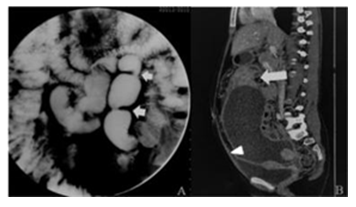
Figure 6: A. Barium meal follow through shows multiple short segment strictures involving the ileum with dilatation and beading of the loops. B. The corresponding CECT sagittal reformation shows circumferential mural thickening involving the small bowel loops. Loculated ascites is seen causing superior displacement of the bowel loops. Peritoneal thickening and enhancement is also noted.
Various signs of ilecaecal tuberculosis have been described on barium enema [38]. Thickening of the lips of ileocaecal valve with wide gaping can be seen. This along with narrowed terminal ileum produces the inverted umbrella or Fleischner sign. The terminal ileum enters into the caecum normally at right angles. In TB the normal ilecaecal angle is lost with the dilated terminal ileum appearing suspended from a pulled up and contracted caecum giving rise to Gooseneck deformity (Figure 7). The caecum is shrunken, and is distorted resulting in a conical caecum which is pulled out of the iliac fossa as a result of fibrosis. Stierlin’s sign is a manifestation of acute inflammation superimposed on a chronically involved segment of bowel. The inflamed segments of ileum, caecum and ascending colon are characterised by lack of barium column with a normal configured column of barium on either side. It appears as narrowing of the terminal ileum with rapid emptying into a shortened, obliterated and rigid caecum. String sign denotes a persistent narrow stream of barium indicating stenosis. Localised stenosis opposite ileocaecal valve with a smooth rounded off caecum and dilated terminal ileum results in purse string stenosis.
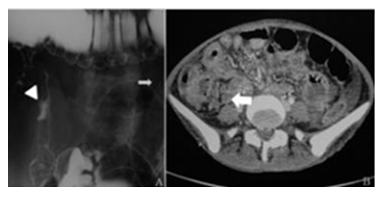
Figure 7: A. Double contrast barium meal follow through showing wide gaping of the ileocaecal valve. The caecum is contracted and pulled up into the right hypochondrium. This produces the classical “goose neck” deformity. B. Corresponding CT image showing diffuse circumferential mural thickening involving the terminal ileal loops. Multiple enlarged necrotic lymph nodes are seen in the adjacent ileocolic mesentery.
Ultrasound depicts bowel wall thickening which is symmetrical and concentric in the region of the ileocaecal region. There can be exudation of fluid from the surface of inflamed bowel loops resulting in “Club Sandwich” or “Sliced Bread” sign as a result of localisation of fluid between radially oriented bowel loops. There can be presence of free or loculated ascites which can be clear or complex with echogenic debris and septae. The thickened ileocaecal region is pulled into a subhepatic location resulting in pseudokidney sign. Often there are multiple enlarged lymph nodes in the mesentry which may be discrete or conglomerate to form large lymph nodal masses. Caseation and calcification within the nodes are highly suggestive of tuberculosis. Omental and peritoneal thickening can also be seen on ultrasound.
CT demonstrates slight and symmetrical thickening of the terminal ileum and caecum in early disease. As the disease progresses there is asymmetrical thickening of the ileocaecal valve and the medial wall of the caecum. Thickening of the bowel loops with adherence of multiple loops combined with lymphadenopathy and mesenteric thickening results in formation of an abdominal lump. Multiple short segment strictures can also be seen in ileum and jejunal loops (Figure 8).
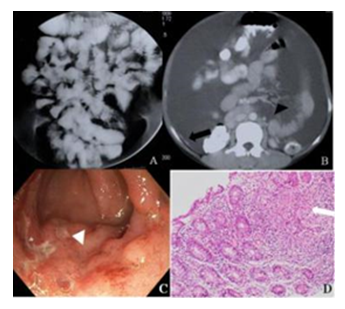
Figure 8: A. Barium meal follow through showing clumping of bowel loops in the central abdomen. Multiple short segment strictures are seen in the visualized small bowel loops. Loss of mucosal architecture is seen in the jejunal loops B. CECT abdomen shows peritoneal involvement with ascites, peritoneal thickening, mesenteric and retroperitoneal lymphadenopathy. C and D. Endoscopy showed multiple ulcers in the ileum, HPE showed granuloma.
Complications like perforation, obstruction and abscess formation is well depicted. Multiple, enlarged discrete or conglomerated nodes with hypodense centres s/o necrosis and peripheral rim enhancement are seen in the mesentery. Other than mesenteric nodes, nodes at porta and peripancreatic region are also seen characteristically. Isolated retroperitoneal lymphadenopathy is less common as compared to lymphoma in which it is commonly seen [39]. Tubercular ascites has a high protein content and thus has high density on CT (25-45HU) than simple ascites. Omental thickening in the form of omental caking is seen. As a result of chronic long-standing inflammation, a thin fibrous wall develops around the omentum resulting in an omental line which is not seen in cases of malignancy [40]. The mesentery appears shaggy and misty with fluid in between the leaves of mesentery and has a stellate appearance.
Crohn’s disease is a strong differential diagnosis of ileocecal tuberculosis and is a cause of great diagnostic dilemma. Though there are no specific features differentiating the two, there are some findings which if present may favour the diagnosis of one over the other [41]. The ulcers in tuberculosis are shallow and superficial and does not cross the muscularis propria whereas in Crohn’s the ulcers penetrate deep resulting in fissures and fistulas which are more often seen in the latter. The tubercular ulcers are typically transversely oriented whereas in Crohn’s they are longitudinal or serpiginous. Anus is a common site of involvement in Crohn’s disease whereas it is rarely involved in tuberculosis. Generally tubercular strictures are short and segmental measuring <3cms in length whereas strictures in Crohn’s are usually long.
6. Tuberculosis of Colon and Rectum
Tuberculosis of the colon and rectum is not as common as the small bowel involvement. Only about 2-3% of patients with abdominal tuberculosis have isolated colonic tuberculosis [42]. In patients of gastro-intestinal tuberculosis, around 10.8%43 to 12% [44] have tuberculosis of the colon. However, the incidence has been on the rise with increasing incidence of HIV, chronic renal diseases, other immunosuppressive disorders and use of immunosuppressive medications [42]. The right side of the colon is more often affected by tuberculosis [45] with caecum and ascending colon being a common site [45, 46] (Figure 9). Caecum being an area of relative physiological stasis with little digestive activity and mainly water and electrolyte absorption make it a preferred site in colon for the tubercle bacilli to inhabit and invade the mucosa [45]. One study by Nagi et al. found the incidence higher in transverse colon, but that too marginally as compared to ascending colon [43].
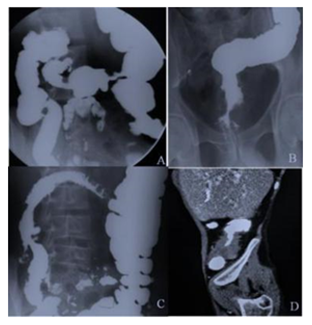
Figure 9: Spectrum of findings seen in tubercular involvement of the colon. A and C. Ileocaecal and transverse colon involvement with tuberculosis showing goose neck deformity, rounded caecum, strictures in the transverse colon. D. CECT image coronal reformation showing mural thickening involving the ascending colon. Malignancy and colitis are close differential diagnosis. B. Rectum and anal canal involvement with stricture and ulceration.
The disease presents with many symptoms and signs, which are mostly non-specific. The most common symptom being abdominal pain, which was seen in more than 80% patients in a study done by Mukewar et al [42, 46]. The nature of the pain may be colicky in case of intestinal obstruction or a dull ache, in case of associated peritoneal inflammation. The other common symptom includes weight loss, which may be due to increased catabolism, mal-absorption, loss of bile salts, anorexia, and/or post-prandial discomfort [45]. Fever, altered bowel habits, bleeding per rectum, abdominal mass, and features of sub-acute or acute intestinal obstruction [46, 47] constitute other common clinical features. Abdominal mass is the most common clinical finding [5] and may arise from thickened colon, lymph nose, mesentery or the omentum [45].
There are notably 2 forms of the disease. The Ulcerative form most often seen in immunocompromised patients and the Hyperplastic form seen in those with a competent immune system [47]. There are various diseases, which are considered in the differential diagnosis of tubercular disease of the colon. The most notable of these are malignancy and Crohn’s disease. The other pathologies, which may bring about a dubiety in diagnosis, include Amoeboma, peri-appendicular abscess, colonic lymphoma, and Ulcerative colitis [45]. Tuberculosis may mimic carcinoma in up to 20% patients [42]. A strong suspicion and histopathological evaluation help in the correct diagnosis.
Crohn’s disease is notorious as a masquerader of tuberculosis making the diagnosis of either difficult at times [42, 44, 45]. Segmental involvement of colon is seen is about 26% cases, making it difficult to differentiate from Crohn’s disease [45, 47]. The various colonoscopic features of tuberculosis include ulcers, nodules, ileocecal valve deformity, polyps, strictures, fibrous bands and erythema [42, 44, 45, 46]. These still however do not categorically help in differentiating it from Crohn’s disease with marked overlapping of these features. In a study by Nagi et al. [43], on imaging, 54% patients were found to have strictures, 34% colitis, and another 7% showed polypoidal lesions. On histopathology, there may be certain differences on the granulomas formed in tuberculosis and Crohn’s disease, but the sensitivity and specificity are not high enough to make a comprehensive diagnosis of either [44, 45]. Other tests, which may be employed, include PCR for M. tuberculosis DNA on endoscopic biopsy specimen (Specificity: 100%, Sensitivity: 26.5 – 70%), ELISA for antibodies against mycobacterial antigens (Specificity: 88%, Sensitivity: 81%), and Anti-cord antibodies (Specificity: 95%, Sensitivity: 85%) [44]. Thus, the differentiation between Crohn’s and tuberculosis is challenging and either differential must be kept in mind while evaluating a patient.
Treatment mainly includes anti-tubercular therapy, which resolves most of the lesions. Surgery however is occasionally required in case of intestinal obstruction, perforation, and fistula [45].
7. Anal tuberculosis
Anal region is a rare site of involvement in the gastrointestinal tract, constituting less than 1% of cases involving the intestines. It occurs more commonly in men (4:1) and mostly in the fourth decade of life. The disease occurs usually concomitant to a pulmonary disease, which may or may not be apparent always [48, 49]. The usual modes of spread include swallowing of bacilli laden sputum; hematogenous spread; lymphatic spread from regional lymph nodes; and direct extension from neighboring areas. Primary lesions are rare [49].
The disease has been classified as ulcerative, verrucous, lupoid and miliary. Anal fistula is also one of the manifestations of anal tuberculosis [50]. However, there is no particular age or site predilection to differentiate it from cryptoglandular fistula. Ulcerative type is the most common of all, typically presenting as superficial ulceration with a hemorrhagic necrotic base, being covered with mucus. Anal fissure may also be a manifestation of the ulcerative type, usually suggested by non-healing lesions which may be multiple and may even be associated with inguinal lymphadenopathy. The verrucous form presents as warty lesions and has an association with M. bovis. Lupoid form is associated with tuberculosis elsewhere in the body, which begins as a reddish nodule, which gradually ulcerates in its Centre. The miliary form is associated with widespread or disseminated tuberculosis [49, 51].
The list of differential diagnosis is extensive, but Crohn’s disease deserves special mention as it may be very difficult to differentiate between the two [48, 49, 50, 51, 52].
|
Infective |
Veneral lesions, amoebiasis, syphilis, herpes simplex, deep mycosis, LGV, anorectal abscess, hidradenitis suppurativa, actinomycosis |
|
Neoplasm |
Benign warts, malignancy |
|
Auto-immune |
Crohn’s disease, Ulcerative colitis, Sarcoidosis |
|
Miscellaneous |
Foreign body lesions, Granuloma pyogenicum, thrombosed hemorrhoids |
Table 1 – Differential diagnosis of anal tuberculosis
For diagnosis, the best detection rates were found by PCR (79.4%), followed by histopathology (73.5%), culture (29–47%) and smear examination (5.8%) [51]. Tuberculin skin test remains positive in about 75% patient [48].
The mainstay of treatment is anti-tubercular therapy, with the need for surgery restricted to obstruction, stenosis, non-healing fistulas and abscess [48, 49].
8. Conclusion
Tuberculosis is a disease with its reaches in every nook and corner of the body. Its ability to mask its presence by the various non-specific symptoms and the long list of differential diagnosis, make it difficult to diagnose the disease. Hence it is important to have a high index of suspicion and appreciate subtle signs and features, which are outlined above. Radiological diagnosis goes a long way in proving or disproving a clinical diagnosis and thus one must always keep tuberculosis as a possible diagnosis and be familiar with the various presentations of the disease.
References
- Robert Koch. Die Atiologie der Tuberculose. Facsimile of the original contribution by Robert Koch in "Berliner Klinische Wochenschrift". Fortschr Med 100 (1982): 539-550.
- World Health Organization. Global tuberculosis report. (Available from: URL: https://www.who.int/tb/publications/global_report/en/) (2019).
- India TB Report 2019. (Available from: https://tbcindia.gov.in/WriteReadData/India%20TB%20Report%202019.pdf).
- Kapoor VK. Abdominal tuberculosis: the Indian contribution. Indian J Gastroenterol 17 (1998): 141-147.
- Debi U, Ravisankar V, Prasad KK, et al. Abdominal tuberculosis of the gastrointestinal tract: revisited. World J Gastroenterol 20 (2014): 14831-14840.
- Sood A, Sood N, Kumar R, et al. Primary tuberculosis of esophagus. Indian J Gastroenterol 15 (1996): 75-88.
- Jain SK, Jain S, Jain M, et al. Esophageal tuberculosis: is it so rare? Report of 12 cases and review of the literature. Am J Gastroenterol 97 (2002): 287-291.
- Khanna V, Kumar A, Alexander N, et al. A case report on esophageal tuberculosis - A rare entity. Int J Surg Case Rep 35 (2017): 41-43.
- Gordon AH, Marshall JB. Esophageal tuberculosis; Definitive diagnosis by endoscopy. Am J Gastroenterol 85 (1990): 174-177.
- Mokoena T, Shama DM, Ngakane H, et al. Oesophageal tuberculosis: a review of eleven cases. Postgrad Med J 68 (1992): 110-115.
- Iwamoto I, Tomita Y, Takasaki M, et al. Esophagoaortic fistula caused by esophageal tuberculosis: report of a case. Surg Today 25 (1995): 381-384.
- Famming AR, Guindi R, Farid A. Tuberculosis of the esophagus. Thorax 24 (1969): 254-256.
- Nagi B, Lal A, Kochhar R, et al. Imaging of esophageal tuberculosis: a review of 23 cases. Acta Radiol 44 (2003): 329-333.
- Welzel TM, Kawan T, Bohle W, et al. An unusual cause of dysphagia: esophageal tuberculosis. J Gastrointestin Liver Dis 19 (2010): 321-324.
- Fujiwara Y, Osugi H, Takada N, et al. Esophageal tuberculosis presenting with an appearance similar to that of carcinoma of the esophagus. J Gastroenterol 38 (2003): 477-481.
- Han XM, Yang JM, Xu LH, et al. Endoscopic ultrasonography in esophageal tuberculosis. Endoscopy 40 (2008): 701-702.
- Godara SC, George RA, Uniyal M. Case report: Gastric tuberculosis - rare manifestation. Indian J Radiol Imaging 14 (2004): 55-56.
- Gupta B, Mathew S, Bhalla S. Pyloric obstruction due to gastric tuberculosis: an endoscopic diagnosis. Postgrad Med J 66 (1990): 63-65.
- Pimentel AM, Rocha R, Santana GO. Crohn’s disease of esophagus, stomach and duodenum. World J Gastrointest Pharmacol Ther 10 (2019): 35-49.
- Chetri K, Prasad KK, Jain M, et al. Gastric tuberculosis presenting as non-healing ulcer: case report. Trop Gastroenterol 21 (2000): 180-181.
- Amarapurkar DN, Patel ND, Amarapurkar AD. Primary gastric tuberculosis—report of 5 cases. BMC Gastroenterology 3 (2003): 170-180.
- Moghadam G, Alborzi A, Pouladfar G, et al. Primary gastric tuberculosis mimicking gastric cancer: a case report. The Journal of Infection in Developing Countries 7 (2013): 355-357.
- Bhansali SK. Abdominal TB-experience with 300 cases. Am J Gastroenterol 67 (1977): 324-337.
- Bhatti A, Hussain M, Kumar D, et al. Duodenal tuberculosis. J Coll Physicians Surg Pak 22 (2012): 111-112.
- Rao YG, Pande GK, Sahni P, et al. Gastroduodenal tuberculosis management guidelines, based on a large experience and a review of the literature. Can J Surg 47 (2004): 364-368.
- Chaudhary A, Bhan A, Malik N, et al. Choledocho-duodenal fistula due to tuberculosis. Indian J Gastroenterol 8 (1989): 293-294.
- Rodney K, Maxted WC, Pahira JJ. Pyeloduodenal fistula. Urology 22 (1963): 536-539.
- Kodaira Y, Shibuya T, Matsumoto K, et al. Primary aortoduodenal fistula caused by duodenal tuberculosis without an abdominal aortic aneurysm: report of a case. Surg Today 27 (1997): 745-748.
- Mukherji B, Singhal AK. Intestinal tuberculosis. Proc Assoc Surg East Afr 2 (1968): 71-75.
- Vij JC, Ramesh GN, Choudhary V, et al. Endoscopic balloon dilation of tuberculous duodenal strictures. Gastrointest Endosc 38 (1992): 510-511.
- Sharma R. Abdominal Tuberculosis. Imaging Science Today 2009: 146. (Available from: URL: http://www.imagingsciencetoday.com/node/146.) (2009).
- Horvath KD, Whelan RL. Intestinal tuberculosis: return of an old disease. Am J Gastroenterol 93 (1998): 692-696 .
- Kentley J, Ooi JL, Potter J, Tiberi S, et al. Intestinal tuberculosis: a diagnostic challenge. Trop Med Int Health 22 (2017): 994-999.
- Lingenfelser T, Zak J, Marks IN, et al. Abdominal tuberculosis; still a potentially lethal disease. Am J Gastroenterol 88 (1993): 744-750.
- Sood R, Baba CS. Diagnosis of abdominal tuberculosis. Role of imaging. J Ind Acad Clin Med 2 (2001): 169-186.
- Kapoor VK, Chattopadhyay TK, Sharma LK. Radiology of abdominal tuberculosis. Austral Radiol 32 (1988): 365-374.
- Kumar N, Aggarwal R. Abdominal tuberculosis. In: API Textbook of Medicine, 7th ed. National Book Depot: Mumbai (2003): 562-570.
- Sharma MP, Bhatia V. Abdominal tuberculosis. Ind J Med Res 20 (2004): 305.
- Gulati MS, Sarma D, Paul SB. CT appearances in abdominal tuberculosis. A pictorial assay. Clin Imaging 23 (1999): 51-59.
- HK Ha, JI Jung, MS Lee, et al. CT differentiation of tuberculosis peritonitis and peritoneal carcinomatosis. Am J Roentgenol 167 (1996): 743-748.
- Tandon HD, Prakash A. Pathology of intestinal tuberculosis and its distinction from Crohn's disease. Gut 134 (1972): 260-269.
- Kumar A, Patodia M, Pandove P, et al. Colonic tuberculosis masquerading as colon cancer. J Surg Case Rep 5 (2012): 10-15.
- Nagi B, et al. Colorectal tuberculosis. European radiology138 (2003): 1907-1912.
- Lau CF, et al. A case of colonic tuberculosis mimicking Crohn's disease. Hong Kong Medical Journal4 (1998): 63-66.
- Shah S, et al. Colonoscopic study of 50 patients with colonic tuberculosis. Gut33 (1992): 347-351.
- Mukewar, Saurabh, et al. Colon tuberculosis: endoscopic features and prospective endoscopic follow-up after anti-tuberculosis treatment. Clinical and translational gastroenterology10 (2012): 24-36.
- Demetriou GA, Manojkumar SN, Navaratnam R. Concurrent caecal and transverse colonic tuberculosis masquerating synchronous colonic carcinoma. BMJ case reports(2013): 36-38.
- Ibn Majdoub Hassani, K, et al. Perianal Tuberculosis: A Case Report and a Review of the Literature. Case reports in infectious diseases(2012): 45-49.
- Soniya M. Anal tuberculosis: report of a case and review of literature. International Journal of Surgery6 (2008): 36-39.
- Sahu M, Mishra JK, Sharma A, et al. A prospective study on tubercular fistula in ano and its management. J Coloproctology 37 (2017): 211-215.
- Yaghoobi Reza, et al. Gastrointestinal tuberculosis with anal and perianal involvement misdiagnosed as Crohn's disease for 15 years. Acta dermato-venereologica3 (2011): 348-349.


 Impact Factor: * 5.31
Impact Factor: * 5.31 Acceptance Rate: 75.63%
Acceptance Rate: 75.63%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 
