Severe Ischemic Cardiogenic Shock: Biventricular Impella®, VA-ECMO and Extracorporeal LVAD as Bridge to a Permanent LVAD
Monika Sadlonova1,2,3 *, Birgit Gerecke1,4, Aschraf El-Essawi1, Lars-Olav Harnisch5, Onnen Moerer5, Konrad Meissner5, Claudius Jacobshagen3,4, Hassina Baraki1,3, Ingo Kutschka1,3
1Department of Thoracic and Cardiovascular Surgery, University of Göttingen Medical Center, Go?ttingen, Germany
2Department of Psychosomatic Medicine and Psychotherapy, University of Göttingen Medical Center, Go?ttingen, Germany
3German Center for Cardiovascular Research (DZHK), partner site Göttingen, Germany
4Department of Cardiology and Pneumology, University of Göttingen Medical Center, Go?ttingen, Germany
5Department of Anesthesiology, University of Göttingen Medical Center, Goottingen, Germany
*Corresponding Author: Monika Sadlonova, MD, Department of Thoracic and Cardiovascular Surgery, Georg-August-Universität Göttingen, Robert-Koch-Str. 40, 37075 Göttingen, Germany
Received: 22 December 2020; Accepted: 05 January 2021; Published: 26 January 2021
Article Information
Citation: Monika Sadlonova, Birgit Gerecke, Aschraf El-Essawi, Lars-Olav Harnisch, Onnen Moerer, Konrad Meissner, Claudius Jacobshagen, Hassina Baraki, Ingo Kutschka. Severe Ischemic Cardiogenic Shock: Biventricular Impella®, VA-ECMO and Extracorporeal LVAD as Bridge to a Permanent LVAD. Archives of Clinical and Medical Case Reports 5 (2021): 171-181.
View / Download Pdf Share at FacebookAbstract
Background: Cardiogenic shock (CS) is the clinical representation of circulatory failure with a state of critical end-organ hypoperfusion due to primary cardiac dysfunction. A severe cardiogenic shock can present with clinical complications such as arrhythmias, ischemia and organ failure and even today is associated with a high mortality of 40-50%. Extracorporeal life support (ECLS) by veno-arterial extracorporeal membrane oxygenation (VA-ECMO), Impella® and other mechanical circulatory support systems can reduce the acute circulatory failure and therefore constitute an important treatment option in the management of severe CS as a bridge to long-term strategies.
Case summary: We present the case of a 38-year-old woman with an acute heart failure due to a coronary artery disease who underwent emergency coronary artery bypass grafting and intraoperative implantation of a veno-arterial extracorporeal membrane oxygenation (VA-ECMO). Over the next 4 months, a multidisciplinary team-approach bridged the patient using first a left ventricular (LV) support system (Impella), then additionally a right ventricular (RV) Impella and finally a temporary paracorporeal continuous flow left ventricular support (Rotaflow). Following a promising neurological recovery, a long-term left ventricular assist device (LVAD) was implanted in a bride to transplant (BTT) concept.
Discussion: The addition of LV Impella and RV support by Impella (BiPELLA) on top of VA-ECMO may support survival of patients with refractory cardiogenic shock. In complex biventricular heart failure, an expert center must be able to provide an early multi-modular intervention with elaborated mechanical circulatory support due to a multidisciplinary expertise.
Keywords
Cardiogenic shock; VA-ECMO; Biventricular impella; LVAD; Case report
Abbreviations articles: AMI- acute myocardial infarction articles; BiPELLA- biventric articles
Cardiogenic shock articles Cardiogenic shock Research articles Cardiogenic shock review articles Cardiogenic shock PubMed articles Cardiogenic shock PubMed Central articles Cardiogenic shock 2023 articles Cardiogenic shock 2024 articles Cardiogenic shock Scopus articles Cardiogenic shock impact factor journals Cardiogenic shock Scopus journals Cardiogenic shock PubMed journals Cardiogenic shock medical journals Cardiogenic shock free journals Cardiogenic shock best journals Cardiogenic shock top journals Cardiogenic shock free medical journals Cardiogenic shock famous journals Cardiogenic shock Google Scholar indexed journals VA-ECMO articles VA-ECMO Research articles VA-ECMO review articles VA-ECMO PubMed articles VA-ECMO PubMed Central articles VA-ECMO 2023 articles VA-ECMO 2024 articles VA-ECMO Scopus articles VA-ECMO impact factor journals VA-ECMO Scopus journals VA-ECMO PubMed journals VA-ECMO medical journals VA-ECMO free journals VA-ECMO best journals VA-ECMO top journals VA-ECMO free medical journals VA-ECMO famous journals VA-ECMO Google Scholar indexed journals Biventricular impella articles Biventricular impella Research articles Biventricular impella review articles Biventricular impella PubMed articles Biventricular impella PubMed Central articles Biventricular impella 2023 articles Biventricular impella 2024 articles Biventricular impella Scopus articles Biventricular impella impact factor journals Biventricular impella Scopus journals Biventricular impella PubMed journals Biventricular impella medical journals Biventricular impella free journals Biventricular impella best journals Biventricular impella top journals Biventricular impella free medical journals Biventricular impella famous journals Biventricular impella Google Scholar indexed journals LVAD articles LVAD Research articles LVAD review articles LVAD PubMed articles LVAD PubMed Central articles LVAD 2023 articles LVAD 2024 articles LVAD Scopus articles LVAD impact factor journals LVAD Scopus journals LVAD PubMed journals LVAD medical journals LVAD free journals LVAD best journals LVAD top journals LVAD free medical journals LVAD famous journals LVAD Google Scholar indexed journals Case report articles Case report Research articles Case report review articles Case report PubMed articles Case report PubMed Central articles Case report 2023 articles Case report 2024 articles Case report Scopus articles Case report impact factor journals Case report Scopus journals Case report PubMed journals Case report medical journals Case report free journals Case report best journals Case report top journals Case report free medical journals Case report famous journals Case report Google Scholar indexed journals treatment articles treatment Research articles treatment review articles treatment PubMed articles treatment PubMed Central articles treatment 2023 articles treatment 2024 articles treatment Scopus articles treatment impact factor journals treatment Scopus journals treatment PubMed journals treatment medical journals treatment free journals treatment best journals treatment top journals treatment free medical journals treatment famous journals treatment Google Scholar indexed journals CT articles CT Research articles CT review articles CT PubMed articles CT PubMed Central articles CT 2023 articles CT 2024 articles CT Scopus articles CT impact factor journals CT Scopus journals CT PubMed journals CT medical journals CT free journals CT best journals CT top journals CT free medical journals CT famous journals CT Google Scholar indexed journals surgery articles surgery Research articles surgery review articles surgery PubMed articles surgery PubMed Central articles surgery 2023 articles surgery 2024 articles surgery Scopus articles surgery impact factor journals surgery Scopus journals surgery PubMed journals surgery medical journals surgery free journals surgery best journals surgery top journals surgery free medical journals surgery famous journals surgery Google Scholar indexed journals Pathogenesis articles Pathogenesis Research articles Pathogenesis review articles Pathogenesis PubMed articles Pathogenesis PubMed Central articles Pathogenesis 2023 articles Pathogenesis 2024 articles Pathogenesis Scopus articles Pathogenesis impact factor journals Pathogenesis Scopus journals Pathogenesis PubMed journals Pathogenesis medical journals Pathogenesis free journals Pathogenesis best journals Pathogenesis top journals Pathogenesis free medical journals Pathogenesis famous journals Pathogenesis Google Scholar indexed journals BiPELLA articles BiPELLA Research articles BiPELLA review articles BiPELLA PubMed articles BiPELLA PubMed Central articles BiPELLA 2023 articles BiPELLA 2024 articles BiPELLA Scopus articles BiPELLA impact factor journals BiPELLA Scopus journals BiPELLA PubMed journals BiPELLA medical journals BiPELLA free journals BiPELLA best journals BiPELLA top journals BiPELLA free medical journals BiPELLA famous journals BiPELLA Google Scholar indexed journals
Article Details
Abbreviations:
AMI- acute myocardial infarction; BiPELLA- biventricular Impella; BTB- bridge-to-bridge; BTD- bridge to decision; BTR- bridge to recovery; BTT- bridge to transplantation; CRRT- continuous renal replacement therapy; CS- cardiogenic shock; CT- computer tomography; ECMO- extracorporeal membrane oxygenation; ECLS- Extracorporeal life support; ´ECPELLA´ or ´ECMELLA´- Impella® device to a veno-arterial extracorporeal membrane oxygenation; IABP- intra-aortal balloon pump; ICD- implantable cardioverter-defibrillator; LV- left ventricular; PROPELLA- prolonged Impella; Lp- lipoprotein; LVAD- left ventricular assist device; MCS- mechanical circulatory support; RV- right ventricular; VA-ECMO- veno-arterial extracorporeal membrane oxygenation
1. Introduction
Cardiogenic shock (CS) is the clinical presentation of circulatory failure with multiorgan dysfunction complicated by hemodynamic, cellular and metabolic abnormalities [1, 2]. The most frequent cause of CS is acute myocardial infarction (AMI) accounting for 80% of cases in registries [2]. In the treatment of severe CS temporary mechanical circulatory support such as ECMO or Impella® can be used as a bridge to recovery (BTR), as a bridge to an implantation of a left ventricular assist device (bridge-to-bridge, BTB), as bridge to decision (BTD) or sometimes as a bridge to transplantation (BTT) [1, 3, 4, 5]. Few studies have shown that the addition of an Impella® device to a VA-ECMO (´ECPELLA´ or ´ECMELLA´) was associated with improved survival of patients with refractory CS [6, 7]. There are small series with different proposals of unloading not only the LV Impella as an ECPELLA or ECMELLA concept, but also with a LV and a RV Impella (BiPELLA) or a prolonged Impella (PROPELLA) with improved survival outcomes [8]. In view of the increasing therapeutic options, a multidisciplinary expert-team approach to patients in cardiogenic shock becomes highly important [9, 10].
2. Description
2.1 Case report
A 38-year-old woman was referred to our institution from a peripheral hospital with progressive dyspnea for months with aggravation in the last three weeks, and bilateral pleural effusions (Table 1; Figure 1); no symptoms of angina, negative family history, risk factor of moderate smoking for many years. Echocardiography revealed a severely reduced left ventricular function with an ejection fraction of 10% with global hypokinesia and apical akinesia and a moderately reduced right ventricular function. Moderate mitral and tricuspid regurgitation were documented. Initial hemodynamic support with dobutamine and noradrenaline was stopped driven by a beginning ischemia of the feet. Under the suspicion of an incipient pneumonia, blood cultures were collected and an antibiotic therapy initiated. Cardiac catheterization revealed a severe three-vessel coronary artery disease.
Emergent coronary artery bypass grafting was performed with a left internal mammary artery graft to the left anterior descending artery, a sequential venous graft to a marginal branch and the marginal branch of the right coronary artery as well as a sequential venous graft to the right coronary artery and the posterior descending artery. As weaning from cardiopulmonary bypass was unsuccessful a peripheral (femoral) veno-arterial extracorporeal membrane oxygenator (VA-ECMO) was implemented.
Table 1: Timeline.
|
Presentation |
The 38-year-old patient was transferred from a peripheral hospital with progressive dyspnea, pleural effusions on low dose inotropes. Echocardiography showed a severely reduced left ventricular function (LV) with an ejection fraction (EF) of 10%. |
|
Initial treatment |
Cardiac catheterization showed a severe three-vessel coronary artery disease. The patient received an emergency coronary artery bypass graft surgery with heart-lung machine (HLM) and VA-ECMO was established. |
|
Day 1 |
Extensive fluid volume substitution and vasoactive therapy. |
|
Day 4 |
Episodes of pulseless, polymorphic and monomorphic ventricular tachycardia. Continuous renal replacement therapy initiated due to acute kidney injury. Diagnosis of dyslipidemia with high levels of Lp(a). |
|
Day 6-8 |
Repeated episodes of ventricular fibrillation following the attempt to reduce ECMO support. Defibrillations and multiple antiarrhythmic drug therapies (magnesium, amiodarone, lidocain and ajmaline). |
|
Day 8 |
Our multidisciplinary heart-team decided to implant a LV Impella (CP®, Abiomed) for LV unloading. |
|
Day 9 |
Implantation of a right ventricular Impella (RP®, Abiomed) to improve RV failure and prepare LVAD implantation (Figure 2). |
|
Day 10 |
Due to extensive hemolysis and an unclear neurological status under biventricular Impella support, we decided to implant a temporary extracorporeal left ventricular assist device (Rotaflow®, Marquet) as a second bridge to long-term. At the same time, we removed the VA-ECMO and LV Impella (CP®, Abiomed) (Figure 3). |
|
Day 13 |
Removal of RV Impella (RP®, Abiomed). |
|
Day 19 |
After the patient had shown adequate neurological reaction implantation of long-term LVAD (HeartMate 3TM, Abbott, USA) as a BTT-concept (Figure 4). Thrombectomy of the right femoral artery with patch plastic after removal of the arterial cannula of the EMCO. |
|
Day 23 |
Computer tomography of the brain showed no focal abnormalities preparing first uneventful extubation. |
|
Day 35 |
Ventricular fibrillations for nearly 20 minutes with re-intubation and mechanical ventilation, defibrillations, multiple antiarrhythmic therapies and cardiopulmonary resuscitation for 20 minutes. Coronary angiography showed open venous coronary artery bypass grafts but occluded LIMA graft (scared target area). |
|
Day 37 |
The hemodynamic situation improved. Catecholamine therapy could be terminated. |
|
Day 36-40 |
Epistaxis with nasal tamponade, second successful weaning and extubation. |
|
Day 41 |
Acute drop in blood pressure, bradycardia with cardiopulmonary resuscitation for approximately 30 minutes. Ventricular fibrillation with the need of intubation and defibrillation. On bronchoscopy multiple clots in trachea that led to hypoxia. |
|
Day 43-44 |
Percutaneous tracheostomy. Due to the right sided hemiparesis clinically detected on weaning, cerebral CT showed no focal abnormalities in the brain parenchyma. |
|
Day 72 |
Implantable cardioverter defibrillator (ICD) was implanted. |
|
Day 95 |
Amputations of I/IV digits of the left feet and debridement of catecholamine induced necrotic tissue from both feet. |
|
Day 114 |
The patient was transferred to a rehabilitation hospital with only minor neurological impairment with complete mobility of the right arm. Renal function had recovered. |
|
Day 154 |
The patient was discharged home. |
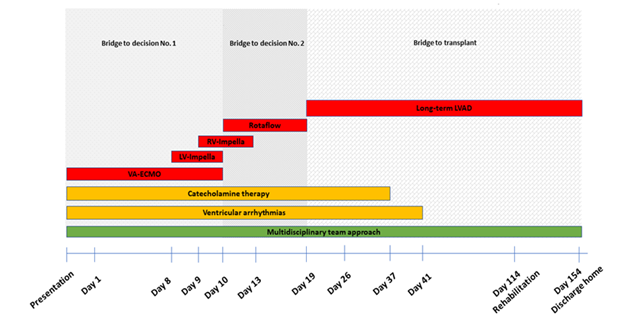
Figure 1: Timeline of devices, catecholamine therapy, ventricular arrhythmias, multidisciplinary team approach.
VA-ECMO=veno-arterial extracorporeal membrane oxygenation, LV-Impella=left ventricular Impella, RV-Impella=right ventricular Impella, LVAD=left ventricular assist device.
Extensive laboratory screening for vasculitis and rheumatologic causes revealed no abnormalities. However, a dyslipidemia with significantly increased lipoprotein (Lp) (a) was detected. Postoperatively the patient developed acute kidney injury with the need for continuous renal replacement therapy (CRRT).
During the next days, the patient suffered repeated episodes of malignant ventricular arrhythmias requiring defibrillations and multiple antiarrhythmic therapies including magnesium, amiodarone, lidocaine, ajmalin and propranolol. LV- function remained poor and reduction of ECMO flow failed. Following a prolonged episode of refractory ventricular arrhythmias on the 7th postoperative day the decision to unload the left ventricle by implantation of a left ventricular Impella (Impella CP®, Abiomed) was made.
Compromised right heart function led to implantation of a right ventricular Impella (Impella RP®, Abiomed) to prepare the implantation of a LVAD-system in a bridge to transplant concept (Figure 2). Spontaneous left sided hemothorax due to thrombocytopenia and severe hemolysis under dual Impella therapy demanded a strategy change. Due to the inconclusive neurological status we were reluctant with a permanent LVAD support at this point. Hence, a temporary paracorporeal left ventricular assist in terms of a 36F inflow cannula inserted via the left ventricular apex, a centrifugal pump (Rotaflow®, Gettinge, Solna, Sweden) and an 19F outflow cannula inserted into a 10 mm Dacron-Prothesis that was anastomosed to the ascending aorta was implanted. Simultaneously, the hemothorax was drained and the LV- Impella as well as the femoral VA-ECMO cannulas were explanted (Figure 3).
3 days later the RV-Impella was weaned and explanted. Under stable hemodynamic conditions the sedation was reduced, the patient remained adynamic but could move her extremities. Furthermore, a cerebral CT showed no evidence of hypoxic sequelae or intracranial bleeding. Thus, a permanent LVAD (HeartMate 3TM, Abbott, USA) was implanted on the 19th day of her stay (Figure 4). Subsequently the patient gradually recovered, was responsive, opened her eyes and adequately moved her extremities.
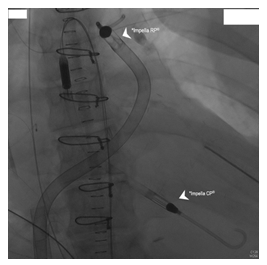
Figure 2: Impella CP® and Impella RP®.
*Impella CP® - Left ventricular Impella was inserted on the 8th day through a standard catheterization procedure through the femoral artery, into the ascending aorta, across the aortic valve into the left ventricle.
*Impella RP® - Right ventricular Impella was inserted on the 9th day thought a standard catheterization procedure through the femoral vein and into the right atrium, across the tricuspid and pulmonic valves into the pulmonary artery.
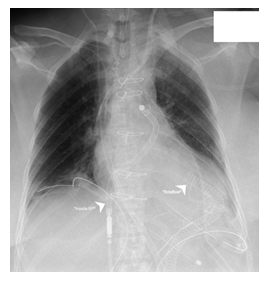
Figure 3: Impella RP® and Rotaflow.
*Impella RP® - Right ventricular Impella.
*Rotaflow® - Temporary paracorporeal left ventricular assist. A 36F inflow cannula was inserted on the 10th day via the left ventricular apex and a 19F outflow cannula into a 10 mm Dacron-Prothesis that was anastomosed to the ascending aorta.
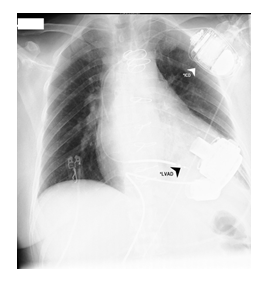
Figure 4: LVAD and ICD.
*LVAD - Long-term left ventricular assist device (HeartMate 3TM) was implanted on the 19th day.
*ICD - implantable cardioverter-defibrillator (Medtronic Evera MRI S VR SureScan) was implanted pectoral left side on the day 72.
On the 23rd day the computer tomography of the brain showed no focal abnormalities and the patient was extubated. On the 35th day the patient suffered of malignant ventricular arrhythmia for nearly 20 minutes with re-intubation and mechanical ventilation requiring 12 defibrillations, multiple antiarrhythmic therapies and cardiopulmonary resuscitation for 20 minutes. Coronary angiography showed open venous coronary artery bypass grafts but occluded LIMA graft. The hemodynamic situation improved during the next days and the patient was successfully weaned and extubated again.
On the 41st day she was resuscitated for approximately 30 minutes following sudden hypotension, bradycardia and respiratory failure. Intubation and successful resuscitation bronchoscopy revealed severe lower airway obstruction by blood clots most likely due to epistaxis. Percutaneous tracheotomy was performed on the next day and cerebral CT scans showed no pathological findings.
On the 59th day the patient was successfully weaned and decannulated. Temporary right sided hemiparesis that had been observed following the resuscitation gradually resolved under intensive physiotherapy. Renal function also recovered so that renal replacement therapy was discontinued.
On the 72nd day an implantable cardioverter-defibrillator (Medtronic Evera MRI S VR SureScan) was implanted. High-dose vasopressor therapy had led to pronounced acral necrosis of both feet. After initial conservative therapy with provision of temporary ortheses and frequent wound management, amputation of the I/IV digits of the left foot were performed in addition to an extensive debridement of necrotic tissue of both feet.
Frequent psychosomatic treatments focusing on strengthening resources, disease acceptance, disease insight, coping with acute somatic events and separation from the family were provided. She was transferred to a qualified rehabilitation facility with only minor neurological residues (limited mobility of the right leg) without any cognitive dysfunction after a total of 114 days after initial admission. She was discharged home on day 154.
3. Discussion
We report a case of a young woman with cardiogenic shock following severe coronary artery disease. Assumingly, the well-balanced combination of different options for ventricular support in early response to the individual course of the patient guided by an interdisciplinary team was the key to successful management of this extraordinary case (Table 1; Figure 1). Mortality in cardiogenic shock is about 40% [2]. In patients with acute heart failure and hemodynamic instability (INTERMACS level 1 und 2) short-term mechanical circulatory support (MCS) may be used to stabilize the patient, to gain time for further decisions on definitive therapy [1]. In a cohort of patients with cardiogenic shock in myocardial infarction Thiele [11] identified a subgroup of 15-25 % that will possibly survive by a MCS device, while 50-60 % will survive without a MCS and 25-35 % will die with or without a MCS (e.g. brain death, sepsis).
Different options for MCS are currently available. However, for a positive outcome the right choice of the device as well as proper timing for initiation as well as for escalation or de-escalation appear to be of paramount importance [5]. VA-ECMO provides complete cardiopulmonary support and is widely used in cardiothoracic surgery [5]. It is independent from the patient´s rhythm and even in malignant arrhythmias will show no flow alterations [2]. However, VA-ECMO in it’s peripheral (femoral) form may cause LV distension due to high afterload. LV distention in turn can exacerbate myocardial ischemia, trigger arrhythmias and may lead to pulmonary edema. Truby [12] found 7% of patients to have a high and 22% to have considerable LV distension on VA-ECMO. In the literature there are suggestions for LV unloading not only by inotropic support, but also by IABP, Impella and other surgical techniques [13].
A retrospective study by Pappalardo et al. [6] showed a significantly lower in-hospital mortality (40% vs. 74%) and a higher rate of a successful bridging to long-term strategies or recovery (62% vs. 30%) in patients treated with concomitant VA-ECMO and Impella® as compared to treatment with VA-ECMO alone. In the largest US-based retrospective study, VA-ECMO (n=36) vs. ECPELLA (n=30) in patients with refractory cardiogenic shock, the combined treatment with Impella® and VA-ECMO was associated with lower all-cause 30-day mortality, lower need for inotropic support and a comparable safety profile [7]. A metanalysis of 17 observational studies published in 2019 showed a lower mortality with different forms of LV unloading (54% vs. 65%), but in patients with additional Impella markedly more hemolysis was noted [14]. Therefore, the combination of VA-ECMO and Impella should be restricted to a limited period of time. LV and RV Impella (BiPELLA) could be indicated in patients with a biventricular failure and contraindications to VA-ECMO. A simultaneous initiation of support with BiPELLA can be associated with improved survival outcomes [8]. A further research is required to provide evidence of the effectiveness of BiPELLA and an implantation of BiPELLA on top of a VA-ECMO.
The implantation of a temporary extracorporeal left ventricular assist device (e.g. Rotaflow®) can bridge over a longer time period to a permanent device in unclear conditions. In the literature one case with a bridge to recovery is reported, in our case it was a bridge-to-bridge concept [15]. The most frequent complications are bleeding, thromboembolism and vascular complications, followed by neurological and respiratory problems [3]. A multidisciplinary heart-team (including cardiothoracic surgeons, cardiologists, anesthesiologists, intensive care specialists, and perfusionists) should join expertise to select and rapidly intervene on patients with severe cardiogenic shock [9, 10]. Tehrani et al [10] suggested the implementation of a multidisciplinary standardized team approach. In their observational study a standardized team approach could significantly increase 30-day MCS survival from 47 to 77% [10]. We highly recommend the additional implementation of psychological and psychosocial support for the patient, the family and the team as well as access to ethical advice and palliative care as proposed in the European Guidelines and in the scientific statement of the American Heart Association [1]. In conclusion, the addition of LV Impella (CP®) implantation and RV support by Impella (RP®) (BiPella) on top of VA-ECMO may support survival outcomes in refractory cardiogenic shock. In uncertain situations a paracorporal (short-term) LVAD (Rotaflow) can be used as a ´second bridge´ to long-term mechanical support with LVAD.
4. Learning Points
- In severe cardiogenic shock due to biventricular cardiac dysfunction, a combined extracorporeal life support (ECLS) with left and right ventricular Impella on top of veno-atrial extracorporeal membrane oxygenation (VA-ECMO) can successfully be installed as a bridge to implantation of a permanent left ventricular assist device (LVAD).
- A staged approach to ventricular support may facilitate decision-making especially in the setting of an unclear neurological status and insufficient left ventricular unloading by VA-ECMO.
- In complex biventricular heart failure, an expert center must be able to provide an early multi-modular intervention with differentiated mechanical circulatory support and individually adapted (high risk) procedures due to a multidisciplinary expertise.
Acknowledgments
The authors confirm that written consent for submission and publication of this case report including images and associated text has been obtained from the patient in line with COPE guidance.
Conflict of Interest
None declared.
Funding
None.
References
- Ponikowski P, Voors AA, Anker SD, et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur H J 37 (2016): 2129-2200.
- Mebazaa A, Combes A, van Diepen S, et al. Management of cardiogenic shock complicating myocardial infarction. Intensive Care Med 44 (2018): 760-73.
- Thiele H, Jobs A, Ouweneel DM, et al. Percutaneous short-term active mechanical support devices in cardiogenic shock: a systematic review and collaborative meta-analysis of randomized trials. Eur Heart J 38 (2017): 3523-3531.
- Bellumkonda L, Gul B, Masri SC. Evolving concepts in diagnosis and management of cardiogenic shock. Am J Cardiol 122 (2018): 1104-1110.
- Guglin A, Zucker MJ, Bazan VM, et al. Venoarterial ECMO for adults JACC scientific expert panel. JACC 73 (2019): 698-716.
- Pappalardo F, Schulte C, Pieri M, et al. Concomitant implantation of Impella® on top of veno-arterial extracorporeal membrane oxygenation may improve survival of patients with cardiogenic shock. Eur J Heart Fail 19 (2017): 404-412.
- Patel SM, Lipinski J, Al-Kindi SG, et al. Simultaneous venoarterial extracorporeal membrane oxygenation and percutaneous left ventricular decompression therapy with impella is associated with improved outcomes in refractory cardiogenic shock. ASAIO J 27 (2018): 27.
- Tschöpe C, Van Linthou S, Klein O, et al. Mechanical unloading by fulminant myocarditis: LV-IMPELLA, ECMELLA, BI-PELLA and PROPELLA concepts. J of Cardiovasc Trans Res 12 (2019): 116-123.
- Doll JA, Ohman EM, Patel MR, et al. A team-based approach to patients in cardiogenic shock. Catheter Cardiovasc Interv 88 (2016): 424-433.
- Tehrani BN, Truesdell AG, Sherwood MW, et al. Standardized team-based care for cardiogenic shock. J Am Coll Cardiol 73 (2019): 1659-1669.
- Thiele H, Ohman EM, de Waha-Thiele S, et al. Management of cardiogenic shock complicating myocardial infarction: an update 2019. Eur H J 40 (2019): 2671-2683.
- Truby LK, Takeda K, Mauro C, et al. Incidence and implications of left ventricular distention during venoarterial extracorporal membrane oxygenation support. ASAIO J 63 (2017): 257-265.
- Meani P, Gelsomino S, Natour E, et al. Modalities and effects of left ventricule unloading on extracorporal life support: a review of the current literature. Eur J Heart Fail 19 (2017): 84-91.
- Russo JJ, Aleksova N, Pitcher I, et al. Left ventricular unloading during extracorporal membrane oxygenation in patients with cardiogenic shock. JACC 73 (2019): 654-662.
- Kashiwa K, Nishimura T, Saito A, et al. Left heart bypass support with the Rotaflow Centrifugal Pump® as a bridge to decision and recovery in an adult. J Artif Organs 15 (2012): 207-210.


 Impact Factor: * 5.3
Impact Factor: * 5.3 Acceptance Rate: 75.63%
Acceptance Rate: 75.63%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 
