A Case of Post-Myocardial Infarction Ventricular Septal Rupture and Intramyocardial Dissection
Jessica Huang1*, Michael Bourne1, Benjamin Rosenfeld2, Federico Mordini3, Christian Nagy3, Andrew Chen3
1George Washington University, Washington, DC, USA
2Georgetown University, Washington, DC, USA
3Washington DC Veteran’s Affairs Medical Center, Washington, DC, USA
*Corresponding Author: Jessica Huang, George Washington University, Washington, DC, USA
Received: 21 April 2023; Accepted: 03 May 2023; Published: 19 May 2023
Article Information
Citation: Jessica Huang, Michael Bourne, Benjamin Rosenfeld, Federico Mordini, Christian Nagy, Andrew Chen. A Case of Post-Myocardial Infarction Ventricular Septal Rupture and Intramyocardial Dissection. Archives of Clinical and Medical Case Reports. 7 (2023): 240-243.
View / Download Pdf Share at FacebookAbstract
Ventricular septal rupture (VSR), albeit rare, is a deadly consequence of ST elevation myocardial infarction (STEMI). Our patient described here presented with both post-infarction VSR and intramyocardial dissection (IMD) of the right ventricle (RV), a subtype of left ventricular free wall rupture (LVFWR). This case highlights the importance of prompt diagnosis of these post-myocardial infarction (MI) complications and the current limitations of surgical and percutaneous repair.
Keywords
<p>Myocardial; Infarction; Ventricular; Septal; Rupture; Intramyocardial; Dissection</p>
Article Details
1. Introduction
1.1 Ventricular septal rupture
With the advent of percutaneous coronary intervention (PCI), the incidence of VSR after STEMI has decreased from 1-3% to 0.17-0.31% [1]. Notably, anterior infarcts more often result in VSRs that demonstrate more anatomic simplicity compared to inferior infarcts that cause complex ruptures. In addition, VSRs after a right coronary artery (RCA) occlusion tend to be more complicated and serpiginous compared to those caused by left coronary artery (LCA) infarcts, which are smaller and more focal [1].
There are three types of free wall rupture defined in the literature – Becker types 1, 2, and 3. Becker type 1 ruptures are characterized by rupture through normal myocardium occurring within 24 hours of MI. These are more often associated with left anterior descending (LAD) occlusion. Becker type 2 presents sub-acutely after MI as a result of breakdown of infarcted myocardium. Finally, Becker type 3 presents late after MI and is caused by tearing of the thinned aneurysmal myocardium; this is more commonly seen when there has been no reperfusion [2]. As illustrated in the Becker categorization, VSR demonstrates a bimodal distribution of presentation occurring either hours or days (3-5 or even more) after initial injury [1]. Notably, anterior infarcts more often result in VSRs that demonstrate more anatomic simplicity compared to inferior infarcts that cause complex ruptures. In addition, VSRs after a right coronary artery (RCA) occlusion tend to be more complicated and serpiginous compared to those caused by left coronary artery (LCA) infarcts, which are smaller and more focal [1].
The gold standard for diagnosis of VSR is transthoracic echocardiogram (TTE) as it is safe, readily accessible, and quickly interpreted in patients that are often hemodynamically unstable. It demonstrates the size of the defect and Color Doppler assess for a left to right shunt [3]. Right heart catheterization (RHC) can aid in diagnosis by showing a “step-up” in oxygen saturation from the right ventricle to the pulmonary artery. Normal oxygen saturation for the RA is 64 to 67% and for the PA is 64-67%. A left to right shunt can be expected in those with a PA oxygen saturation >75% and if is there a >5% increase in oxygen saturation from the RA to PA [4, 5]. Cardiac magnetic resonance imaging (CMR) and computed topography (CT) can be used in hemodynamically stable patients to show infarcted tissue and a ruptured septum [6].
Delayed surgical repair of the VSR is preferred; a study from the Society of Thoracic Surgeons National Database found surgical repair of VSR has a 30-day mortality of 42.9%, which improves to 18.4% if repair is delayed after 7 days [1]. The improved outcomes with delayed surgical repair may be related to survival bias and possibly remodeling of the friable myocardium into more repairable tissue. In those who are not candidates for surgical repair, transcatheter septal closure (TSC) is recommended. This process involves femoral arterial and femoral or internal jugular venous access, crossing the septal rupture from left to right, placing the wire in the pulmonary artery, and snaring it from the venous circulation to create an arteriovenous rail. This is used to place a device-delivery sheath across the defect which deploys a septal occluder composed of two discs that sit on each side of the defect. TSC is limited by the size of the defect, with the optimal defect size being <15 mm [1]. Occasionally, TSC can also be used as an adjunct to surgical repair and/or as salvage therapy after initial surgery. Still, those who receive TSC have an 89% success rate and 32% chance of 30-day or in-hospital mortality [1]. This is contrast to those who are neither surgical nor TSC candidates and are treated with medical therapy alone, which is associated with a mortality rate of 90% [7].
In the case of isolated post-infarct VSR, mortality varies depending on clinical status and timing of VSR surgery. In the case of patients similar to ours – with cardiogenic shock requiring percutaneous VSR closure – mortality approaches 88%. This is compared to those percutaneously repaired without shock who have a mortality rate of 38% [8]. Notably, however, this includes those who have not suffered intramyocardial dissection.
1.2 Intramyocardial dissection
Even more uncommon than postinfarction VSR is intramyocardial dissection (IMD), also known as intramyocardial hematoma (IMH). IMD is an exceedingly rare sub-category of left ventricular free wall rupture (LVFWR) with few cases reported in the literature. LVFWR is the most common post-infarction mechanical complication, occurring 6-8 times more often than VSR or rupture of the papillary muscles, and usually results in a rupture into the pericardium [9]. However, in rare instances the rupture proceeds through a tract in the myocardium, causing an intramyocardial dissection [9, 10]. Unlike ventricular pseudoaneurysms, IMD is defined as being contained within the myocardial wall, maintaining communication with the ventricles, and demonstrating blood follow in conjunction with the heartbeat [10]. It is frequently underreported given the difficulty in diagnosis, often requiring intraoperative or post-mortem diagnosis [9]. VSR with concomitant IMD is more commonly associated with RCA insult and inferior MI [1, 11].
Cases with LV involvement, particularly of the apical LV free wall, demonstrate improved outcomes. Contrastly, IMD that extends into the septum or into the RV is both rarer and deadlier [12]. It is of paramount importance that IMD be recognized as soon as possible. TTE is the quickest method for diagnosis and should demonstrate at least three of the following: a neocavity within an echo-lucent center, a thinned and mobile endomycardial border surrounding the cavity, ventricular myocardium outside of the cystic area, heterogenous echogenicity of the neocaviity, partial or complete absorption of the cystic structure, continuity between the dissection and one of the ventricles, communication between the ventricles through the dissection, and Doppler flow within dissected myocardium [12, 13]. CMR and/or CT may also be used. Confirmation can be obtained via coronary angiography [14-16].
Treatment for IMD is not well understood given its rarity and frequent association with tenuous hemodynamics. In those who are hemodynamically stable with small, apical IMD in the absence of VSR, there is a role for conservative management and observation for spontaneous reabsorption. This approach is also more successful in patients who have already been revascularized [11]. However, even in those who are stable, this can be a controversial approach as these patients can quickly deteriorate into cardiogenic shock. In the few reported cases of VSR associated with RV IMD, some authors advocate for urgent surgical repair, which includes using a patch with either bovine or prosthetic material [7, 15].
2. Case Presentation
An 86-year-old gentleman with a history of hypertension, chronic obstructive pulmonary disease (COPD), and 100 pack year smoking history presented with progressive dyspnea and one week of neck pain and dizziness.
In the emergency room, he had a blood pressure of 104/74, heart rate 85, respiratory rate 27, and SpO2 95% on 3 liters of nasal cannula. He was in no acute distress but physical exam revealed jugular venous distention to the mandible and mild bilateral wheezing. Labs were notable for a leukocytosis of 11, hemoglobin 11.8, creatinine 2.59, Pro-BNP 12376, and AST/ALT 348/342. Troponin was found to be 7.39 with an elevated lactate of 4.7. Electrocardiogram (ECG) showed normal sinus rhythm with second degree atrioventricular (AV) block Mobitz type 1 and ST depression in anterior leads. Chest x-ray showed pulmonary edema. He received aspirin 325 mg, clopidogrel 300 mg, and IV furosemide 80 mg. The patient continued to be symptomatic and thus sent for urgent left heart catheterization (LHC).
2.1 Clinical evaluation
LHC revealed a large caliber right coronary artery (RCA) with 80% mid vessel stenosis followed by 100% occlusion. Left ventriculogram showed a basal inferior wall aneurysm and an estimated ejection fraction of 55%, end diastolic pressure of 33 mmHg, and no mitral regurgitation. The RCA occlusion was not stented as he was unable to tolerate the remainder of the procedure due to dyspnea and worsened hypoxia. Subsequently, the patient was admitted to the medical intensive care unit (MICU) for medical stabilization.
In the MICU, the patient was placed on a heparin infusion and underwent placement of a central line. Of note, during central line insertion he sustained a 20 second episode of asystole which spontaneously resolved. Venous oxygen saturation (SvO2) from the central line was 35%. Overnight, labs showed worsening creatinine from 2.5 to 3.5 and troponin 7.3 to 14.3.
The following day, TTE demonstrated a basal inferoseptal that had not been well-visualized on LHC. The defect appeared to be 12 mm in diameter and was associated with severe left-to-right shunting during systole and a small right to left shunt during diastole. Right ventricular systolic function was moderately reduced. Cardiothoracic surgery was consulted for surgical VSR repair but given the patient’s age and comorbidities, he was deemed to be at prohibitive surgical risk. A percutaneous VSR repair option was subsequently explored. For pre-procedure planning, a CMR was performed to assess the extent of necrotic or friable tissue surrounding the VSR, accurately size the defect closure device, and determine if there were adequate rims for percutaneous closure. The study confirmed a large VSR with a large infarct extending into the inferolateral wall with extensive necrosis and a dissected tissue flap from the right ventricular endocardium, consistent with an intramyocardial dissection (IMD) (Figure 1).
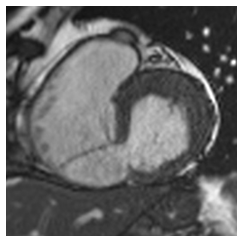
Figure 1: Cardiac MRI demonstrating ventricular septal rupture and intramyocardial dissection involving RV endocardium.
Insertion of an intra-arterial balloon pump (IABP) was attempted as a bridge to TSC, but the patient was unable to tolerate the procedure due to agitation. He was then intubated and placed on Norepinephrine for right heart catherization (RHC). RHC revealed a step-up from the right atrium (RA) to right ventricle (RV) of 58% to 80%, Qp:Qs of 2.33, and cardiac index of 2.10, findings consistent with severe left-to-right shunting and cardiogenic shock. During RHC, a wire could not be passed through the VSR due to obstruction by the RV myocardial flap from the IMD. Therefore, the decision was made to forego percutaneous VSR repair. Family was engaged in a goals of care discussion and opted for a palliative route. The patient was compassionately extubated and expired the following day.
3. Discussion
As demonstrated in this case, although post-MI VSR is an uncommon occurrence in the PCI era, it is still associated with high mortality. It remains an outcome with significant treatment complications, particularly in those presenting in cardiogenic shock. In patients with serpiginous VSR, they may have an associated, albeit rare, intramyocardial dissection. Prompt diagnosis in these patients is key. TTE remains the cornerstone for diagnosis and can be supported by CMR, CT, and/or coronary angiography. Surgical repair of VSR and IMD are preferred, however percutaneous transcatheter septal closure of VSR can be explored for those that are not surgical candidates. As in our case, however, clinical and anatomic obstacles are often encountered in these critically ill patients and can hinder both surgical and percutaneous options. For these patients, a conservative approach with medical management alone is also an option. Ultimately, management of hemodynamically unstable patients with structurally complicated VSR and IMD is challenging and requires clarification.
Conflicts of Interest
None of the authors have any conflicts of interest to report.
References
- Amit Goyal, Venu Menon. Contemporary Management of Post-MI Ventricular Septal Rupture. American College of Cardiology (2022).
- Satoshi Honda, Yasuhide Asaumi, Takafumi Yamane, et al. Trends in the Clinical and Pathological Characteristics of Cardiac Rupture in Patients With Acute Myocardial Infarction Over 35 Years. Journal of the American Heart Association. 3 (2022): e000984.
- Sharma E, Beale C, Ehsan A, et al. Plugging the Hole: Diagnosis and Management of Post–Myocardial Infarction Ventricular Septal Defect. CASE Cardiovasc Imaging Case Rep 4 (2020): 283-287.
- Themes UFO. Complications of Acute Myocardial Infarction. Thoracic Key (2016).
- Rosenkranz S, Preston IR. Right heart catheterisation: best practice and pitfalls in pulmonary hypertension. Eur Respir Rev 24 (2015): 642-652.
- Mahajan K, Shah N, Patel H. Post Infarct Ventricular Septal Rupture. In: StatPearls. StatPearls Publishing (2022).
- Chan W, Yan B, Warren R, Goldblatt J, Aggarwal A. A rare complication of left ventricular rupture-Right ventricular intramyocardial dissection with left-to-right shunting. Int J Cardiol 121 (2007): e19-e21.
- Thiele H, Kaulfersch C, Daehnert I, et al. Immediate primary transcatheter closure of postinfarction ventricular septal defects. Eur Heart J 30 (2008): 81-88.
- Ercan A, Gurbuz O, Kumtepe G, et al. Intramyocardial Dissection following Postinfarction Ventricular Wall Rupture Contained by Surrounding Postoperative Adhesions. Case Rep Surg (2015): e584795.
- Liu CC, Wang LS, Su ZP, et al. Intramyocardial dissection with concomitant left ventricular aneurysm as a rare complication of myocardial infarction: a case report. J Geriatr Cardiol JGC 13 (2016): 632-635.
- Hajsadeghi S, Amirfarhangi A, Pakbaz M, et al. Postinfarction intramyocardial dissection, an interesting case report and systematic review. Echocardiography 37 (2020): 124-131.
- Vargas-Barrón J, Roldán FJ, Romero-Cárdenas Á, et al. Dissecting Intramyocardial Hematoma: Clinical Presentation, Pathophysiology, Outcomes and Delineation by Echocardiography. Echocardiography 26 (2009): 254-261.
- Soriano CJ, Pérez-Boscá JL, Canovas S, et al. Septal rupture with right ventricular wall dissection after myocardial infarction. Cardiovasc Ultrasound 3 (2005): 33.
- Basarici I, Erbasan O, Kemaloglu D, et al. Exceptional Ventricular Septal Rupture Associated with Intramyocardial Dissection Throughout the Right Ventricle. Echocardiography 27 (2010): 460-465.
- Roslan A, Jauhari Aktifanus AT, Hakim N, et al. Intramyocardial Dissecting Hematoma in Patients with Ischemic Cardiomyopathy: Role of Multimodality Imaging in Three Patients Treated Conservatively. CASE Cardiovasc Imaging Case Rep 1 (2017): 159-162.


 Impact Factor: * 5.3
Impact Factor: * 5.3 Acceptance Rate: 75.63%
Acceptance Rate: 75.63%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 
