Nutraceutical Approach for the Management of Diabetic Neuropathy
Giovanni Ragozzino1, Umberto Di Maio2, Maria Potenza3*
1Endocrinology, Diabetology and Clinical Nutrition Unit, DiStaBiF, University of Campania "Luigi Vanvitelli", Naples, Italy
2Shedir Pharma Group SpA, Via Bagnulo, 95 - 80063 Piano di Sorrento (NA), Italy
3Neilos Srl, Via Bagnulo, 95 - 80063 Piano di Sorrento (NA), Italy
*Corresponding Author: Maria Potenza, Neilos Srl, Via Bagnulo, 95 - 80063 Piano di Sorrento (NA), Italy
Received: 14 October 2025; Accepted: 21 October 2025; Published: 04 November 2025
Article Information
Citation: Giovanni Ragozzino, Umberto Di Maio, Maria Potenza. Nutraceutical Approach for the Management of Diabetic Neuropathy. Archives of Clinical and Medical Case Reports. 9 (2025): 225-230.
View / Download Pdf Share at FacebookAbstract
Painful diabetic neuropathy (pDN) is a common type of peripheral neuropathic pain and it's defined as pain that is a direct consequence of abnormalities in the peripheral somatosensory system in individuals with diabetes. Antidiabetic drugs improve the glycemic profile but do not act on the symptoms of pDN, while the drugs commonly used for the symptoms of diabetic neuropathy (antidepressants and anticonvulsants) cause several side effects including dizziness, constipation, somnolence and loss of appetite, so it’s important to have nutraceutical tools capable of delaying or reducing the pharmacological approach. The aim of the present retrospective clinical survey was to evaluate the effect of Epiderali® plus in patients undergoing therapy with hypoglycemic drugs showing symptoms of diabetic neuropathy not well controlled with the pharmacological treatments (T0 score for motion questionnaires). After three months of treatment and still up to six months with Epiderali® plus of treatment the blood parameters were controlled without collateral interactions with pharmacological treatments. Moreover, using three different standardized questionnaires all the symptomatology related to motion, pain and quality of life was significantly improved. After a more complex clinical investigation it could be possible to consider Epiderali® plus as novel therapeutic tool for the management of motorial neuropathy and pain in patients with diabetes neuropathy.
Keywords
<p>Diabetic neuropathy; Pain treatment; Fatty acids; Nutraceutical</p>
Article Details
1. Introduction
Diabetes mellitus (DM) is a common disorder characterized by persistent hyperglycaemia. Elevated glucose primarily harms cells with limited control over their glucose absorption, such as vascular cells, Schwann cells, and neurons in both the peripheral and central nervous systems. The persistence of a glycaemic imbalance leads to an inflammatory status mediated by an hyperactivation of mast cells and macrophages, that could determine severe complications such as retinopathy, nephropathy, hypertension, and neuropathy. Not all diabetic patients develop diabetic polyneuropathy. The risk factors for neuropathy, include poor glycaemic control and common cardiovascular risk factors such as hypertension, high triglycerides, obesity, and smoking. Genetic factors may also play a role in the development of diabetic polyneuropathy (dPNP). Painful diabetic neuropathy (pDN) is a common type of peripheral neuropathic pain. It's defined as "pain that is a direct consequence of abnormalities in the peripheral somatosensory system in individuals with diabetes" [1-2]. Among patients with chronic diabetes mellitus (DM), up to 60% are affected by distal symmetric polyneuropathy (dPNP), and even 7-10% of newly diagnosed patients already have some form of neuropathy; in fact, specifically in the case of autonomic neuropathy often the symptoms (such as cardiovascular, gastro- intestinal or genitourinary impairments) may manifest even before of DM diagnosis [2]. Pain deriving from diabetic neuropathy results directly from a lesion or disease in the somatosensory nervous system. Consequently, drugs used for neuropathic pain should be effective in treating painful pDN [3-4]. Gabapentinoids are the first-choice drugs for treating diabetic neuropathy, they attach to a specific part of voltage-gated calcium channels, which in turn stops the release of neurotransmitters.
In addition, gabapentinoids encourage the uptake of glutamate (an excitatory neurotransmitter), and inhibit descending serotonergic facilitation, stimulating descending inhibition, providing anti-inflammatory benefits [3-5]. SNRIs (serotonin-norepinephrine reuptake inhibitors) are also commonly used drugs for treating diabetic neuropathy. The drug class known as SNRIs has shown promise in treating painful diabetic neuropathy because they inhibit the reuptake of serotonin and norepinephrine and consequently reduce associated pain [3-6]. Duloxetine, a specific type of SNRI, has been proven effective in multiple studies and Venlafaxine, another SNRI, is also used for treating this kind of pain [3-7]. However, SNRIs can cause more severe side effects than other pain medications like gabapentinoids such as pregabalin and mirogabalin. These side effects include dizziness, constipation, somnolence and loss of appetite [3-8-9-10-11]. The possibility of having nutraceutical tools that reduce pDN by delaying the pharmacological approach or to enhance the drug efficiency and reduce the side effects it’s is a challenge in the scientific field for different therapeutic areas [12 13 14 15].
Epiderali® plus is a food supplement based on the patented technology F.A.G.® a specific qualitative and quantitative mixture of specific fatty acids with strong anti-inflammatory activity. Epiderali® plus is specifically formulated to counteract the increasing symptomatology related to motorial, sensitivity and autonomic diabetic neuropathy induced. In fact, the formulation is enriched with vitamin B12, lycopene and spirulina. In fact, vitamin B12 supplementation is correlated with reduction of neuropathic symptoms and the use of antioxidant like lycopene and spirulina could also improve the efficacy in patients with pDN [16-17-18].
This study aims to evaluate the clinical experience with Epiderali® plus in adult population with diabetic neuropathy focusing on its efficacy and safety within its intended scope of application, particularly evaluating the effect on motorial neuropathy in terms of pain and patients’ quality of life. The study was conducted considering a group of patients taking Epiderali® plus in association with conventional pharmacological treatments in order to evaluate the efficacy and safety of the nutraceutical approach.
2. Materials and Methods
2.1 Settings
The clinical survey has been conducted by an Italian medical specialist and it is based on its clinical experience in patients taking Epiderali® plus tablets. The retrospective observational survey was conducted in accordance with the Standards of Good Clinical Practice of the European Union and the ethical principles expressed in the Declaration of Helsinki. Data were retrospectively collected in the period January 2023 - October 2023 by the medical specialist. Ethical approval was not necessary according to National Code on Clinical Trials declaration because this data derives from a real-life retrospective study [19]. The aim of the present study was to evaluate the effect of Epiderali® plus oral administration in combination with pharmacological treatments at T0 (enrolment phase), after three months (T3) and six months (T6) of treatment.
2.2 Study Population, treatment and evaluated parameters
An Italian medical doctor enrolled participants in the period January 2023 - October 2023 evaluating their clinical manifestations during medical examination. In particular, the participants were selected according to defined inclusion criteria that were related to type 2 diabetes mellitus associated with sensory and motor neuropathy. Considering these clinical conditions, a total of 21 patients were enrolled in the present survey; at the first medical examination, the doctor reported for each patient its age, gender, comorbidity and use of pharmacological treatments. Then, the patient’s clinical condition was assessed with plasmatic analysis for glycaemia and glycated haemoglobin and in addition with three different questionnaires: Michigan Neuropathy Screening Instrument Questionnaire (MNSI-Q), Walking Impact Scale (Walk-12) and Visual Analogue Scale (VAS).
Then, each enrolled participant administered Epiderali® plus according to the method of use and posology indicated on the package leaflet and to re-show for a follow-up visit after 3 months (T3) and after 6 months (T6). Patients took Epiderali® plus in association with their pharmacological treatments traditionally used for diabetes mellitus such as hypoglycaemics metformin, pioglitazone, GLP-1 analogues, SGLT2 and insulin; and in some cases also in association with antidepressants and anticonvulsants like duloxetine and pregabalin as reported in Table 1.
|
Total |
Male |
Female |
|
|
Gender |
21 |
14 |
7 |
|
Average Age (years) |
62.52 |
62.57 |
62.43 |
|
In treatment with SGLT-2 |
2 |
2 |
/ |
|
In treatment with SGLT2 and pregabalin |
1 |
1 |
/ |
|
In treatment with SGLT2 and Metformin |
1 |
/ |
1 |
|
In treatment with SGLT2 and GLP-1 analogue |
2 |
/ |
2 |
|
In treatment with SGLT2 and pioglitazione |
1 |
1 |
/ |
|
In treatment with SGLT2 and insulin |
1 |
1 |
/ |
|
In treatment with GLP-1 analogue |
7 |
5 |
2 |
|
In treatment with GLP-1 analogue and Doluxetine |
3 |
1 |
2 |
|
In treatment with Pioglitazione and Metformin |
1 |
1 |
/ |
|
No pharmacological treatments recorded |
2 |
2 |
/ |
Table 1: Patients’ population enrolled in the retrospective survey.
3. Results and Discussion
Diabetes mellitus is a global public health issue, that affects up to half a million people worldwide; diabetic neuropathy is a common complication of diabetes mellitus including various patterns of neuropathy as categorised by the location of nerve damage. However, despite many researchers all around the world aimed to identify the real of diabetic neuropathy, its underlying mechanisms remain complicated and unclear so the prevalence of DN continues to grow up and there is a very minimal enhancement in the management of DN [20-21-22]. The present retrospective clinical survey aimed to evaluate the efficacy and safety of a nutraceutical specifically formulated for diabetic neuropathy in order to counteract specifically the symptomatology related to the motion impairment caused by the diabetic neuropathy; in fact, the main pharmacological treatments, such as insulin, SGLT-2, DPP-4, GLP-1 are not specific for this goal. In fact, all the enrolled patients taking specific drugs as reported in table 1, at T0 presented a significant severity of symptoms related to motion capacity according to MNSI and Walk-12 questionnaire as well as according to VAS scale (Figure 3).
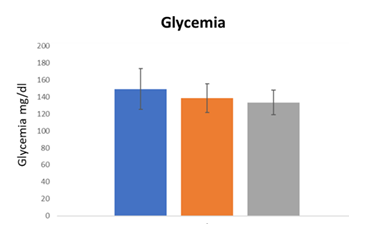
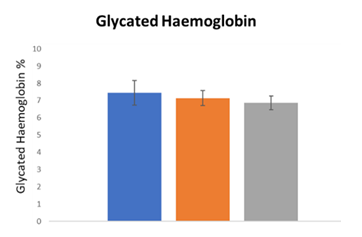
These data indicated that the prescribed drugs for these patients were not able to control the symptomatology specifically related to motion capacity in diabetic neuropathy also worsening the quality of life. As first step, hematic parameters (glycemia and glycated haemoglobin) were monitored at the enrolment (T0) and after the treatment with Epiderali® plus (T3 - T6) in combination with conventional drugs prescribed (table 1) in order to understand if this association altered the hematic parameters. As reported in Figure 1 and 2, for both glycemia and glycated haemoglobin Epiderali® plus maintained the efficacy of pharmacological treatments demonstrating a good control of the hematic parameters maybe improving the effect of conventional oral hypoglycemics.
Conventional treatments used for diabetes are able to control hematic parameters like glycemia and glycated haemoglobin, but are poor effective in controlling the symptomatology of motorial neuropathy, so for these reason three different questionnaires related to motion ability, pain control and quality of life were evaluated after the treatment with Epiderali® plus.
Interestingly, Epiderali® plus is able to reduce the symptomatology related to motorial neuropathy with optimum results for all patients considering three different questionnaires. In fact, MNSI questionnaire is used widely for the evaluation of distal symmetrical peripheral neuropathy in diabetes, the Walk-12 Questionnaire was designed to examine patients' perception of their walking ability in multiple sclerosis and also applied to peripheral neuropathy and Vas scale is used for the determination of autonomic neuropathy and quality of life. Particularly, MNSI questions refer to numb hands and feet, hypersensitivity to touch, feet ulcers etc. while the Walk-12 questions are specifically related to the ability to walk, the distance travelled, the possible need for assistance. Finally, the VAS scale is more generally related to an overall quality of life (appetite, quality of sleep, nausea, diarrhoea, etc.) [23-24-25].
It’s interesting to point out that, in the present study, the use of Epiderali® plus revealed able to reduce the symptomatology of motorial neuropathy differently from commonly prescribed drugs (at T0 all patients treated with drugs presented high scores for motion neuropathy according to motion questionnaires score), while also maintaining an excellent profile of blood parameters such as glycemia and glycated hemoglobin (Figure 1).
The F.A.G. patented biotechnology in Epiderali® plus is also able to ameliorate the nerve cells function and also thanks to its anti-inflammatory effect reduce the pain improving the quality of life. In addition, thanks to the presence of B12 vitamin in the formulation, Epiderali® plus also counteracts the B12 deficiency that is often undermine from oral hypoglycaemic such as metformin [26].
4. Conclusion
The present retrospective clinical survey was conducted with the aim to analyse the post-marketing clinical experience concerning the use of Epiderali® plus in patients with diabetes neuropathy. A total of 21 patients were enrolled in the present retrospective clinical survey and were treated with Epiderali® plus considering to different time points for the follow-up visit (three and six months). After three months of treatment and still up to six months of treatment with Epiderali® plus the blood parameters were controlled and still improved with respect to the T0 data (patients treated only with the oral hypoglycaemic) without collateral interactions with pharmacological treatments. Moreover, using three different standardized questionnaires, the symptomatology related to motorial neuropathy, pain and quality of life was significantly improved with the respect to the treatment only with hypoglycaemics and/or antidepressants (T0 score). More consistent randomized and placebo-controlled clinical trials are necessary to confirm Epiderali® plus as a novel therapeutic tool for the control of the symptomatology related to motion neuropathy in patients with diabetes neuropathy.
Conflicts of Interest
We declare that Umberto Di Maio is a Shedir Pharma Group S.p.A. member and Maria Potenza is a Neilos S.rl. member.
Funding
This research was funded by a grant from Neilos S.r.l.
Authors’ Contributions
All authors contributed equally to the manuscript and read and approved the final version of the manuscript.
References
- Tesfaye S, et Diabetic neuropathies: update on definitions, diagnostic criteria, estimation of severity, and treatments. Diabetes Care 33 (2010): 2285-2293.
- Rosenberger DC, Blechschmidt V, Timmerman H, et al. Challenges of neuropathic pain: focus on diabetic neuropathy. J Neural Transm (Vienna) 127 (2020): 589-624.
- Jang HN, Oh TJ. Pharmacological and Nonpharmacological Treatments for Painful Diabetic Peripheral Diabetes Metab J 47 (2023): 743-756.
- Raja SN, Carr DB, Cohen M, et The revised International Association for the Study of Pain definition of pain: concepts, challenges, and compromises. Pain 161 (2020): 1976-1982.
- Hincholkar M. Analgesic mechanisms of gabapentinoids and effects in experimental pain models: a narrative Br J Anaesth 120 (2018): 1315-1334.
- Bates D, Schultheis BC, Hanes MC, et al. A comprehensive algorithm for management of neuropathic Pain Med 20 (2019): S2-12.
- Pop-Busui R, Boulton AJ, Feldman EL, et al. Diabetic neuropathy: a position statement by the American Diabetes Diabetes Care 40 (2017):136-54.
- Goldstein DJ, Lu Y, Detke MJ, Lee TC, Iyengar Duloxetine vs. placebo in patients with painful diabetic neuropathy. Pain 116 (2005): 109-118.
- Rowbotham MC, Goli V, Kunz NR, et al. Venlafaxine extended release in the treatment of painful diabetic neuropathy: a double-blind, placebo-controlled study. Pain 110 (2004): 697-706.
- Parsons B, Li C. The efficacy of pregabalin in patients with moderate and severe pain due to diabetic peripheral Curr Med Res Opin 32 (2016): 929-937.
- Vinik A, Rosenstock J, Sharma U, et al. Efficacy and safety of mirogabalin (DS- 5565) for the treatment of diabetic peripheral neuropathic pain: a randomized, double-blind, placebo- and active comparator-controlled, adaptive proof-of-concept phase 2 study. Diabetes Care 37 (2014): 3253-3261.
- Sabha M, Di Maio U, Bagnulo A, et al. Effect of Zenasinil® Spray in Patient with Upper Respiratory Tract Diseases (2025).
- Falvo OF, Di Maio U, Bagnulo A, et al. Effectiveness and Safety of Zenasinil® Nasal Drops in Patients with Upper Airways Inflammation. Biomed J Sci & Tech Res 63 (2025): 2025.
- Camerlingo A, U Di Maio, A Cerciello. Pain relief effect of Crackdol® fast foam in osteoarticular "J Clin Stud Med Case Rep 193 (2023): 2.
- Di Maio, Umberto, Antonino Bagnulo, and Andrea Cerciello. COLPLATIR® as a Novel Therapeutic Adjuvant for the Management of Irritable Bowel Syndrome (IBS). Journal ISSN 2766 (2023):
- Karedath J, Batool S, Arshad A, et The Impact of Vitamin B12 Supplementation on Clinical Outcomes in Patients With Diabetic Neuropathy: A Meta-Analysis of Randomized Controlled Trials. Cureus 14 (2022): e31783.
- Abdel-Daim MM, Shaaban Ali M, et al. Oral Spirulina Platensis Attenuates Hyperglycemia and Exhibits Antinociceptive Effect in Streptozotocin-Induced Diabetic Neuropathy Rat J Pain Res 13 (2020): 2289-2296.
- Zhang FF, Morioka N, Kitamura T, et al. Lycopene ameliorates neuropathic pain by upregulating spinal astrocytic connexin 43 Life Sci 155 (2016): 116-122.
- Kıraç Is ethics approval necessary for all trials? A clear but not certain process. Mol Imaging Radionucl Ther; Volume 22 (2013): 73-75.
- Ismail Issues and challenges in diabetic neuropathy management: A narrative review. World J Diabetes 14 (2023): 741-757.
- Feldman EL, Nave KA, Jensen TS, et al. New Horizons in Diabetic Neuropathy: Mechanisms, Bioenergetics, and Neuron 93 (2017): 1296-1313.
- Liu YP, Shao SJ, Guo Schwann cells apoptosis is induced by high glucose in diabetic peripheral neuropathy. Life Sci 248 (2020): 117459.
- Herman WH, Pop-Busui R, Braffett BH, et Use of the Michigan Neuropathy Screening Instrument as a measure of distal symmetrical peripheral neuropathy in Type 1 diabetes: results from the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications. Diabet Med 29 (2012): 937-944.
- Graham RC, Hughes Clinimetric properties of a walking scale in peripheral neuropathy. J Neurol Neurosurg Psychiatry 77 (2006): 977-979.
- Takemoto S, Ushijima K, Honda K, et al. Precise evaluation of chemotherapy-induced peripheral neuropathy using the visual analogue scale: a quantitative and comparative analysis of neuropathy occurring with paclitaxel-carboplatin and docetaxel-carboplatin Int J Clin Oncol 17 (2012): 367-372.
- Sayedali E, Yalin AE, Yalin Association between metformin and vitamin B12 deficiency in patients with type 2 diabetes. World J Diabetes 14 (2023): 585-593.



 Impact Factor: * 5.3
Impact Factor: * 5.3 Acceptance Rate: 75.63%
Acceptance Rate: 75.63%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 
