A Case of Saccharomyces cerevisiae Blood Infection in a Full-Term Newborn Unrelated to Feeding
Lupande Mwenebitu David MD, MSc, PhD1,2,3*, Eric Hendwa BSc1, Kulimushi Eliezer MD4, Mulumeoderhwa Kahasha Pierrot MD5, Mbusa Kambale Richard MD, MSc, PhD4,6
1Service of Laboratory, Microbiology Unit, Hôpital Provincial Général de Référence de Bukavu, P.O Box 285, Bukavu, Democratic Republic of Congo
2Laboratory Department, Center for Tropical Diseases and Global Health (CTDGH), Faculty of Medicine, Université Catholique de Bukavu, P.O Box 285, Bukavu, Democratic Republic of Congo
3Global Health and Infectious Diseases Research Center (GHIDRC), Institut Supérieur des Techniques Médicales de Bukavu, Bukavu, Democratic Republic of Congo
4Department of Paediatry, Service of Neonatology, Hôpital Provincial Général de Référence de Bukavu, Bukavu, P.O Box 285, Democratic Republic of Congo
5Department of Pathology, Hôpital Provincial Général de Référence de Bukavu, P.O Box 285, Bukavu, Democratic Republic of Congo
6Section of Midwifery Science, Institut Supérieur des Techniques Médicales de Bukavu, Bukavu, Democratic Republic of Congo
*Corresponding Author: Lupande Mwenebitu David, Service of Laboratory, Microbiology Unit, Hôpital Provincial Général de Référence de Bukavu, P.O Box 285, Bukavu, Democratic Republic of Congo.
Received: 20 August 2025; Accepted: 02 September 2025; Published: 10 October 2025
Article Information
Citation: Lupande Mwenebitu David, Eric Hendwa, Kulimushi Eliezer, Mulumeoderhwa Kahasha Pierrot, Mbusa Kambale Richard. A Case of Saccharomyces cerevisiae Blood Infection in a Full-Term Newborn Unrelated to Feeding. Archives of Clinical and Medical Case Reports. 9 (2025): 190-192.
View / Download Pdf Share at FacebookAbstract
Introduction: Cerevisiae is a probiotic micro-organism whose use in food is known to have beneficial effects on digestion and several gastrointestinal disorders. However, while probiotics are commonly used, they can be dangerous for certain people, especially those with immuno-compromised systems, who can develop fungemia and subsequently die. This case report describes a case of fungemia in a newborn baby that was not diet-related (lactation).
Case presentation: We present for the first time a case of S. cerevisiae fungemia isolated in a blood culture from a two-hours-old newborn who presented with vomiting and a disturbance of vital and biological signs immediately after taking formula milk powder containing no probiotics (Nestlé NAN 1). Despite this less severe clinical picture, the symptoms disappeared rapidly after three days, without the need to administer antifungal agents. The isolated S. cerevisiae was probably wild type, as it was sensitive to the antifungal agents tested, and the symptoms resolved after five days, with the baby being discharged from hospital on day 7.
Conclusion: The risk of S. cerevisiae fungemia remains present in all newborns with signs of infection, whether they are on an S. cerevisiaebased diet. Transmission of S. cerevisiae that is not diet-related, remains a real challenge, and several studies need to be carried out to elucidate its involvement in fungemia.
Keywords
<p><em>Saccharomyces cerevisiae</em>; Fungemia; Newborn; Bukavu</p>
Article Details
1. Introduction
Saccharomyces cerevisiae (S. cerevisiae) is a budding yeast that is generally considered safe for human consumption, having been used in food for many years. It is used in the agro-food industry, particularly in the production and composition of fermented foods (bread) and beverages (beer, wine, spirits), as well as biofuel. It is also a real cell factory, producing many important pharmaceutical and biochemical compounds [1,2]. There is evidence to date that Saccharomyces boulardii is an asporogenic strain of S. cerevisiae, with moderate virulence demonstrated in mouse models of systemic infection. S. boulardii is currently used in several food formulations for newborns and infants [3,4]. We report here a case of S. cerevisiae fungemia in a newborn without a direct link to a Saccharomyces boulardii-based diet.
2. Case Presentation
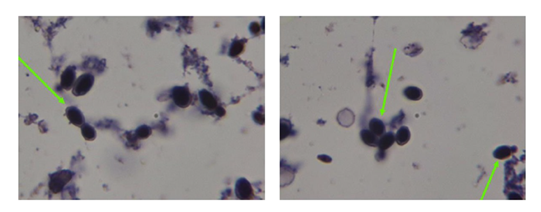
This was a female newborn, born at full term (37 weeks), admitted to the neonatology department of the Hôpital Provincial Général de Référence de Bukavu (HPGRB), Bukavu, in the east of the Democratic Republic of Congo, for vomiting two hours after taking powdered artificial milk (Nestlé NAN1, Switzerland) because her mother did not know how to breastfeed her. Her birth weight was 2690g. She was treated with ondasetron, and her digestive tract was rested for 48 hours. Enteral nutrition was started on the third day of life in conjunction with parenteral nutrition using an infusion of G10% combined with calcium gluconate. As a baseline biological investigation, we had procalcitonin at 0.85 ng/dl, complete blood count within normal limits, blood cultures were indicated after initiating levofloxacin and amikacin treatment. Vomiting improved. Control procalcitonin one day after: 0.21ng/l. All the clinical and biological data indicated an improvement. Blood cultures taken from Render bottles (Render Biotech. Co, Shenzhen-China) grew on fresh blood agar after 72 hours of incubation ((on the 5th day after the onset of symptoms), with Gram staining showing images suggestive of yeast (Figure 1). Using the Render MA-120 Automated Identification System (Render Biotech. Co, Shenzhen-China), we were able to identify S. cerevisiae, which was sensitive to many anti-fungal agents, as shown in Table 1 (Render MA-120 offer minimum inhibitory concentration results regarding EUCAST/CLSI standards).
The diagnosis of fungemia was formally made 5 days after the onset of symptoms, during which time the newborn had already been discharged from hospital. Data from the Neonatology inpatient case follow-up register showed that the clinical and biological evolution had been favourable after the first 48 hours, which is why the baby was discharged from hospital with a follow-up appointment scheduled for 2 weeks later. Two weeks later, the newborn was seen again in consultation, with a good evolution, and was already in perfect health.
Table 1: Susceptibility data on S. cerevisiae MIC Drugs.
|
Drugs |
MIC (µg/mL) |
Profile |
|
Flucytosine |
≤ 2 |
susceptible |
|
Amphotericin |
= 0,5 |
susceptible |
|
Captofungin |
≤ 0,12 |
susceptible |
|
Fluconazole |
≤ 2 |
susceptible |
|
Itraconazole |
≤ 0,06 |
susceptible |
|
Variconazole |
≤ 0,12 |
susceptible |
MIC: Minimum inhibitory concentration
3. Discussion
Increasing numbers of S. cerevisiae bloodstream infections have been reported, with weakened immune systems and the use of probiotics containing S. cerevisiae (S. boulardii) as the most common explanatory factors [4-7] Infants and children are particularly vulnerable to fungemia in general and S. cerevisiae fungemia, as their immune systems are fragile and immature [8].
Curiously, although it is well documented that fungal infections often have a very high mortality rate, our newborn's clinical picture was not severe, and he progressed well over the next few days [6] As S. cerevisiae is present both in the environment and in certain foods, including certain milk formulations, the origin of the contamination was not found in our newborn and no probiotics were used in his diet. The aggressiveness of the gastric acid on S. cerevisiae further strengthens the rejection of a probable hypothesis of contamination via the digestive tract. To find its way into the bloodstream, S. cerevisiae must have taken another relatively easy route, the pulmonary route, which cannot be ruled out after these vomiting episodes, given the role of the aerodigestive crossroads [9].
Our isolate was sensitive to the antifungal drugs tested, suggesting that it was probably of wild origin, with relatively low minimum inhibitory concentration (MICs). This led us to believe that we were dealing with diploid chromosomes, especially as it had been shown in the experiment by Anderson James et al. that diploidy is less prone to resistance than haploidy [10]. Genomic characterization of the conserved strain will provide further details soon.
As major limitations of our observation, we were unable to completely rule out the possibility of contamination during sampling in the neonatal unit or during laboratory analysis, given that S. cerevisiae can be found in both the newborn's and the mother's environment. We were also unable to access the milk consumed by the baby before the onset of symptoms to search for pathogens, including S. cerevisiae. Furthermore, the mother's history of dysimmunities (e.g. HIV) had not been documented.
4. Conclusion
cerevisiae, which is well known in the food industry, can also cause devastating clinical symptoms, so it should be used with caution in immuno-compromised patient, but its presence should now be suspected in these fragile people when faced with infectious symptoms in our environment, especially as we can now identify it well with our RENDER MA-120 automatic identification system, because with conventional identification methods many fungi remain unknown despite clinical symptoms and isolation on agar or broth.
Ethics approval and consent to participate
The anonymous biological analyses reported in this study are an integral part of the routine monitoring of blood cultures as part of hospital hygiene and were recorded retrospectively following the requirements of the Declaration of Helsinki. Informed consent could not be obtained.
Clinical trial number:
Not applicable.
Consent for publication
Retrospective and recorded anonymous data, we can’t get consent for publication.
Data availability
Materials and data provided in this case report are available from the corresponding author on reasonable request.
Conflicts of Interest
All authors have no conflicts of interest to declare.
Funding
None to declare
Author Contributions
EH, D.L.-M, drafted the manuscript. Writing, review and editing, D.L.-M; R.K.-M, EH, SN, EK, P.M.-K, G.M.-B supervision, D.L-M; All authors have read and agreed to the published version of the manuscript.
Acknowledgments
My thanks to the nurses of the Paediatric Department and the lab Tech team at Hôpital Provincial Général de Référence de Bukavu for helping with clinical information and collection of the isolate.
References
- Belda I, Ruiz J, Santos A, et al. Saccharomyces cerevisiae. Trends Genet 35 (2019): 956-957.
- Parapouli M, Vasileiadis A, Afendra AS, et al. Saccharomyces cerevisiae and its industrial applications. AIMS Microbiol 6 (2020): 1-31.
- McCullough MJ, Clemons KV, McCusker JH, et al. Species Identification and Virulence Attributes of Saccharomyces boulardii (nom. inval.). J Clin Microbiol 36 (1988): 2613-2617.
- Perapoch J, Planes AM, Querol A, et al. Fungemia with Saccharomyces cerevisiae in two newborns, only one of whom had been treated with ultra-levura. Eur J Clin Microbiol Infect Dis 19 (2000): 468-470.
- Diop C, Descy J, Sacheli R, et al. Saccharomyces cerevisiae fungemias: how heterogenous is their management? Diagn Microbiol Infect Dis 109 (2024): 116343.
- Muñoz P, Bouza E, Cuenca-Estrella M, et al. Saccharomyces cerevisiae fungemia: an emerging infectious disease. Clin Infect Dis 40 (2005): 1625-1634.
- Poncelet A, Ruelle L, Konopnicki D, et al. Saccharomyces cerevisiae fungemia: Risk factors, outcome and links with S. boulardii-containing probiotic administration. Infect Dis Now 51 (2021): 293-295.
- Basha S, Surendran N, Pichichero M. Immune responses in neonates. Expert Rev Clin Immunol 9 (2014): 1171-1184.
- Edwards-Ingram L, Gitsham P, Burton N, et al. Genotypic and physiological characterization of Saccharomyces boulardii, the probiotic strain of Saccharomyces cerevisiae. Appl Environ Microbiol 73 (2007): 2458-2467.
- Anderson JB, Sirjusingh C, Parsons AB, et al. Mode of Selection and Experimental Evolution of Antifungal Drug Resistance in Saccharomyces cerevisiae. Genetics 163 (2003): 1287–1298.


 Impact Factor: * 5.3
Impact Factor: * 5.3 Acceptance Rate: 75.63%
Acceptance Rate: 75.63%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 
