Association Between Lipid Profile and The Clinical Course of Influenza
Maria Lagadinou1,2, Eleni Polyzou1, Christos Michailides1, Marina Amerali1, Ioanna Marlafeka1, Leonidia Leonidou1,2,3, Dionysios Chartoumpekis2,4, Markos Marangos1,2,3*
1Department of Internal Medicine, University Hospital of Patras, 265 04 Patras, Greece
2Medical School of Patras, University of Patras, 265 04 Patras, Greece
3Division of Infectious Diseases, University Hospital of Patras, 265 04 Patras, Greece
4Department of Endocrinology, University Hospital of Patras, 265 04 Patras, Greece
*Corresponding Author: Prof. Markos Marangos, Department of Internal Medicine, University Hospital of Patras, 265 04 Patras, Greece
Received: 14 July 2025; Accepted: 22 July 2025; Published: 13 October 2025
Article Information
Citation: Maria Lagadinou, Eleni Polyzou, Christos Michailides, Marina Amerali, Ioanna Marlafeka, Leonidia Leonidou, Dionysios Chartoumpekis, Markos Marangos. Association Between Lipid Profile and The Clinical Course of Influenza. Archives of Clinical and Medical Case Reports. 9 (2025): 201-209.
View / Download Pdf Share at FacebookAbstract
Background: Influenza remains a major cause of morbidity and mortality worldwide. Identifying biomarkers associated with disease severity could improve risk stratification and patient management.
Aim: The aim of this study was to investigate the association between lipid profile and the clinical outcomes of influenza infection, especially among adults who were either admitted to the hospital or were treated as outpatients after being diagnosed with influenza in the emergency department (ED).
Results: The median age of the total cohort was 59.9 ± 20.7 years, with 47.8% being male and 52.2% female. Patients in the hospitalized group were significantly older than those managed as outpatients (70.2 ± 15.6 vs 40.1 ± 23.1 years, p=0.002). Among all lipid parameters, only Low-Density Lipoprotein (LDL) levels were significantly lower in hospitalized patients compared to outpatients (76.6 ± 30.3 vs 98.4 ± 22.6 mg/dL, p=0.038). To assess the impact of treatment, lipid values measured upon admission were compared with those recorded after clinical recovery. No significant changes were detected in any lipid marker, including Total Cholesterol (TC), LDL, High-Density Lipoprotein (HDL), and Triglycerides (TG), indicating that the treatment course did not substantially alter lipid levels. No significant correlations were identified between White Blood Count (WBC) and TC, HDL, LDL, or TG, with Pearson correlation coefficients ranging from -0.08 to 0.17. When lipid levels were analyzed in relation to patient age, TG demonstrated a weak positive correlation with age (r=+0.08). Triglycerides were the best severity predictor (AUC=0.87), followed by C-Reactive Protein (CRP) (AUC=0.85). A comparison between the influenza cohort and 32 healthy control subjects revealed significantly lower levels of TC, HDL, and LDL among patients with influenza (all p < 0.001 for TC and LDL, and p=0.002 for HDL). Triglyceride levels did not differ significantly between the two groups (p=0.681).
Conclusion: LDL shows a consistent inverse correlation with hospitalization and age. Triglycerides have high discriminatory ability for severe influenza infection. These findings highlight the potential role of lipid profile -particularly LDL and TG—as indicators of disease severity and proliferated immune response in influenza infection. The observed differences warrant further investigation in larger and more diverse patient populations.
Keywords
<p>Influenza; Severity; Lipid profile; Prognostic factors</p>
Article Details
1. Introduction
Among viral respiratory pathogens, influenza remains a leading cause of morbidity and mortality worldwide. This persistent public health burden highlights the urgent need for improved strategies to predict disease severity and reduce adverse outcomes [1,2]. An emerging area of interest is lipid profiling, which reflects underlying processes in inflammation, immune regulation, and metabolic homeostasis. Lipid profiling has gained attention as a promising and rapidly evolving research tool that may offer valuable insights into the pathogenesis of high-priority respiratory infections. Notably, distinct lipid clusters in human sputum have been associated with specific viral pathogens such as Influenza A (H1N1, H3N2) and Rhinovirus [3].
Recent research has increasingly focused on the role of lipids in modulating host–pathogen interactions and shaping the immune response. Although lipids have long been recognized as key players in the pathophysiology of lung infections, the diversity and complexity of lipid species have historically limited a detailed understanding of their precise functions. Advances in analytical techniques have begun to address these challenges, enabling deeper exploration into the roles of specific lipid molecules [3].
For instance, Morita et al. demonstrated that lipid mediators produced in the respiratory tract during infection contribute to both pro- and anti-inflammatory responses. When inflammation becomes excessive and viral replication is uncontrolled, disease severity increases. Lipidomic analyses can capture global changes in lipid metabolites associated with severe infection and inflammation, potentially leading to the identification of novel biomarkers for pulmonary disease [1].
The objective of the present study was to identify lipid parameters associated with the severity of influenza infection, particularly in adult patients who were either hospitalized or treated as outpatients after being diagnosed with influenza in the emergency department.
2. Patients and Methods
We conducted a single-center case-control study involving patients diagnosed with influenza. A total of 37 patients were enrolled between December 2024 and March 2025 at the General University Hospital of Patras. The exclusion criteria were: (1) pregnancy, (2) age under 18 years, (3) severe medical conditions such as malignancy, acquired immunodeficiency syndrome (AIDS), or liver cirrhosis, (4) current use of lipid-lowering therapy. Additionally, 32 healthy individuals with documented lipid profile abnormalities were included as a control group. These participants were matched to influenza patients based on age, gender, and comorbidities.
The Research Ethical Committee of the hospital granted approval to waive the requirement for informed consent, considering the observational, non-interventional nature of the study.
2.1 Laboratory Analysis
Influenza was confirmed using a commercially available rapid antigen test combined with the clinical sample collection, processing, and laboratory testing which were based on WHO guidelines. Blood samples were obtained upon each patient´s admission in the emergency department. All samples were processed in the same manner, namely centrifuged for 10 minutes and measured during the first three hours.
2.2 Lipid profile determination
Blood samples were collected upon admission, and a lipid panel was performed, including measurements of TC, HDL, LDL, and TG. Lipid levels were analyzed using an immunoanalytical method on the Roche/Hitachi Cobas c501 automated analyzer. Comparisons of lipid levels were made between severity groups (hospitalized vs. outpatient), as well as before and after treatment, particularly in hospitalized patients. All patients received treatment with antibiotics and oseltamivir, in accordance with international guidelines for the management of influenza-related lung infections and their complications and our hospital’s population-based guidelines. Additionally, potential correlations between lipid profiles and clinical or laboratory parameters—such as age, WBC count, CRP—were evaluated.
2.3 Statistical Analysis
Statistical analysis was conducted using SPSS version 25 (IBM SPSS Statistics 25). Normally distributed continuous data were presented as mean ± standard deviation (SD). A p-value of less than 0.05 was considered statistically significant. Pearson’s correlation coefficient was employed to examine relationships between variables, while the Mann–Whitney U test was used to assess differences between independent groups.
3. Results
3.1 Lipid Levels in hospitalized and outpatients
A total of 37 Influenza patients were included in the study. Based on disease severity, patients were divided into two groups: hospitalized (Group 1) and non-hospitalized (Group 2, outpatients). The median age of all patients was 59.9 ± 20.7 years old. 47.8% were men and 52.2% were women. The median age of the hospitalized cases was significantly higher than moderate ones (70.2 ± 15.6 vs 40.1 ± 23.1 years old, p= 0.002). The most common symptoms upon admission were fever, cough and dyspnea.
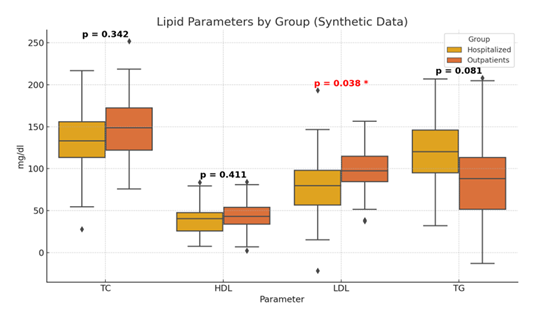
We examined the plasma levels of TC, HDL, LDL, TG, CRP, and WBC before and after treatment. Analysis of all patients revealed that LDL levels were significantly lower in hospitalized patients compared to outpatients (75.2 ± 29.9 vs 98.4 ± 22.6, p=0.03). No other lipid parameters showed statistically significant differences between groups. All the above-mentioned measurements are shown in Table 1 and Figure 1.
Table 1: Lipid measurements in hospitalized patients vs outpatients.
|
Parameter |
Hospitalized |
Outpatients |
p value |
|
TC mg/dl |
138.6 ± 42.3 |
151.2 ±32.7 |
0.342 |
|
HDL mg/dl |
38.9± 16.4 |
45± 17.3 |
0.411 |
|
LDL mg/dl |
76.6 ±30.3 |
98.4 ±22.6 |
0.038 |
|
TG mg/dl |
118.1± 40.6 |
84.7 ±46.9 |
0.081 |
3.2 Lipid Levels before and after recovery in hospitalized patients
To evaluate the impact of treatment on lipid levels, further analysis was performed between lipid values measured on admission and after clinical recovery (hospital discharge). No statistically significant differences were observed in TC, LDL, HDL, or TG values before and after treatment. All measurements are shown in Table 2.
Table 2: Lipid Levels: Before vs After Treatment.
|
Parameter |
Before |
After |
p-value |
|
TC mg/dl |
162.4± 36.3 |
162.1± 23 |
0.967 |
|
LDL |
96.2 ±31 |
98.5± 21.8 |
0.667 |
|
HDL |
44.±20.3 |
41.8± 14.3 |
0.588 |
|
TG |
108.7 ±48.2 |
114.6± 41.8 |
0.438 |
3.3 Correlation Between Lipid Levels and WBC upon admission
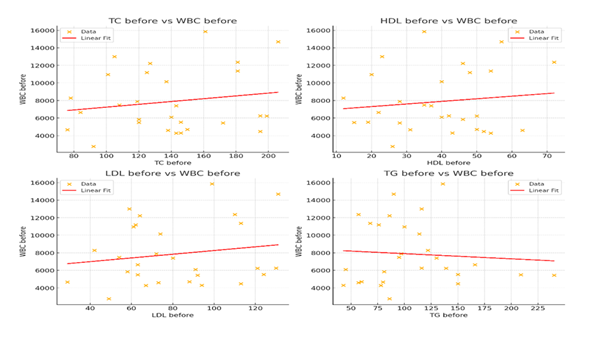
We further examined the correlation between lipid Levels and WBC upon admission to all patients enrolled in this study. No statistically significant correlations were found between TC, HDL, LDL, TG and WBC (Pearson correlation 0.17, 0.13, 0.17, and -0.08 respectively). Scatterplots confirmed the weak relationships, showing no clear linear trend. (Figure 2).
No significant correlations were observed between WBC and TC, LDL, HDL, or TG levels. The scatterplots reveal no clear linear trends, confirming the absence of association between lipid markers and WBC count. Abbreviations: TC: Total Cholesterol, HDL: High Density Lipoprotein, LDL: Low Density Lipoprotein, TG: Triglycerides, WBC: White Blood Cells.
3.4 Correlation Between Lipid Levels and CRP
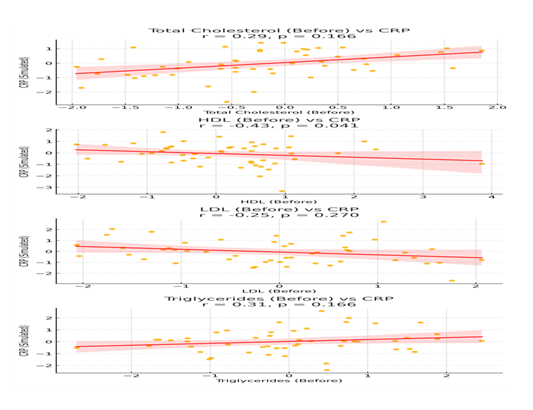
We further examined the correlation between Lipid Levels and CRP upon admission to all patients enrolled in the study. Strong positive correlations were observed between CRP and TC before treatment. All correlations are shown in Table 3 and Figure 3.
Table 3: Correlation between lipid levels and CRP upon admission.
|
Parameter |
Pearson Correlation with CRP |
p-value |
|
Total Cholesterol mg/dl |
0.29 (weak positive) |
0.166 |
|
HDL (before) mg/dl |
-0.43 (moderate negative) |
0.041 |
|
LDL (before) mg/dl |
-0.246 (moderate negative) |
0.270 |
|
Triglycerides (mg/dl) |
+0.306 (moderate positive) |
0.166 |
The scatterplots reveal positive linear trends, between TC, Triglycerides and CRP. Abbreviations: TC: Total Cholesterol, HDL: High Density Lipoprotein, LDL: Low Density Lipoprotein, TG: Triglycerides, WBC: White Blood Cells.
3.5 Correlation between Lipid Levels and age
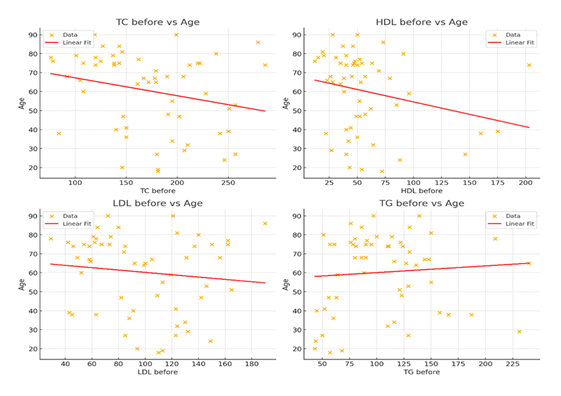
We also analyzed the relationship between age and several lipid parameters upon admission. Triglycerides show a weak positive trend with older individuals tending to have higher TG levels. HDL (High-Density Lipoprotein) demonstrates a moderate negative correlation. Table 4 and Figure 4 show the Pearson correlation coefficients.
Figure 4: Scatterplots showing the relationship between age and lipid profile. Each plot includes a linear regression line to demonstrate the trend between age and individual lipid values. Triglycerides (TG) show a positive correlation with age, while TC and HDL exhibit a strong and moderate negative correlation, retrospectively. LDL shows weak trends.
Table 4: Pearson Correlation between age and lipid profile upon admission.
|
Lipid parameter |
Pearson Correlation with age |
P values |
|
TC before (mg/dl) |
-0.25 (strong negative) |
0.056 |
|
HDL before (mg/dl) |
-0.24 (moderate negative) |
0.063 |
|
LDL before (mg/dl) |
-0.12 (weak negative) |
0.384 |
|
TG before (mg/dl) |
+0.08 (weak positive) |
0.75 |
3.6 Predictive Value for Hospitalization
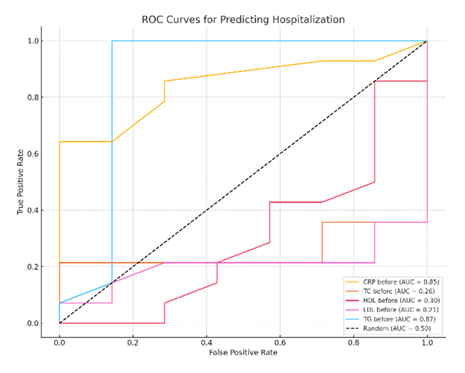
The evaluation of the Area Under the Receiver Operating Characteristic Curve (AU-ROC) further substantiated the discriminatory power of lipid parameters and CRP in predicting hospitalization. Among the biomarkers assessed, TG exhibited the highest prognostic accuracy (AUC=0.87), indicating very good predictive ability. CRP also showed strong discriminatory capacity (AUC=0.85). In contrast, other lipid markers, including TC, LDL, HDL, demonstrated poor predictive performance, with AUC values of 0.26, 0.30, and 0.21, respectively (Figure 5). In the severity context (hospitalization), TGs had the highest prognostic ability (AUC=0.87, very good), CRP had comparable predictive value (AUC=0.85) while other lipid markers had low AUC. The AUC values for TC, LDL, HDL were 0.26, 0.30, and 0.21, respectively, mentioning a poor predictive value (Figure 5).
The diagnostic performance of CRP and lipid profile components (TC, LDL, HDL, TG) is illustrated. TGs demonstrated the highest area under the curve (AUC=0.87), followed by CRP (AUC=0.85). TC, LDL, and HDL had poor discriminatory value.
3.7 Comparison of Lipid Levels with Control group
A total of 32 healthy participants were included in the study, comprising controls (n=32) as well. They were compared in terms of lipid profile values before treatment with influenza group. Statistically significant differences were observed in the following pre-treatment lipid parameters: TC: Controls had significantly higher TC levels compared to influenza patients (207.8 ± SD vs 141.1 ± SD, p <0.001), HDL: controls also had higher HDL levels (66.6 ± 39.3 vs 41.4 ± 16.6, p=0.002). Similarly, LDL levels were significantly higher in controls (124.2 ± 35.7 vs 82.2 ± 29.2, p <0.001). TG did not differ significantly between groups (103 ± 61.6 vs 109 ± 44.3, p=0.681). All differences are shown in Table 5.
Table 5: Comparison of lipid levels between influenza patients and control group.
|
Parameter |
Control Group |
Influenza Patients |
p-value |
|
Total Cholesterol (TC) mg/dl |
207.8 |
141.1 |
< 0.001 |
|
High-Density Lipoprotein (HDL) mg/dl |
66.6 ± 39.3 |
41.4 ± 16.6 |
0.002 |
|
Low-Density Lipoprotein (LDL) mg/dl |
124.2 ± 35.7 |
82.2 ± 29.2 |
< 0.001 |
|
Triglycerides (TG) mg/dl |
103 ± 61.6 |
109 ± 44.3 |
0.681 |
4. Discussion
Influenza continues to pose a substantial public health burden, with seasonal outbreaks and periodic pandemics leading to significant morbidity and mortality [1]. It results in a variety of clinical symptoms, usually mild and self-limiting, such as fatigue, sore throat, fever and runny nose. Laboratory investigations, such as the complete blood count and inflammatory biomarkers, are frequently used to assess and define the severity of influenza infection. However, no distinct laboratory pattern has been shown to be specifically linked to the severity of influenza. Even more, no study has yet examined LDL/TG in adult influenza patients. This study provides a comprehensive analysis of the impact of demographic and lipid-related factors on the severity and of patients with influenza.
In our study, age emerged as a key risk factor for hospitalization, consistent with previous literature indicating worse outcomes among older adults. Coleman et al. reported that young adults were significantly less likely to be admitted to hospital compared to older adults in both high-income countries and low-middle income countries and in both pandemic and non- pandemic seasons [4].
This study offers a focused analysis of the association between lipid parameters and influenza severity in a well-defined cohort of adult patients. Overall, we observed statistically significant decreases in total cholesterol, HDL-C, and LDL-C levels in influenza patients compared to the control group. These findings are consistent with the existing literature. It is well established that patients infected from a variety of causative microorganisms including gram-positive and gram-negative bacteria, viruses, tuberculosis, and parasites—exhibit similar alterations in plasma lipid profiles. Specifically, total cholesterol, LDL-C, and HDL-C levels tend to decrease, while plasma triglyceride levels are often elevated or remain inappropriately normal, considering the patients’ typically poor nutritional status [5].
Furthermore, our findings suggest that LDL cholesterol levels are significantly lower in hospitalized patients, supporting a possible inverse relationship between LDL and disease severity. Although the common lipid alterations include a decrease in total cholesterol (TC) levels and an increase in the concentration of triglyceride-rich lipoproteins, when comparing the lipid profiles of influenza patients between hospitalized and outpatient groups, no significant differences in total cholesterol (TC), high density lipoprotein (HDL) and triglycerides levels (TG) were found in our study. Especially for triglycerides we found higher triglycerides in hospitalised patients compared to outpatients. This may be even more clearly interpreted considering the findings by Zhai et al., who demonstrated that hypertriglyceridemia in severe influenza is closely associated with multiple inflammatory mediators such as plasma levels of interferon-γ, interleukin-18, and interleukin-1 receptor antagonist [6]. Additionally, no statistically significant differences were found in lipid profile before and after influenza treatment. Lipids have a close relationship with lung biology and the pathophysiology of viral infection, but few studies have focused on alterations in lipid profiles in the context of viral pneumonia [7].
Our data regarding LDL levels are consistent with reports from COVID-19 research, a comparable viral respiratory illness. Both Zhang et al. and Chen Qin et al. demonstrated that LDL cholesterol was significantly lower in patients with severe COVID-19 compared to those with milder presentation [7,8]. This suggests some potential shared pathophysiological mechanism across viral infections, possibly related to systemic inflammation or viral exploitation of host lipid pathways.
Our results align with prior studies demonstrating that acute infections can disrupt lipid metabolism, although the direction and magnitude of these changes can vary. For instance, Filippas-Ntekouan et al. noted that acute infections commonly induce a decrease in TC and HDL, and an increase in TG levels, yet our findings showed only partial concordance with this pattern. All lipid parameters are significantly deranged during acute infection [9].
HDL levels decrease with age, which is generally considered unfavorable for cardiovascular health. In the present study, it is noteworthy the decrease in median values in HDL we observed in severe cases compared to non-severe ones (hospitalized vs outpatients) regardless of age, although not statistically significant. This trend may reflect infection-driven functional changes, as Van Lenten et al. reported that HDL loses its anti-inflammatory properties during acute influenza A infection [10].
The present study also evaluated the ability of lipid profile variables to predict influenza severity. Our study found that only triglycerides had moderate discriminatory ability, with a very good AUC of 0.87, like CRP (0.85). In a study conducted by Ochoa-Ramirez et al. which included COVID-19 patients, it was found that all lipid profile components had moderate predictive ability with AUC values ranging from 0.79 to 0.89 [11].
Additionally, combining the advanced age of patients hospitalized with influenza and the high diagnostic value of triglycerides, we investigated a strong positive correlation between these factors suggesting that age-related changes in lipid metabolism may contribute to disease vulnerability. This aligns with existing knowledge suggesting that plasma TG metabolism itself is altered by age in humans and rodents [12]. Aging is marked by a chronic, low-grade inflammatory state even in the absence of infection, and this condition is recognized as a significant risk factor for morbidity and mortality in elderly [13]. This inflammation is closely linked to adipose tissue dysfunction and serves as a key connection between obesity and dyslipidemia in the aging population [12].
It is important to note that hypolipidemia affects various body systems, including the immune system, where it contributes to both activation and regulation. Reduced lipid levels may be associated with diminished immune system activation resulting in infection with increased severity and morbidity [14]. This may partially explain the decreased levels of Total Cholesterol TC), HDL and LDL we found in hospitalized patients compared to those treated in an outpatient setting.
While this study highlights important associations between lipid profile abnormalities and influenza severity, making a meaningful contribution to the existing body of literature, it has also some limitations. Firstly, it is a single-center study with a small sample size which may limit the generalizability of our findings. Secondly, the lack of control groups introduces potential biases and limits the generalizability of the results. Third, potential confounding factors such as undiagnosed metabolic conditions or diet could not be fully accounted for. Another limitation of the study is that there was no detailed analysis of the content of cholesterol and triglycerides in the various lipoprotein subclasses or any functional assay specifically for HDL. The non-continuous sampling and missing data due to extreme workload which disrupted the data collection is also a limitation. The inability to collect data regarding somatometric characteristics such as BMI and to count adipose tissue and inflammation markers enhances the lack of connections. The unavailability of PCR testing for all patients who presented with influenza-like symptoms also decreased our patients’ volume.
To strengthen future research with larger and more diverse patient populations and appropriate control groups is needed. The use of advanced statistical methods to account for confounding variables, along with mechanistic studies, will help refine our understanding and support the development of targeted interventions, ultimately enhancing the global scientific relevance of this field.
In conclusion, LDL cholesterol demonstrated a consistent inverse association with hospitalization and age in patients with influenza. Although other lipid markers did not reach statistical significance, triglycerides showed moderate predictive value for severe disease. These findings underscore the potential utility of lipid profiling in risk stratification for influenza and highlight the need for further investigation in larger, multi-center studies. Understanding lipid-related pathways in viral infections may also offer novel targets for therapeutic intervention.
References
- Uppala R, et al. Clinical characteristics and prognostic markers for hospitalization in children with influenza infection in an emergency setting. BMC Pediatrics 24 (2024): 399.
- García-Sastre A. Lessons from lipids in the fight against influenza. Cell 154 (2013): 14-15.
- Humes ST, Iovine N, et al. Association between lipid profiles and viral respiratory infections in human sputum samples. Respiratory Research 23 (2022): 177.
- Coleman BL, et al. Hospitalizations for influenza among young and older adults in high- and low-income countries. Influenza and Other Respiratory Viruses 12 (2018): 22-29.
- Feingold Kenneth R., and Grunfeld Carl, The Effect of Inflammation and Infection on Lipids and Lipoproteins, National Institute of Health (2022).
- Zhai T, Wu X, Nannan Xu Z, et al. Inflammatory risk factors for hypertriglyceridemia in patients with severe influenza, Journal of International Medical Research 48 (2020): 1-13.
- Qin C, et al. Alteration of lipid profile and value of lipids in the prediction of the length of hospital stay in COVID-19 pneumonia patients. Food Science & Nutrition 8 (2020): 6144-6152.
- Zhang K, et al. Causal associations of blood lipid levels with COVID-19 risk: A Mendelian randomization study. medRxiv [Preprint]. (2020). doi:10.21203/rs.3.rs-86425/v1.
- Filippas-Ntekouan S, Liberopoulos E, Elisaf M. Lipid testing in infectious diseases: Possible role in diagnosis and prognosis. Infection 45 (2017): 575-588.
- Van Lenten BJ, Wagner AC, Nayak DP, et al. High-density lipoprotein loses its anti-inflammatory properties during acute influenza A infection. Circulation 103 (2001): 2283-2288.
- Ochoa-Ramírez LA, et al. Association between lipid profile and clinical outcomes in COVID-19 patients. Scientific Reports 14 (2024): 12139.
- Spitler KM, Davies BSJ. The role of lipids in host–pathogen interactions. Journal of Lipid Research 61 (2020): 1370-1382.
- Franceschi C, Garagnani P, Parini P, Giuliani C, Santoro A. Inflammaging: A new immune–metabolic viewpoint for age-related diseases. Nature Reviews Endocrinology 14 (2018): 576-590.
- Qasim A, et al. The role of hypolipidemia in the immune response to infections. Journal of Clinical and Translational Research 6 (2020): 198-202.


 Impact Factor: * 5.3
Impact Factor: * 5.3 Acceptance Rate: 75.63%
Acceptance Rate: 75.63%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 
