Colorectal Cancer Screening History, Methods and Future Perspectives
Manasa Anipindi*, Shriya Doreswamy, Rafal Ali, Aysha Jilani and Daniel Bitetto
Einstein Medical Center Montgomery, East Norriton, PA, USA
*Corresponding Author: Manasa Anipindi, Einstein Medical Center Montgomery, East Norriton, PA, USA
Received: 02 May 2023; Accepted: 12 May 2023; Published: 20 May 2023
Article Information
Citation: Manasa Anipindi, Shriya Doreswamy, Rafal Ali, Aysha Jilani, Daniel Bitetto. Colorectal Cancer Screening History, Methods and Future Perspectives. Archives of Clinical and Medical Case Reports. 7 (2023): 216-239.
View / Download Pdf Share at FacebookAbstract
Colorectal cancer [CRC] is the third most diagnosed but preventable cancer worldwide. The guidelines for CRC screening were first introduced formally in the 1990s, and over the past three decades, the screening rate slowly increased. As per the American Cancer Society [ACS], in the year 2020, about 69.7% of adults between 50 and 75 had colorectal cancer screening, with the lowest screening rate in adults between 50 and 64 years of age. The incidence of colorectal cancer is also rising in the younger population worldwide. According to recent American College of Gastroenterology guidelines, the starting age for screening is 45 years instead of 50. The rising incidence and low compliance with screening in the younger population make it hard to improve CRC-related mortality. As per ACS, there is almost 30% of the eligible unscreened population in 2020, so we believe there is a need for effective screening programs worldwide.
We understand that there are modifiable and non-modifiable factors for non-compliance. Some of them are lack of awareness, fear about screening, their previous experience with screenings, and overprescription of Colonoscopies in open or direct access systems. Overprescription of Colonoscopies in open or direct access systems [OAC] can lead to longer wait times due to fewer appointments for people who need them. Implementing patient navigation or tracking systems with reminders can help recruit people for cancer screening, overcome the barriers to accessing clinical services, and provide appropriate counseling. Executing clinical care pathways can help reduce the risks of overprescription. Monitoring practice progress by establishing a baseline screening rate and a future goal is also essential. Developing quality improvement projects around these goals can help discover system deficits and ideas to overcome them. The primary purpose of this review, even though it is not new, is to increase awareness among physicians regarding the rising incidence of CRC in the younger population and the need to increase screening rates. We also believe there is a need for more effective CRC screening tests that are easy to administer, with minimal discomfort to the participant.
Keywords
<p>Colorectal cancer; Colon cancer; Cancer</p>
Article Details
1. Introduction
Cancer is the second leading cause of death worldwide. Colorectal or colon cancer [CRC] is the third most common cancer diagnosed in men and women. It is one of the few preventable cancers worldwide. As per the American Cancer Society, there will be 106 970 new cases in 2023 compared to 106,180 new cases of colon cancer in the year 2022, showing increasing incidence [1]. In 2020, it led to 935,173 deaths worldwide and 37,930 deaths in the United States [2, 3]. The incidence and death rates from Colon cancer in the older population have decreased in recent years due to the wide availability of screening and early polypectomy with the help of Colonoscopy [4, 5]. However, the incidence and death rate has increased in younger people under 50 [6, 7]. CRC incidence increased by 51% in people younger than 50 between 1974 and 2013. Studies showed increasing adenoma detection in people between 45 and 49 years of age, even after excluding family and personal history of polyps and colorectal cancer [8]. Patients between the ages of 40 and 49 have a 14-16% risk of adenoma detection and a 3-6% risk of large polyp detection [9-11]. Some studies advise starting the screening at 40, considering increasing the detection of CRC in these individuals [12, 13]. Younger individuals also risk delayed diagnosis and misdiagnosis due to the focus on more common illnesses by the physicians in this population [14, 15]. Colon cancer has different screening tests, but 31% of the eligible population is not current with their screening, as per 2018 statistics. These statistics are from before the Coronavirus disease of 2019 [COVID-19] pandemic era [16]. One-third of the patients did not get screening, and approximately 46%-63% of deaths due to colorectal cancer are in this unscreened population [17, 18]. During the COVID pandemic, the number screened decreased further [19, 20]. 1.2 million Americans who have Lynch syndrome remain undiagnosed; this syndrome increases the risk for not only colorectal but also for the ovarian, endometrial, small intestine, stomach, urinary bladder, and breast cancer in females, so it is essential to diagnose this syndrome earlier to avoid unnecessary morbidity and mortality [21-24]. National Comprehensive Cancer Network [NCCN] recommends Universal screening for Lynch syndrome in patients with CRC or endometrial cancer [25].
1.1 Risk factors and prognosis
Colorectal cancer has an excellent prognosis with a 5-year survival of 91% if localized, but 5-year survival is only 14% if cancer has spread to distant organs [26]. The risk factors are both modifiable and non-modifiable. The common risk factors are family history, de novo mutations, smoking, alcohol use, inadequate physical activity, salty food consumption, excess body weight, and excess calorie diet [27-31]. It is interesting to note that in a study, the risk of colon cancer in young women increased by 20% with five points increase in BMI and by almost 69% with more than 14 hours of television watching per week [32, 33]. A recent retrospective study did not show any relation between increased CRC risk in young individuals and obesity, diabetes, or smoking [34]. Some studies found associations of early- onset CRC with antibiotic use, high fructose corn syrup, and changes in gut microbiota [35-37]. Many medications like aspirin, statins, NSAIDs [Non-Steroidal Anti-inflammatory drugs], and hormone therapy decrease the risk of colon cancer and adenomatous polyps based on evidence. The harm associated with the long-term use of these medications limits their use [38, 39].
1.1.1 Family history and genetic syndromes: Having a family history of CRC in first-degree relatives increases the risk by 2 to 4 times [40]. Increased risk of CRC is also common in individuals with distant relatives with a history of CRC [41]. A family history of adenomas also increases the risk of CRC [42]. Almost 25% of younger patients with CRC or adenomas have a first-degree relative with CRC, and 16% have hereditary syndrome, mainly Lynch syndrome [43, 44]. Family history documentation is vital, but less than 50% of Primary Care Physicians document complete family history due to a lack of guidelines and awareness. Less than 22% of medical records register information on family history necessary for genetic counseling [45, 46].
1.1.2 Prevention: Controlling all the risk factors and implementing primordial, primary, or secondary prevention is impossible for any cancer. However, colorectal cancer is one of the few cancers that qualify for prevention with the help of available screening methods. Identifying colorectal polyps is possible nowadays with available screening tests but the beginnings of these tests and the discovery of polyp date to the 1930s. The hard work of dedicated physicians for years led to the development of these screening tests [47]. Colonoscopy screening primarily aims to identify and remove adenomas and sessile serrated lesions. These tests can also detect early-stage colorectal cancer preventing mortality.
1.2 History of colorectal cancer screening tests
1.2.1 Adenoma Carcinoma sequence: The development of colon cancer screening tests took many years. Lockhart–mammary et al. illustrated the relationship between residual adenomas and colorectal cancers in 1927. In the early 1930s, physicians discovered precancerous adenomas changed to colorectal cancer in St. Mark's Hospital, London and developed the first staging system for colorectal cancers. After observing polyp growth, they noted that it takes at least five and more than ten years to become cancer. Morson called the name polyp–cancer sequence for the first time, and this concept got accepted more than 70 years after being proved by the National Polyp study in the 1990s [48, 49] and the decades of hard work from physicians led to the development of additional screenings based on this sequence. Screening methods, including Colonoscopy, Sigmoidoscopy, CT Colonography, and stool-based tests, can detect advanced adenomas, but Colonoscopy can also detect sessile serrated lesions [50] [Figure 1].
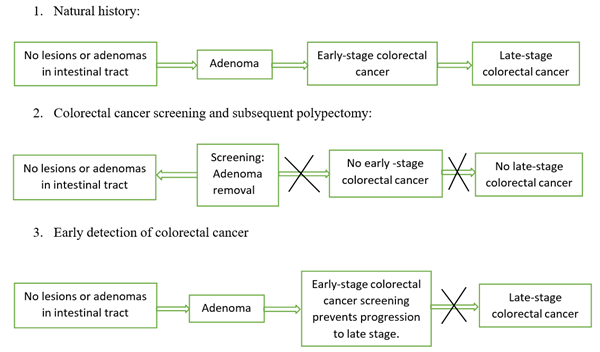
Figure 1: Adenoma carcinoma sequence and importance of colorectal cancer screening
1.3 Rigid sigmoidoscopy origin
Rigid sigmoidoscopy use dates to 1895. Initially, its use was for defects identified with barium enema in the distal colon. It was tough to see till sigmoid flexure except by experienced colorectal surgeons, and patients were under general anesthesia but still had significant discomfort. The use of rigid Sigmoidoscopy as a screening investigation to avoid colorectal cancer in healthy adults was an observation on 21,500 people done at the University of Minnesota in 1948. They demonstrated significant results [51]. So, in 1960, a Randomized Controlled Trial [RCT] was done with 26,000 asymptomatic patients on Sigmoidoscopy as a screening procedure, demonstrating 90% survival in patients with colorectal cancer screening [52]. The early rigid Sigmoidoscopy was not used often due to extreme patient discomfort and the surgeon's inexperience. Eventually, they could insert longer scopes up to splenic flexure under general anesthesia. It was not a very good experience for patients and physicians. With early rigid sigmoidoscopy screening, the morbidity and mortality rate was very high as it required frequent hospital admissions due to complications and polyp removal. Even though it showed improved mortality with screening, rigid Sigmoidoscopy was not a screening performed by many due to the amount of work involved.
1.3.1 Flexible Sigmoidoscopy [FS]: Flexible Sigmoidoscopy is easy to use without sedation. Bowel preparation is also much more manageable than traditional Colonoscopy, consisting of only enemas. Randomized controlled trials showed a reduction of CRC incidence by 18%-23% and CRC mortality by 22%-31% in patients who had screening only once on 10-13 years of follow- up; in participants screened every 3-5 years, CRC incidence and mortality decreased by 20% and 27% respectively [53-57]. The systemic review showed more reduction in mortality with Sigmoidoscopy in CRC affecting distally. Recent pooled flexible sigmoidoscopy trials and updated Norwegian NORCCAP trials showed no reduction with FS in CRC incidence or mortality in women [58]. Flexible Sigmoidoscopy is a screening test for individuals unwilling to undergo Colonoscopy or FIT.
1.3.2 Guaiac card test origin [gFOBT]: Patients with colon cancer usually present with bleeding. There was a suspicion that gross bleeding precedes occult bleeding. Initially, patients got whole stool specimens with benzidine, which is carcinogenic. In 1967, a primary care physician, Dr. Greegor, identified a new home card for colorectal cancer screening that tests occult blood in the stools. He proposed identifying cancers earlier using these guaiac fecal occult blood test cards [gFOBT]. This test required a person to be on a high-fiber diet for three days and follow dietary restrictions like avoiding meat, aspirin, and peroxide-rich foods [59]. Randomized controlled trials that followed patients for 11-30 years showed an 18% reduction in colon cancer-related mortality in patients screened with guaiac fecal occult blood tests [60-66]. Annual FOBT testing showed a 33% reduction in CRC mortality over 30 years and a 20% reduction in CRC incidence with 18 years follow-up in the Minnesota FOBT trial [67, 68].
1.4 Colonoscopy introduction
In the 1970s, colonoscopy introduction was a real game changer and made life easier. Eventually, Colonoscopy was used not only as a screening procedure but also as a treatment to remove the polyps identified. In the 1990s, trials using FOBT cards in combination with Colonoscopy revealed reduced colorectal cancer mortality. In these trials, patients who tested positive on an gFOBT card got screened with a Colonoscope. In 1997 after accepting that colorectal cancer originated from pre-existing adenomas, guidelines for colorectal screening were introduced [69]. This screening was offered to all patients 50 years or older as the tumor was seen in these patients most commonly. The guidelines recommend Colonoscopy once every ten years based on the observation that it takes at least 10 to 15 years on average for a polyp to transform into cancer and should be as conservative as possible [70, 71]. Based on observational studies, it reduces colorectal cancer incidence by 69% and colorectal cancer- related mortality by 65-88% [72]. Sensitivity changes with the physician performing the procedure, and it has a chance of missing 27% of serrated polyps, 26% of adenomas, and 9% of advanced adenomas [73]. Colonoscopy was associated with a 68% reduction in colorectal cancer mortality compared to no screening, and individuals with polypectomy had a 43% reduction in the incidence of colorectal cancer [74]. No randomized controlled trial results are available for our review showing Colonoscopy's effect on the incidence and mortality of colorectal cancer.
However, cohort and case-control studies showed decreased colorectal cancer mortality and the incidence with lower endoscopy [75-77]. NorDICC study, a randomized controlled trial, is currently being conducted comparing Colonoscopy with no screening, the results of which are pending, and only one population-based RCT showed increased cancer detection rates in patients who underwent Colonoscopy screening [78, 79].
1.4.1 Fecal Immunohistochemical test origin [FIT]: Fecal Immunohistochemical Test checks for Hemoglobin in the stool with monoclonal or polyclonal antibodies directed against globin moiety in Hemoglobin. These tests started as early as the 1970s and almost wholly replaced the gFOBT test, considering the increased sensitivity of FIT compared to gFOBT [80, 81, 82]. As per studies, the FIT test has increased sensitivity for detecting colorectal cancer and advanced adenomas compared to the gFOBT test [83]. It is available in qualitative and quantitative forms. In the United States, qualitative testing reporting positive or negative results is used. A meta- analysis of 19 studies showed a sensitivity of 79% and a specificity of 94%; another said 91% sensitivity and 90% specificity [84, 85]. No results of randomized control trial data on CRC mortality reduction with the FIT test are available, and one RCT trial showed no mortality benefit [86, 87]. A recent meta-analysis comparing fecal tests, including highly sensitive gFOBT or FIT with no screening, showed a 12% relative reduction in CRC mortality over 15 years [88, 89]. According to the recent American college of physicians [ACP] guidelines, yearly fecal- based testing is an option, like a Colonoscopy every ten years [90].
1.4.2 Cologuard origin: Cologuard is a quantitative test that checks for aberrantly methylated BMP3 and NDRG4 promoter regions, mutant KRAS, beta-actin, and fecal Hemoglobin [91]. It is also called the Fecal Immunohistochemical and Deoxynucleic acid [DNA] test [FIT-DNA TEST]. In 2014, an article on Stool DNA testing in combination with fecal Hemoglobin compared with Colonoscopy was published. This study included 9989 participants who had Colonoscopies as a reference. Multitarget stool DNA and fecal hemoglobin testing have higher sensitivity for detecting colorectal cancer, advanced precancerous adenomas, and Sessile Serrated Lesions [SSLs]. It had fewer false-positive results than traditional fecal immunoglobulin testing [92]. But no randomized controlled studies are available to test CRC-related mortality or incidence. It has higher sensitivity for detecting CRC and advanced adenomas than gFOBT, as per observational studies [93, 94]. As an add-on benefit, no need for bowel preparation or dietary or medication restrictions. In 2014, Food Drug Administration [FDA] approved Cologuard as a screening test [95]. There is an ongoing Voyage prospective cohort trial on Cologuard, and the interim analysis will be available next year [96]. Recent studies reported that annual FIT and colonoscopy every ten years are more effective than Cologuard every three years [97]. American College of Gastroenterology [ACG] recommends no further testing for asymptomatic individuals with positive Cologuard and negative Colonoscopy.
1.4.3 CT Colonography origin: Computed Tomography Colonography [CTC], also called virtual Colonoscopy, was first reported in 1994 as a test that can detect both colon polyps and colorectal cancer. This imaging creates 3D pictures with the help of CT technology [98]. Magnetic Resonance Imaging [MRI] technology can also produce similar pictures [99]. The dark lumen technique, where the colon lumen is pictured black with wall and polyps as bright T1-enhanced images, was first described in 2001 [100, 101]. An observational study on 122 subjects showed that dark lumen Colonography has 93% sensitivity and 100% specificity compared with Colonoscopy [102]. In studies that compared CT colonography with Colonoscopy, the sensitivity, and specificity were between 70 and 100%. However, small polyps' sensitivity is very low, even with newer multi-slice CT technology [103, 104]. Flat and depressed colonic lesions are not easily seen in CT Colonography but are easy to notice on Colonoscopy [105-108]. It can easily detect advanced proximal colon tumors compared to flexible Sigmoidoscopy and FIT. However, it has a lower detection rate for distal neoplasia than flexible Sigmoidoscopy [109, 110]. No long-term RCTs data in our search evaluating its effect on colorectal cancer incidence and mortality. Colon polyps with a size more than or equal to 10 mm need a Colonoscopy as a follow-up study, between 6- and 9-mm size need CTC follow-up, or a Colonoscopy and polyps less than or equal to 5 mm need routine screening. However, precise guidelines have yet to exist [111]. It is associated with fewer complications than Colonoscopy; CTC is an alternative for advanced neoplasia.
1.5 Development of colorectal cancer screening guidelines
In the 1970s, after the availability of excellent colonoscopes, patients who had positive gFOBT tests underwent Colonoscopies for accurate diagnostic workups. The advantage of removing polyps was also proposed in 1973, adding more benefits to this testing [112]. Several randomized controlled trials administered Colonoscopy in patients with positive gFOBT test proved the reduction in mortality with screening [113, 114]. Confirmation from several studies showing the benefit of colorectal cancer [CRC] screening in people 50 and older led to the development of guidelines between 1993 and 1996 recommending the options of gFOBT or flexible Sigmoidoscopy at different intervals [115]. Based on the benefit of clinical studies, screening Colonoscopy was added to guidelines in 1997 by Gastrointestinal Consortium [GI Consortium] and American Cancer Society [ACS] [116]. Colonoscopy is recommended every ten years in asymptomatic individuals based on the time required for a small polyp to grow into CRC [117]. Cologuard was included in colorectal cancer screening guidelines in 2016 by U.S. Preventive Taskforce and the National Comprehensive Cancer Network [Figure 2].
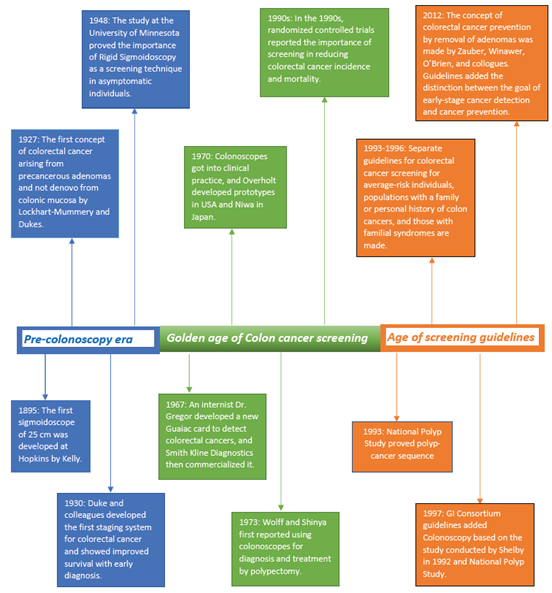
Figure 2: History of colorectal cancer screening
1.6 International colorectal cancer screening guidelines and recommendations
The highest incidence of colorectal cancer is in the Asia Pacific region, with more than 50% of cases and mortality related to this cancer [118]. In this region, organized screening programs available in countries including Australia, Brunei, Hong Kong, Japan, South Korea, New Zealand, Singapore, and Taiwan use fecal immunohistochemical test [FIT] every year or every two years as the primary screening test or Colonoscopy every ten years in some situations. In contrast, Sigmoidoscopy is not used [119]. The Asia Pacific working group in this region recommended using the screening for the population over 50 years of age—the decisions for screening patients over 75 years depend on overall health status and preferences. Screening could be stopped for the population over 85 years of age if the recent screening test was negative.
Patients with two first-degree relatives [FDR] with colorectal cancer or advanced adenoma at any age should start screening ten years before the diagnosis of their FDR or 40 years, whichever is earlier, and then repeat every five years [120]. In the United States, organized settings use Colonoscopy and FIT tests for screening. Other tests, including CTC, FS, Cologuard, and Colon Capsule, are reserved for people unwilling or unable to get Colonoscopy or FIT test and in individuals with incomplete Colonoscopy. The United States Preventive Services Task Force [USPSTF] screening recommendations exclude blood-based tests, urine tests, and capsule endoscopies. USPSTF includes stool-based tests with high sensitivity [high sensitivity gFOBT, FIT, DNA-FIT] and direct visualization tests [Colonoscopy every ten years, computed tomography [CT] Colonography every five years, flexible Sigmoidoscopy every five years, or flexible Sigmoidoscopy every ten years plus FIT every year] in the recommendations considering the available evidence. However, we included most screening tests that are available now in our review [121]. American College of Gastroenterology recommends colorectal cancer screening at 45 [122] [Table 1]. Many European countries do not have a conventional screening program in place; European Council recommends starting colorectal cancer screening between ages 50 and 74 years and then repeating every two years if the screening test used earlier was Fecal Occult Blood Test [FOBT] or Fecal Immunohistochemical test [FIT] or every ten years or more if the screening used was Colonoscopy or flexible Sigmoidoscopy [123, 124]. The quantitative fecal immunohistochemical test is the preferred screening test for colorectal cancer, as per European Health Union [125]. Patients with abnormal FIT or FOBT and large or multiple polyps on Sigmoidoscopy require follow-up Colonoscopy [126]. A population-based European study by Cardoso and colleagues also noted the recent increase in colon cancer in the younger population [127]. The lack of well-established health insurance makes it hard for proper colon cancer screening in Sub-Saharan Africa [128].
|
Age to start or stop screening |
Evidence of recommendation |
|
Start at 45 years |
Conditional recommendation |
|
Continue screening between 50 and 75 years |
Strong recommendation |
|
Stop screening >75 years |
Conditional recommendation (decision should be dependent on life expectancy and adverse risks) |
|
Start at 40 years or 10 years before the age of youngest affected relative |
If they have a first degree relative (FDR) with CRC or advanced polyps before age 60. Two first degree relatives with CRC or advanced polyps after age 60 |
Table 1: American College of Gastroenterology screening recommendations.
1.7 Colorectal cancer screening types
The screening methods for colorectal cancer are both non-invasive and invasive. Non-invasive techniques include stool-based and radiological testing. The stool-based tests check for blood or hemoglobin in the stools or newer fecal DNA, and these tests are fecal occult blood test [gFOBT], fecal immunohistochemical test [FIT], and Cologuard [FIT-DNA]. The radiological investigations are CT colonography and capsule endoscopy. Invasive tests are sigmoidoscopy and Colonoscopy help us visualize the colon directly by inserting the scope. A Colonoscopy is the most accurate screening test as it screens the entire colon and can stop the adenoma- carcinoma sequence by removing polyps [129]. Stool-based screening tests like Cologuard and FIT tests cannot test high-risk patients who have a family history of colorectal cancer, IBD, personal history of polyps, and those who have GI symptoms [130]. Screening tests can be 1-step or direct, like a Colonoscopy, which is diagnostic and therapeutic. They can also be 2-step tests that need a Colonoscopy if positive to know if it is genuinely positive. The 2-step tests include stool-based tests, flexible sigmoidoscopy [FS], CT colonography [CTC], or colon capsule test [CC] [131]. All the screening tests except Colonoscopy are 2-step tests, requiring Colonoscopy follow-up if positive.
1.7.1 Perceived advantages and disadvantages of individual screening tests Colonoscopy: Colonoscopy is the gold standard screening investigation and is still the top in the list of screenings as it offers screening, diagnosis, and treatment if needed. It is also valid for at least ten years. It can be a primary screening or secondary when other tests are positive, and it can visualize the entire colon. The downside is that it needs bowel preparation with purgatives and dietary modification. It requires general anesthesia; some patients cannot tolerate the bowel preparation and require assistance from others to transport them back home after the procedure [132]. Poor bowel preparation can lead to poor colon visualization, with missing opportunities to detect adenomas and polyps [133]. Sometimes multiple attempts are needed due to poor bowel preparation. Many newer bowel preparations, with low volume and pleasant flavor, are now available to improve palatability and comfort [134]. It is operator dependent and has a complication rate of 4-8 in 10,000. Colonoscopy-related complications include bleeding, perforation in older individuals, splenic injury due to tension on the splenocolic ligament or colon manipulation in combination with preexisting adhesions, electrolyte imbalances, bowel preparation-related nephropathy, and cardiopulmonary events due to sedation [135-137]. There is also a concern for post Colonoscopy detected CRC [PCCRCs] due to missed diagnosis as it is operator dependent; therefore, there is a necessity for good quality improvement programs to reduce PCCRCs [138, 139]. PCCRCs are common in the proximal colon and account for about 3-9% of CRCs.
1.7.2 Flexible Sigmoidoscopy [FS]: Flexible sigmoidoscopy allows us to see the distal colorectal portion and helps with biopsy and removal of polyps. It needs less bowel preparation than the Colonoscopy in the form of enemas, does not require sedation, and needs follow-up every five years. However, it is limited to detecting adenomas or cancers only in the distal colon [140]. It is also less invasive and has a lower risk of complications than a Colonoscopy. Rates of CRC screening with sigmoidoscopy are low in the United States as the equipment is similar to Colonoscopy; it cannot examine the entire colon and is limited to the distal colon. The lack of sedation makes it a very uncomfortable procedure. Recent studies also revealed no reduction in CRC-related mortality and lower use of FS in women. If positive, it needs to follow up with a Colonoscopy like every other 2-step screening test. It is like Colonoscopy in scheduling, but no specialist consultation is required. Its use and availability are low in the United States [141]. We expect small blood in bowel movements after FS, but more severe bleeding and intestinal puncture are rare.
1.7.3 Non-endoscopic radiologic screening tests: Individuals who do not want to or cannot undergo a Colonoscopy or FIT or those who couldn't complete a Colonoscopy can get these screening tests.
1.7.3.1 CT Colonography: It is one of the screening tests like flexible sigmoidoscopy and Colonoscopy that directly visualizes the colon polyps and cancers. It takes three-dimensional images of the bowel using CT or MRI technology in 10 minutes in lying back and prone positions. It needs colonic insufflation through a small flexible rectal catheter with air or carbon dioxide for distension just before the imaging. Patients need laxative bowel preparation before this procedure, like Colonoscopy, but no sedation or analgesia is required [142-145]. The bowel preparation might be considered unpleasant by some patients. It is less invasive than a Colonoscopy and works well in patients who cannot tolerate anesthesia. There is also an advantage to seeing other organs besides the colonic mucosa [146]. It works when Colonoscopy is impossible due to obstructing tumors or adhesions. Those circumstances allow us to detect and stage the tumors [147-149]. The other indications of its use are in Inflammatory bowel disease [IBD] and follow-up on patients with treated colorectal cancer. It can detect stenosis and fissures in IBD and see tumors and extracolonic lesions during treated colorectal cancer follow-up [150- 152]. It is a primary screening in some centers but has lower sensitivity, as per some previous studies [153]. Accurate reading of CT is radiology reader dependent. It is sometimes also not covered by insurance and not available widely. Patients may feel bloated and have cramps due to air in the colon and rectum, but these should be relieved once air passes away. The risk of puncture or perforation is much less than Colonoscopy [154].
1.7.3.2 Colon Capsule endoscopy [CC]: Capsule endoscopy is a technique in which a patient swallows a pill-like capsule containing a camera. This camera takes pictures of the entire intestine and sends them to a patient's recorder on their shoulder or waist, which the radiologist can later read. The capsule swallowed will be out with a bowel movement. It has a sensitivity of 81%, specificity of 93% for polyps greater than or equal to 6mm, and 87% for polyps >9 mm [155, 156]. It is minimally invasive and does not require sedation. Patients can do newer version tests at home, and it requires bowel preparation. Positive CC requires a Colonoscopy, which involves cleansing the bowels before administering. There are no screening guidelines for repeat intervals. FDA approved the use of CC in patients with incomplete Colonoscopy or in individuals who cannot get a Colonoscopy due to lower gastrointestinal bleeding. However, it cannot be a standard screening test. A recent study comparing CTC and CC reported a sensitivity of 32% and 84%, respectively, for polyps more than or equal to 6 mm and a sensitivity of 53% and 84%, respectively, for polyps more than or more than equal to 10 mm [157]. Further testing and clinical trials are needed to characterize the repeat time interval and if it can be a routine primary screening test.
1.7.3.3 Stool-based tests: Guaiac FOBT test: Guaiac fecal occult blood testing is the first stool-based test included in screening, and it needs repeatition yearly. It tests the pseudo-peroxidase activity to detect blood in the stool. It has a very low positive predictive value. This test has confounding results with a specific diet containing peroxidases, including red meat, plants with enzyme peroxidase, medications like non-steroidal anti-inflammatory drugs, and vitamin C [158]. It also requires patients to submit three stool samples at home. Office or hospital-based testing is not for screening as it tests only once and is unreliable [159, 160]. The downside of this test is that the patient should be motivated to follow dietary restrictions, there is laboratory variability, it needs repetition every year, and a positive result requires follow-up with a Colonoscopy [161]. Screening with the gFOBT test showed a reduction in colon cancer-related mortality.
1.7.3.4 FIT test: FIT tests for globin in the feces and is susceptible to even a small amount of hemoglobin in the feces [162]. This test has no interaction with dietary factors and can detect advanced neoplasia compared to the gFOBT test but has very low sensitivity for detecting polyps [163, 164]. This test requires only one stool sample, and patients were more adherent than gFOBT [165]. If the FIT test is positive, patients should get a Colonoscopy for further surveillance to look for and treat adenomas. Patients with hemorrhoids and other perianal issues cannot be tested accurately with the FIT test, and patients should get it annually for screening.
The positive result needs follow-up with a Colonoscopy like every other two-step test [166]. Research also showed the importance of quantifying the amount of hemoglobin concentration with a qFIT [quantitative FIT] test to distinguish between neoplasia versus non-neoplasia-related gastrointestinal bleeding [167, 168]. In the United States, the qualitative FIT is the approved test for screening. It has low sensitivity for advanced adenomas and does not detect serrated lesions. As discussed, no randomized control trials testing FIT were available to prove its effectiveness. However, observational studies showed a 62% reduction in colorectal cancer-related mortality with the routine FIT [169].
1.7.3.5 Cologuard or FIT-DNA: Cologuard tests fecal DNA biomarkers with occult hemoglobin. The patient can perform it at home without going to the clinician's office [170]. No bowel preparation or sedation is needed. No dietary or medication restrictions are required [171]. The sensitivity of the Cologuard test depends on the size of the tumor, and it can detect only less than half of the precancerous polyps, as per research. If any non-invasive tests are positive, patients should get the Colonoscopy for further surveillance; it has lower specificity with a 12% false positive rate, and patients with a normal Colonoscopy after a positive result on the Cologuard test might require aggressive surveillance [172]. It is expensive compared to FIT and Colonoscopy, as per a 2016 study. If Cologuard is positive, some insurances can consider the follow-up Colonoscopy diagnostic rather than screening, leading to an increased cost burden on the patients [173, 174]. Stool tests are accessible at home, but some patients reported aversion to sampling their stool. False positive results of stool-based tests lead to anxiety, sometimes leading to Colonoscopy- related harm [175].
1.7.3.6 Blood DNA tests or Liquid biopsy: Septin9: Septin9 [SEPT9] gene is a cell cycle protein belonging to the cytoskeletal GTPase family [176, 177]. It is involved in polarization, cytokinesis, vesicle trafficking, DNA repair, apoptosis, cell migration, and cell division [178]. An abnormal SEPT9 gene can cause problems with cell division—mutation in the SEPT9 gene is common in many malignancies. SEPT9 mRNA is low when progressing from colorectal adenoma to CRC, and protein expression is lower in CRC cells than in normal epithelial cells [179]. Overexpression of SEPT9 transcripts v2, v4, v4*, and v5 in colorectal cancer cells is seen [179]. This gene is a tumor suppressor gene, and hypermethylation of the promoter gene can inhibit its tumor suppressor and cell autophagy actions promoting the development of CRC [180, 181]. SEPT9 gene methylation results in microsatellite instability [MSI]. As per studies, 72% of CRC patients have it, so they confirmed its importance as a specific diagnostic marker of CRC [182]. SEPT9 gene knockout also led to tissue cancer development, as per studies [183]. SEPT9 methylation detection peripheral blood tests, as per studies, had low sensitivity in adenomas and polyps but had higher detection rates in all stages of CRC, showing the importance of this test in detecting early CRC [184].
An increase in necrosis and apoptosis as colorectal cancer progresses is one reason for increasing methylation rates in stages II, III, and IV compared to stage I. The use of methylated SEPT9 in peripheral blood in screening, diagnosis, prognosis, and monitoring of CRC is under research [185]. It can be an add-on test to the routine blood draw and a minimally invasive test. It has low sensitivity and, if positive, will need a Colonoscopy. When done together, the fecal occult blood test and SPET9 gene methylation have a sensitivity of 88.7%-97.8% and a specificity of 52.9%- 78.8% per study [186, 187]. However, this test should be combined with other tests to improve test sensitivity and specificity, so further research is needed [188]. No evidence from studies is available that it reduces CRC incidence or mortality. FDA-approved SEPT9 test, Epi proColon test, or Septin9 test to screen adults aged 50 years and older at average risk for CRC and declined other tests for CRC screening. It cannot replace routine colorectal cancer screening tests.
1.7.3.7 Prophylaxis to prevent CRC: Primary prevention strategies are being studied for all cancers to avoid the development of neoplasia altogether. Aspirin reduces the risk of colon cancer in the long term but does not minimize CRC risk in the first years of initiating therapy, as per studies [189]. However, it cannot serve as a replacement for routine screening. The mechanism of aspirin's effect on tumors is not entirely known, but it has both direct and indirect impacts on tumors. Prostaglandin E2 [PGE2] is involved in CRC tumorigenesis, and aspirin decreases the production of PGE2 [190]. It inhibits the WNT signaling pathway [WNT-wingless-related integration site], PIK3CA/AKT pathway [Phosphatidylinositol-4,5-Bisphosphate 3-Kinase Catalytic Subunit Alpha, Ak strain transforming] and Ras/Raf/MAP kinase [MEK]/ERK pathway [Rat sarcoma virus /rapidly accelerated fibrosarcoma /Mitogen-activated protein kinase/ERK kinase [MEK]/extracellular- signal-regulated kinase [ERK]] by decreasing PGE2 production [191-194]. Aspirin inhibits the NF-kB [Nuclear factor kappa B] and AMPK/mTOR [mechanistic [or mammalian] target of rapamycin [mTOR] and the adenosine monophosphate-activated protein kinase [AMPK]] pathways and stimulates the apoptosis pathway [195-197]. It has indirect effects through platelet inhibition and decreasing inflammation [198, 199]. Case-control and cohort studies noted that aspirin use decreases the risk of CRC [200]. Aspirin, at least 16 times per month, reduced the risk of colon cancer mortality by 40% in 6 years in early analysis of Cancer Prevention Study II Cohort [201]. The use of 325 mg aspirin daily for five years showed a lower incidence of CRC. The use of aspirin at least two times a week reduced CRC risk by 21% in 18 years of follow-up and 23% in 20 years of follow-up [202-204]. In RCTs and meta-analysis, aspirin initiation was beneficial 5-7 years after initiation [205]. In the Women Health Study [WHS] low dose of aspirin at 50 mg was also found effective [206]. Aspirin use also showed decreased colorectal adenomas in placebo-controlled RCTs. Meta-analysis of several trials showed a 17% reduction in the risk of colorectal adenomas over 33 years of follow-up with aspirin at any dose from 81 mg to 325 mg [207-211]. Long-term use of aspirin increases the risk of gastrointestinal bleeding, and adding a proton pump inhibitor, especially in patients on dual antiplatelet therapy, was helpful, as per some studies [212, 213]. There were concerns that aspirin affects the FIT test by increasing physiological and pathological blood loss [214, 215]. The recent meta-analysis did not show any effect of aspirin on FIT results [216]. A randomized, double-blind, controlled trial conducted on patients with Lynch syndrome showed a reduced risk of CRC in the aspirin group compared to the placebo group with per-protocol analysis but no difference with intention-to- treat analysis [217]. USPSTF recommends aspirin for primary prevention of CRC in patients with a 10-year cardiovascular risk of more than 10% and ages between 50 and 59 years of age [218, 219].
1.7.3.8 Screening in younger population: CRC incidence rates have doubled in patients aged between 20 and 49 years, and most of these individuals do not have a family history of colorectal cancer [220, 221]. American Cancer Society in 2018 recommended the change to start colorectal cancer screening at an earlier age of 45 years instead of 50 years based on age-cohort epidemiological evidence of a 51% increase in CRC incidence among individuals younger than 50 years [222]. As per modeling studies, starting screening at 45 years of age instead of 50 years results in 25 more life years per 1000 individuals screened [223]. The increase in the incidence rate of colorectal cancer is partly due to the birth cohort effect. People born around 1990 have approximately 2-4 times the risk of colorectal cancer compared to people born around 1950, showing the impact of exposures in early life [224-226]. People living in regions with poverty, unemployment, and poor access to healthcare have the highest incidence of early-onset CRC [227]. As usual, the reasons for this increase in early-onset CRC are multiple, including lifestyle-related factors like diet and obesity, occupational exposures like mineral dust and trace elements, and environmental factors including agricultural runoff and industrial pollution [228].
Considering the increasing evidence of increased CRC incidence in this age group, it is crucial to consider the diagnosis of CRC in younger adults. Routine screening at 45 should be propagated among all adults even before they turn 45, especially during their regular PCP visits. Early detection of adenomas in the age group 45-49 years leads to reduced CRC incidence in those 50 and older. It is also essential to know that younger individuals are usually diagnosed with distal colon or rectal cancers, while proximal colon cancers are common in older patients [229]. Most of these increased numbers in younger population are due to rectal cancer, which has increased by more than 90% since the 1990s compared to colon cancer which only increased by 40% with a higher incidence in women. The younger population diagnosed with CRC usually lacks a family history of CRC, hereditary syndromes, or germline mutations but has genetic mutations unrelated to CRC [230, 231]. However, screening for CRC in the younger generation has its disadvantages due to the use of resources in this population instead of elderly individuals who are at more risk.
1.8 Reasons for noncompliance with the recommended screening guidelines
Screening for colon cancer has been essential to any annual wellness visit in family practice or primary care physician’s office. Adherence to screening guidelines is being monitored in many countries. Patients can decline the screening even with excellent education background and after listening to the importance of screening [232]. The reasons for deferral are many; they are not specific, and there is no general answer. The factors influencing colorectal cancer screening participation are modifiable and non-modifiable. Modifiable factors include patients' knowledge of screening and their views on it, providers' views, and barriers to screening, whereas non- modifiable factors include demographics, education, income, and insurance [233]. There can be socioeconomic or psychosocial factors, or variations in healthcare provider recommendations [234]. In younger individuals, time spent at their job and caring for family risks their health.
Anxiety about CRC and feeling reassured with the screening are essential in determining CRC screening participation [235]. Decision style, individual attitude towards screening, and education level play a crucial role in non-participation [236]. A recent publication in 2022 showed anxiety about the invasive procedure as the first concern in the first-time screening population. In contrast, in previous participants, anxiety about colon preparation and test accuracy played an essential role in participation. The effect of cost and insurance coverage was also seen [237].
About 50% of direct-access or open-access Colonoscopies are inappropriate, indirectly leading to longer Colonoscopy waiting times, especially in patients with positive stool tests or alarming symptoms. The reasons for excess Colonoscopy referrals include lack of awareness of post- polypectomy surveillance guidelines among physicians, inadequate bowel preparation, new clinical signs, suspicion for synchronous or metachronous lesions, patient or referring physician insistence, and sometimes medico-legal issues. An open access system also has many no-shows; this, along with overprescription of Colonoscopies, leads to non-adherence with recommended screening guidelines [238]. The decreased use of screening tests leads to late diagnosis and increasing colorectal cancer related mortality. Out of all the screening tests, gFOBT and FIT tests are cheap, readily available, and easy to perform [239]. As the benefits of polypectomy are delayed by 7-10 years after screening, the use of colon cancer screening is low in people with diminished life expectancy which could also be a reason for low compliance in some groups.
1.9 Compliance with follow-up Colonoscopy in individuals with positive stool-based tests
Numerous studies also showed suboptimal follow-up with Colonoscopy in individuals with positive FIT tests [240, 241]. The adherence to follow-up Colonoscopy after a positive stool- based test is between 50% and 87%. Compliance is higher in larger health systems and lower in safety net settings [242]. In observational studies with information from four large health centers between 2010 and 2012, adherence was 79.6% at three months and 58.1% to 83.8% at six months [243, 244]. At VA clinics in California using 2014-2016 data, the follow-up Colonoscopy completion rate after a positive stool test was 62.1% at six months [245]. Large safety net systems reported only 51.5% to 57.7% adherence at 6-12 months follow-up. In Netherlands and Spain, a study conducted by National screening programs reported a compliance rate of 90% [246, 247]. The compliance rates with follow-up Colonoscopy were 84.9% with positive FIT-DNA testing and 42.6% with routine positive FIT test [248]. A retrospective study done on 15,469 patients at the Mayo clinic showed 87% compliance with follow-up Colonoscopy in patients with a positive FIT-DNA test [249]. Studies showed that every extra month until Colonoscopy increases mortality in patients with positive stool tests, so implementing a fast-track Colonoscopy within one month is crucial [250, 251]. Implementation of screening programs after a positive FIT test by Kaiser Permanente showed an increase in screening rate from 38% to 82%, along with a decrease in CRC incidence and mortality by 25% and 52%, respectively [252]. If any two-step screening tests are positive, they will need a follow- up with a Colonoscopy. Insurance might not cover the follow up costs after positive stool-based tests as they consider this Colonoscopy a diagnostic instead of screening. So, physicians need to consider insurance issues and be able to find a solution for the patients when stool-based tests are positive to avoid non-compliance with screening.
1.10 Methods to improve colorectal Cancer screening compliance
To improve compliance with the colorectal cancer screening, many qualitative studies performed in different medical centers showed the importance of using standard terms with straightforward language and avoiding medical jargon [253, 254]. Per a study published in the American Journal of Gastroenterology [ACG], patients are more likely to get Colonoscopy if their physicians recommend it [255]. They suggested interventions targeting patients, providers, and organizations for improving CRC screening. Another study at the University of Florida also proved that compliance with CRC screening increases with patient-provider discussions on the importance of screening [256]. Individuals most likely choose non-invasive tests over invasive tests like Colonoscopy [257].
Using healthcare management tools developed by national healthcare systems like clinical care pathway [CCP] has fewer Colonoscopy over prescriptions. CCP also has reduced wait times compared to open-access Colonoscopies, suggesting the need for additional surveillance of open- access systems to improve wait times and adherence. Introducing navigation services, including outreach services with letters or calls, educational materials, or sessions, addressing barriers to screening, appointment scheduling and reminders, mailed supplies, assistance with bowel preparation, and transportation assistance into practice improves colorectal cancer screening rates [258]. Patient navigators are vital in enrolling people in cancer screening, increasing screening quality, increasing screening rates in populations at risk for noncompliance, and increasing the number of follow-ups [259, 260]. Even though developed countries have a wide availability of screening tests and opportunities, advertising the importance of colon cancer screening tests is essential, considering the increasing death rate in the younger population and mortality rate in unscreened patients [261].
1.11 Future suggestions for improving compliance
Improving the taste of bowel preparation, making it individualized based on a patient’s ability to tolerate it, and understanding their comorbidities which could limit their mobility, can improve compliance with invasive tests like Colonoscopy. Communicating well with the patients about the procedure with visual aids can help them understand it better. In the future discovery or invention of a small one-time pill that can serve the purpose of bowel preparation can be helpful as well. The development of quantitative FIT tests that distinguish between non-neoplastic and neoplastic GI bleeding can reduce the number of required Colonoscopies. Decreasing the number of stool samples needed to increase specificity for gFOBT and FIT tests can improve patient outcomes and ease. Banning cost sharing by the states for Colonoscopies after a positive Cologuard screen can be helpful for patients and can be beneficial for being more compliant with screening guidelines. Newer techniques like color coding, fecal tagging, computer-directed diagnosis, or virtual pathology might increase the sensitivity of CT Colonography, making virtual Colonoscopy a primary screening test in the future. CT Colonography avoids sedation and bowel preparation making it more cost-effective [262]. Additional training and evaluations for reading Colonography to prevent variation in the practice and missing polyps can help. Low dose scanning and newer reconstruction techniques can reduce the risk of radiation from CTC, but the benefits of screening outweigh the risks from radiation [263, 264]. Randomized controlled trials are needed to compare other available screenings to know if we can switch from one screening to another to improve patient ease. We need more RCTs on CT Colonography and Cologuard to see if they influence CRC incidence and mortality.
1.12 Future of colorectal cancer screening
Blood, fecal-based micro-RNA, circulating tumor DNA tests, and gut microbiome-related markers are a possibility that can make future screening much easier than before. Developing more blood-related tests like Septin-9 that work equally well in high-risk and low-risk individuals can improve screening compliance. Blood tests are easier to get than procedures that are too uncomfortable, time-consuming, and expensive. It also avoids aversion to stool-based testing and the false positives associated with them [265]. There is ongoing research to develop new blood tests with the help of circulating tumor cells [CTCs], nucleic acids including RNA, DNA, messenger RNA, microRNAs and cytokines, antibodies, and proteins [266-269].
Research on urine-based metabolites to check for colorectal polyps and tumors is ongoing [270- 272]. Research to improve the detection of biomarkers and increase the sensitivity of stool-based tests is continued [273]. Creating a risk score, especially for younger patients, to know if they need to get screened helps reduce CRC incidence and mortality. Considering the lack of evidence on the benefits and harms of initiating CRC screening at an earlier age, precision cancer screening can better understand risk assessment and make personalized screening recommendations based on the risk score [274]. These models documented starting screening from age 38 in a young patient with a family history of CRC to 71 years in women with no family history and lower scores [275]. They can also predict the choice of a screening test based on risk score and other factors [276]. Blood-based screening tests which can check DNA/RNA/mRNA changes or mutations might be the future of cancer screening, diagnosis of adenomas/precancerous lesions/cancers, and monitoring of treatment.
2. Conclusion
Colorectal cancer is preventable with effective screening, but only 68.8% of adults aged between 50 and 75 underwent screening in 2018, as per CDC statistics [277]. The current screening rates are only 57.9% in ages 50-64 and 62.4% in ages 50-75 [278]. American cancer society now also recommends colorectal cancer screening from age 45 years instead of 50 years due to the recent increase in cancer-related mortality in younger adults [279]. The exact number screened between 45 and 50 is still to be determined, considering recent addition to the guidelines. Screening patients at a younger age of 45 years is cost-effective but achieving a target of 80% screening between 50 and 75 years will reduce CRC-related mortality by three times at a lesser cost [280]. Many countries are yet to include the screening starting at 45 years. Further research is needed to precisely account for the usefulness of beginning screening at a younger age of 45 years. Research is also necessary to see if we can shift from one screening test to another to improve patient ease. Further investigations are essential to improve the current screening methods and to develop more accessible blood, urine, and stool-based tests, which are much more comfortable for the entire population at a reasonable cost.
Treatment of colorectal cancer improved significantly in recent years, leading to improved morbidity and mortality. But even with the availability of excellent treatment regimens, after the detection of colon cancer, patients still undergo colectomy or get chemotherapy depending on the stage of cancer [281]. Not all stage III and IV patients can get chemotherapy, as the treatment depends on their baseline functional status. Therefore, effective screening prevents colorectal cancer mortality and morbidity by removing polyps and precancerous lesions [282]. In office visits, it is essential to create a personalized screening plan for the patients depending on their risk, lifestyle, needs, and comorbidities instead of providing overwhelming information about all available screening tests. Interventions to help include patient navigators, provision of follow-up appointments, providers reminders or performance data, telephone calls, group, and one-to-one education, small or mass media, reducing client out-of-pocket costs, reducing structural barriers, and improving quality improvement efforts to improve the screening rate are necessary [283, 284]. National tracking of everyone for colorectal cancer screening might help increase screening rates through patient outreach with notifications, support tools, and other recommendations. The goal of the National Colorectal Cancer Roundtable is 80% of screening which is hard to achieve worldwide without aggressive efforts. We want to conclude our paper by informing that implementing organized CRC screening programs is crucial in every community worldwide to avoid colorectal cancer-related mortality.
References
- Key statistics of colon cancer
- Colon cancer deaths worldwide
- GLOBOCAN 2020 USA
- Edwards BK, Ward E, Kohler BA, Eheman C, Zauber AG, Anderson RN, et al. Annual report to the nation on the status of cancer, 1975-2006, featuring colorectal cancer trends and impact of interventions (risk factors, screening, and treatment) to reduce future rates. Cancer: Interdisciplinary International Journal of the American Cancer Society 116 (2010): 544-73.
- Zauber AG, Winawer SJ, O'Brien MJ, Lansdorp-Vogelaar I, van Ballegooijen M, Hankey BF, et al. Colonoscopic polypectomy and long-term prevention of colorectal-cancer deaths. N Engl J Med 23 (2012): 687-96.
- Bhandari A, Woodhouse M, Gupta S. Colorectal cancer is a leading cause of cancer incidence and mortality among adults younger than 50 years in the USA: a SEER-based analysis with comparison to other young-onset cancers. Journal of Investigative Medicine 65 (2017): 311-5.
- Siegel RL, Miller KD, Jemal A. Colorectal cancer mortality rates in adults aged 20 to 54 years in the United States, 1970-2014. Jama 318 (2017): 572-4.
- Karsenti D, Tharsis G, Venezia P, Cattan P, Tordjman G, Gillet A, et al. Adenoma detection rate according to age: colonoscopy screening should start at 45 years old. United European Gastroenterology Week 30 (2017): A1-60.
- Thoma MN, Castro F, Golawala M, Chen R. Detection of colorectal neoplasia by colonoscopy in average-risk patients age 40–49 versus 50–59 years. Digestive diseases and sciences 56 (2011): 1503-8.
- Lieberman DA, Williams JL, Holub JL, Morris CD, Logan JR, Eisen GM, et al. Race, ethnicity, and sex affect risk for polyps> 9 mm in average-risk individuals. Gastroenterology 147 (2014): 351-8.
- Rundle AG, Lebwohl B, Vogel R, Levine S, Neugut AI. Colonoscopic screening in average-risk individuals ages 40 to 49 vs 50 to 59 years. Gastroenterology 134 (2008): 1311-5.
- Bailey CE, Hu CY, You YN, Bednarski BK, Rodriguez-Bigas MA, Skibber JM, Cantor SB, Chang GJ. Increasing disparities in the age-related incidences of colon and rectal cancers in the United States, 1975-2010. JAMA surgery 150 (2015): 17-22.
- Siegel RL, Jemal A, Ward EM. Increase in incidence of colorectal cancer among young men and women in the United States. Cancer Epidemiology Biomarkers & Prevention 18 (2009): 1695-8.
- You YN, Xing Y, Feig BW, Chang GJ, Cormier JN. Young-onset colorectal cancer: is it time to pay attention?. Archives of internal medicine 172 (2012): 287-9.
- Mitchell E, Macdonald S, Campbell NC, Weller D, Macleod U. Influences on pre-hospital delay in the diagnosis of colorectal cancer: a systematic review. British journal of cancer 98 (2008): 60-70.
- Davidson KW, Barry MJ, Mangione CM, Cabana M, Caughey AB, Davis EM, et al. Screening for colorectal cancer: US Preventive Services Task Force recommendation statement. Jama 325 (2021): 1965-77.
- White A, Thompson TD, White MC, Sabatino SA, de Moor J, Doria-Rose PV, et al. Cancer screening test use—United States, 2015. Morbidity and Mortality Weekly Report 66 (2017): 201.
- Meester RG, Doubeni CA, Lansdorp-Vogelaar I, Goede SL, Levin TR, Quinn VP, et al. Colorectal cancer deaths attributable to nonuse of screening in the United States. Annals of epidemiology 25 (2015): 208-13.
- Mazidimoradi A, Tiznobaik A, Salehiniya H. Impact of the COVID-19 pandemic on colorectal cancer screening: a systematic review. Journal of Gastrointestinal Cancer (2021): 1-5.
- Harber I, Zeidan D, Aslam MN. Colorectal Cancer Screening: Impact of COVID-19 Pandemic and Possible Consequences. Life 11 (2021): 1297.
- Møller P, Seppälä T, Bernstein I, Holinski-Feder E, Sala P, Evans DG, et al. Cancer incidence and survival in Lynch syndrome patients receiving colonoscopic and gynaecological surveillance: first report from the prospective Lynch syndrome database. Gut 66 (2017): 464-72.
- Win AK, Jenkins MA, Dowty JG, Antoniou AC, Lee A, Giles GG, et al. Prevalence and Penetrance of Major Genes and Polygenes for Colorectal CancerPrevalence and Penetrance of Major Genes and Polygenes for CRC. Cancer Epidemiology, Biomarkers & Prevention 26 (2017): 404-12.
- Bansidhar BJ. Extracolonic manifestations of lynch syndrome. Clinics in Colon and Rectal Surgery 25 (2012): 103-10.
- Barrow E, Robinson L, Alduaij W, Shenton A, Clancy T, Lalloo F, et al. Cumulative lifetime incidence of extracolonic cancers in Lynch syndrome: a report of 121 families with proven mutations. Clinical genetics 75 (2009): 141-9.
- Gupta S, Provenzale D, Llor X, Halverson AL, Grady W, Chung DC, et al. NCCN guidelines insights: Genetic/familial high-risk assessment: Colorectal, version 2.2019: Featured updates to the NCCN guidelines. Journal of the National Comprehensive Cancer Network 17 (2019): 1032-41.
- https://www.cancer.net/cancer-types/colorectal-cancer/statistics
- Xue K, Li FF, Chen YW, Zhou YH, He J. Body mass index and the risk of cancer in women compared with men: a meta-analysis of prospective cohort studies. European Journal of Cancer Prevention 26 (2017): 94-105.
- Carter BD, Abnet CC, Feskanich D, Freedman ND, Hartge P, Lewis CE, et al. Smoking and mortality—beyond established causes. New England journal of medicine 372 (2015): 631-40.
- McNabb S, Harrison TA, Albanes D, Berndt SI, Brenner H, Caan BJ, et al. Meta-analysis of 16 studies of the association of alcohol with colorectal cancer. International journal of cancer 146 (2020): 861-73.
- Vieira AR, Abar L, Chan DS, Vingeliene S, Polemiti E, Stevens C, et al. Foods and beverages and colorectal cancer risk: a systematic review and meta-analysis of cohort studies, an update of the evidence of the WCRF-AICR Continuous Update Project. Annals of Oncology 28 (2017): 1788-802.
- Boyle T, Keegel T, Bull F, Heyworth J, Fritschi L. Physical activity and risks of proximal and distal colon cancers: a systematic review and meta-analysis. Journal of the national cancer institute 104 (2012): 1548-61.
- Liu PH, Wu K, Ng K, Zauber AG, Nguyen LH, Song M, et al. Association of obesity with risk of early-onset colorectal cancer among women. JAMA oncology 5 (2019): 37-44.
- Nguyen LH, Liu PH, Zheng X, Keum N, Zong X, Li X, et al. Sedentary behaviors, TV viewing time, and risk of young-onset colorectal cancer. JNCI cancer spectrum (2018): pky073.
- Gausman V, Dornblaser D, Anand S, Hayes RB, O'Connell K, Du M, et al. Risk factors associated with early-onset colorectal cancer. Clinical Gastroenterology and Hepatology 18 (2020): 2752-9.
- Dik VK, van Oijen MG, Smeets HM, Siersema PD. Frequent use of antibiotics is associated with colorectal cancer risk: results of a nested case–control study. Digestive diseases and sciences 61 (2016): 255-64.
- Goncalves MD, Lu C, Tutnauer J, Hartman TE, Hwang SK, Murphy CJ, et al. High-fructose corn syrup enhances intestinal tumor growth in mice. Science 363 (2019): 1345-9.
- Cao Y, Wu K, Mehta R, Drew DA, Song M, Lochhead P, et al. Long-term use of antibiotics and risk of colorectal adenoma. Gut 67 (2018): 672-8.
- Chottanapund S, Chamroonsawasdi K, Tunyasitthisundhorn P, Aekplakorn W, Silpasuwan P, Anantachoti P, et al. Modifiable Factors and Colon Cancer Risk in Thai Population. Asian Pacific Journal of Cancer Prevention: APJCP (2021): 37.
- Giovannucci E. Modifiable risk factors for colon cancer. Gastroenterology Clinics 31 (2002): 925-43.
- Lowery JT, Ahnen DJ, Schroy III PC, Hampel H, Baxter N, Boland CR, et al. Understanding the contribution of family history to colorectal cancer risk and its clinical implications: a state-of-the-science review. Cancer 122 (2016): 2633-45.
- Samadder NJ, Smith KR, Hanson H, Pimentel R, Wong J, Boucher K, et al. Increased risk of colorectal cancer among family members of all ages, regardless of age of index case at diagnosis. Clinical Gastroenterology and Hepatology 13 (2015): 2305-11.
- Tuohy TM, Rowe KG, Mineau GP, Pimentel R, Burt RW, Samadder NJ. Risk of colorectal cancer and adenomas in the families of patients with adenomas: A population-based study in Utah. Cancer 120 (2014): 35-42.
- Pearlman R, Frankel WL, Swanson B, Zhao W, Yilmaz A, Miller K, et al. Prevalence and spectrum of germline cancer susceptibility gene mutations among patients with early-onset colorectal cancer. JAMA oncology 3 (2017): 464-71.
- Chen FW, Sundaram V, Chew TA, Ladabaum U. Low prevalence of criteria for early screening in young-onset colorectal cancer. American journal of preventive medicine 53 (2017): 933-4.
- Flynn BS, Wood ME, Ashikaga T, Stockdale A, Dana GS, Naud S. Primary care physicians' use of family history for cancer risk assessment. BMC family practice 11 (2010): 1-8.
- Wood ME, Kadlubek P, Pham TH, Wollins DS, Lu KH, Weitzel JN, et al. Quality of cancer family history and referral for genetic counseling and testing among oncology practices: a pilot test of quality measures as part of the American Society of Clinical Oncology Quality Oncology Practice Initiative. Journal of Clinical Oncology 32 (2014): 824.
- Winawer SJ. The history of colorectal cancer screening: a personal perspective. Digestive diseases and sciences 60 (2015): 596-608.
- T, Bussey HJ, Morson BC. The evolution of cancer of the colon and rectum. Cancer 36 (1975): 2251-70.
- Winawer SJ, Zauber AG, Ho MN, O'brien MJ, Gottlieb LS, Sternberg SS, et al. Prevention of colorectal cancer by colonoscopic polypectomy. New England Journal of Medicine 329 (1993): 1977- 81.
- Knudsen AB, Rutter CM, Peterse EF, Lietz AP, Seguin CL, Meester RG, et al. Colorectal Cancer Screening: An Updated Decision Analysis for the US Preventive Services Task Force.
- Gilbertsen VA, Nelms JM. The prevention of invasive cancer of the rectum. Cancer 41 (1978): 1137-9.
- Hertz RE, Deddish MR, Day E. Value of periodic examinations in detecting cancer of the rectum and colon. Postgraduate medicine 27 (1960): 290-4.
- Schoen RE, Pinsky PF, Weissfeld JL, Yokochi LA, Church T, Laiyemo AO, et al. Colorectal-cancer incidence and mortality with screening flexible sigmoidoscopy. New England Journal of Medicine 366 (2012): 2345-57.
- Atkin W, Wooldrage K, Parkin DM, Kralj-Hans I, MacRae E, Shah U, et al. Long term effects of once-only flexible sigmoidoscopy screening after 17 years of follow- up: the UK Flexible Sigmoidoscopy Screening randomised controlled trial. The Lancet 389 (2017): 1299-311.
- Segnan N, Armaroli P, Bonelli L, Risio M, Sciallero S, Zappa M, et al. Once-only sigmoidoscopy in colorectal cancer screening: follow-up findings of the Italian Randomized Controlled Trial—SCORE. Journal of the National Cancer Institute 103 (2011): 1310-22.
- Atkin WS, Edwards R, Kralj-Hans I, Wooldrage K, Hart AR, Northover JM, et al, UK Flexible Sigmoidoscopy Trial Investigators. Once-only flexible sigmoidoscopy screening in prevention of colorectal cancer: a multicentre randomised controlled trial. The Lancet 375 (2010): 1624-33.
- Holme Ø, Schoen RE, Senore C, Segnan N, Hoff G, Løberg M, et al. Effectiveness of flexible sigmoidoscopy screening in men and women and different age groups: pooled analysis of randomised trials. Bmj 13 (2017): 356.
- Holme Ø, Løberg M, Kalager M, Bretthauer M, Hernán MA, Aas E, et al. Long-term effectiveness of sigmoidoscopy screening on colorectal cancer incidence and mortality in women and men: a randomized trial. Annals of internal medicine 168 (2018): 775-82.
- Greegor DH. Diagnosis of large-bowel cancer in the asymptomatic patient. Jama 201 (1967): 943-5.
- Holme Ø, Bretthauer M, Fretheim A, Odgaard-Jensen J, Hoff G. Flexible sigmoidoscopy versus faecal occult blood testing for colorectal cancer screening in asymptomatic individuals. Cochrane Database of Systematic Reviews 9 (2013).
- Hardcastle J. Randomized control trial of faecal occult blood screening for colorectal cancer: results for the first 144,103 patients. European Journal of Cancer Prevention 1 (1991): 21-2.
- Hardcastle JD, Chamberlain JO, Robinson MH, Moss SM, Amar SS, Balfour TW, et al. Randomised controlled trial of faecal-occult-blood screening for colorectal cancer. The Lancet 348 (1996): 1472-7.
- Scholefield JH, Moss S, Sufi F, Mangham CM, Hardcastle JD. Effect of faecal occult blood screening on mortality from colorectal cancer: results from a randomised controlled trial. Gut 50 (2002): 840-4.
- Kronborg O. Screening for early colorectal cancer. World journal of surgery 24 (2000): 1069-74.
- Kronborg O. Faecal occult blood testing in the secondary prevention of colorectal cancer. European Journal of Cancer Prevention 10 (2001): 167-8.
- Kronborg O, Jørgensen OD, Fenger C, Rasmussen M. Randomized study of biennial screening with a faecal occult blood test: results after nine screening rounds. Scandinavian journal of gastroenterology 39 (2004): 846-51.
- Shaukat A, Mongin SJ, Geisser MS, Lederle FA, Bond JH, Mandel JS, Church TR. Long- term mortality after screening for colorectal cancer. New England Journal of Medicine 369 (2013): 1106-14.
- Mandel JS, Church TR, Bond JH, Ederer F, Geisser MS, Mongin SJ, et al. The effect of fecal occult-blood screening on the incidence of colorectal cancer. New England Journal of Medicine 343 (2000): 1603-7.
- Winawer SJ, Fletcher RH, Miller L, Godlee F, Stolar MH, Mulrow CD, et al. Colorectal cancer screening: clinical guidelines and rationale. Gastroenterology 112 (1997): 594-642.
- Stryker SJ, Wolff BG, Culp CE, Libbe SD, Ilstrup DM, MacCarty RL. Natural history of untreated colonic polyps. Gastroenterology 93 (1987): 1009-13.
- Hoff GA, Foerster A, Vatn MH, Sauar J, Larsen S. Epidemiology of polyps in the rectum and colon: recovery and evaluation of unresected polyps 2 years after detection. Scandinavian journal of gastroenterology 21 (1986): 853-62
- de Kanter C, Dhaliwal S, Hawks M. Colorectal Cancer Screening: Updated Guidelines from the American College of Gastroenterology. American Family Physician 105 (2022): 327-9.
- Zhao S, Wang S, Pan P, Xia T, Chang X, Yang X, et al. Magnitude, risk factors, and factors associated with adenoma miss rate of tandem colonoscopy: a systematic review and meta-analysis. Gastroenterology 156 (2019): 1661-74.
- Nishihara R, Wu K, Lochhead P, Morikawa T, Liao X, Qian ZR, et al. Long-term colorectal-cancer incidence and mortality after lower endoscopy. New England Journal of Medicine 369 (2013): 1095-105.
- Kahi CJ, Imperiale TF, Juliar BE, Rex DK. Effect of screening colonoscopy on colorectal cancer incidence and mortality. Clinical gastroenterology and hepatology 7 (2009): 770-5.
- Manser CN, Bachmann LM, Brunner J, Hunold F, Bauerfeind P, Marbet UA. Colonoscopy screening markedly reduces the occurrence of colon carcinomas and carcinoma-related death: a closed cohort study. Gastrointestinal endoscopy 76 (2012): 110-7.
- Kahi CJ, Pohl H, Myers LJ, Mobarek D, Robertson DJ, Imperiale TF. Colonoscopy and colorectal cancer mortality in the veterans affairs health care system: a case–control study. Annals of Internal Medicine 168 (2018): 481-8.
- Kaminski MF, Bretthauer M, Zauber AG, Kuipers EJ, Adami HO, van Ballegooijen M, et al. The NordICC Study: rationale and design of a randomized trial on colonoscopy screening for colorectal cancer. Endoscopy 44 (2012): 695-702.
- Bretthauer M, Kaminski MF, Løberg M, Zauber AG, Regula J, Kuipers EJ, et al. Population-based colonoscopy screening for colorectal cancer: a randomized clinical trial. JAMA internal medicine 176 (2016): 894-902.
- Barrows GH, Burton RM, Jarrett DD, Russell GG, Alford MD, Songster CL. Immunochemical detection of human blood in feces. American Journal of Clinical Pathology 69 (1978): 342-6.
- Allison JE, Sakoda LC, Levin TR, Tucker JP, Tekawa IS, Cuff T, et al. Screening for colorectal neoplasms with new fecal occult blood tests: update on performance characteristics. Journal of the National Cancer Institute 99 (2007): 1462-70.
- Robertson DJ, Lee JK, Boland CR, Dominitz JA, Giardiello FM, Johnson DA, et al. Recommendations on fecal immunochemical testing to screen for colorectal neoplasia: a consensus statement by the US Multi-Society Task Force on Colorectal Cancer. Gastroenterology 152 (2017): 1217-37.
- Imperiale TF, Gruber RN, Stump TE, Emmett TW, Monahan PO. Performance characteristics of fecal immunochemical tests for colorectal cancer and advanced adenomatous polyps: a systematic review and meta-analysis. Annals of internal medicine 170 (2019): 319-29.
- Lee JK, Liles EG, Bent S, Levin TR, Corley DA. Accuracy of fecal immunochemical tests for colorectal cancer: systematic review and meta-analysis. Annals of internal medicine 160 (2014): 171-81.
- Imperiale TF. Quantitative immunochemical fecal occult blood tests: is it time to go back to the future?. Annals of internal medicine 146 (2007): 309-11.
- Whitlock EP, Lin JS, Liles E, Beil TL, Fu R. Screening for colorectal cancer: a targeted, updated systematic review for the US Preventive Services Task Force. Annals of internal medicine 149 (2008): 638-58.
- Zheng S, Chen K, Liu X, Ma X, Yu H, Chen K, et al. Cluster randomization trial of sequence mass screening for colorectal cancer. Diseases of the colon & rectum 46 (2003): 51-8.
- Jodal HC, Helsingen LM, Anderson JC, Lytvyn L, Vandvik PO, Emilsson L. Colorectal cancer screening with faecal testing, sigmoidoscopy or colonoscopy: a systematic review and network meta-analysis. BMJ open 9 (2019): e032773.
- Buskermolen M, Cenin DR, Helsingen LM, Guyatt G, Vandvik PO, Haug U, et al. Colorectal cancer screening with faecal immunochemical testing, sigmoidoscopy or colonoscopy: a microsimulation modelling study. Bmj 367 (2019).
- Qaseem A, Crandall CJ, Mustafa RA, Hicks LA, Wilt TJ, Clinical Guidelines Committee of the American College of Physicians*. Screening for colorectal cancer in asymptomatic average-risk adults: a guidance statement from the American College of Physicians. Annals of internal medicine 171 (2019): 643-54.
- Imperiale TF, Ransohoff DF, Itzkowitz SH, Turnbull BA, Ross ME. Fecal DNA versus fecal occult blood for colorectal-cancer screening in an average-risk population. New England journal of medicine 351 (2004): 2704-14.
- Tepus M, Yau TO. Non-invasive colorectal cancer screening: an overview. Gastrointestinal tumors 7 (2020): 62-73.
- Allison JE, Tekawa IS, Ransom LJ, Adrain AL. A comparison of fecal occult-blood tests for colorectal-cancer screening. New England Journal of Medicine 334 (1996): 155-60.
- Shapiro JA, Bobo JK, Church TR, Rex DK, Chovnick G, Thompson TD, et al. A comparison of fecal immunochemical and high-sensitivity guaiac tests for colorectal cancer screening. The American journal of gastroenterology 112 (2017): 1728.
- https://wayback.archive-it.org/7993/20170112222835/http:/www.fda.gov/NewsEvents/Newsroom/PressAnnouncem ents/ucm409021.html
- Olson JE, Kirsch EJ, Kirt CR, Kneedler B, Laffin JJ, Weaver AL, et al. Colorectal cancer outcomes after screening with the multi-target stool DNA assay: protocol for a large-scale, prospective cohort study (the Voyage study). BMJ Open Gastroenterology 7 (2020): e000353.
- Naber SK, Knudsen AB, Zauber AG, Rutter CM, Fischer SE, Pabiniak CJ, et al. Cost-effectiveness of a multitarget stool DNA test for colorectal cancer screening of Medicare beneficiaries. PloS one 14 (2019): e0220234.
- Vinging DJ, Gelfand D, Bechtola RE. Technical feasibility of colon imaging with helical CT. AJR (American Journal of Roentgenology) 1629 (1994): 104.
- Herfarth H, Schreyer AG. The virtuosity of virtuality or how real is virtual colonography. Gut 52 (2003): 1662-4.
- Lauenstein TC, Herborn CU, Vogt FM, Göhde SC, Debatin JF, Ruehm SG. Dark lumen MR-colonography: initial experience. InRöFo-Fortschritte auf dem Gebiet der Röntgenstrahlen und der bildgebenden Verfahren 173 (2001): 785-789.
- Debatin JF, Lauenstein TC. Virtual magnetic resonance colonography. Gut 52 (2003): iv17-22.
- Ajaj W, Pelster G, Treichel U, Vogt FM, Debatin JF, Ruehm SG, Lauenstein TC. Dark lumen magnetic resonance colonography: comparison with conventional colonoscopy for the detection of colorectal pathology. Gut 52 (2003): 1738-43.
- Wessling J, Fischbach R, Domagk D, Lügering N, Neumann E, Heindel W. Colorectal polyps: Detection with multi-slice CT colonography. InRöFo-Fortschritte auf dem Gebiet der Röntgenstrahlen und der bildgebenden Verfahren 173 (2001): 1069-1071.
- Yee J, Akerkar GA, Hung RK, Steinauer-Gebauer AM, Wall SD, McQuaid KR. Colorectal neoplasia: performance characteristics of CT colonography for detection in 300 patients. Radiology 219 (2001): 685-92.
- Rembacken BJ, Fujii T, Cairns A, Dixon MF, Yoshida S, Chalmers DM, Axon AT. Flat and depressed colonic neoplasms: a prospective study of 1000 colonoscopies in the UK. The Lancet 355 (2000): 1211-4.
- Saitoh Y, Waxman I, West AB, Popnikolov NK, Gatalica Z, Watari J, et al. Prevalence and distinctive biologic features of flat colorectal adenomas in a North American population. Gastroenterology 120 (2001): 1657-65.
- Fidler JL, Johnson CD, MacCarty RL, Welch TJ, Hara AK, Harmsen WS. Detection of flat lesions in the colon with CT colonography. Abdominal imaging 27 (2002): 292- 300.
- Spinzi G, Belloni G, Martegani A, Sangiovanni A, Del Favero C, Minoli G. Computed tomographic colonography and conventional colonoscopy for colon diseases: a prospective, blinded study. The American journal of gastroenterology 96 (2001): 394-400.
- Regge D, Iussich G, Segnan N, Correale L, Hassan C, Arrigoni A, et al. Comparing CT colonography and flexible sigmoidoscopy: a randomised trial within a population-based screening programme. Gut 66 (2017): 1434-40.
- Sali L, Mascalchi M, Falchini M, Ventura L, Carozzi F, Castiglione G, et al. Reduced and full-preparation CT colonography, fecal immunochemical test, and colonoscopy for population screening of colorectal cancer: a randomized trial. Journal of the National Cancer Institute 108 (2016): djv319.
- De Haan MC, Pickhardt PJ, Stoker J. CT colonography: accuracy, acceptance, safety and position in organised population screening. Gut 64 (2015): 342-50.
- Wolff WI, Shinya H. Polypectomy via the fiberoptic colonoscope: removal of neoplasms beyond reach of the sigmoidoscope. New England Journal of Medicine 288 (1973): 329-32.
- Mandel JS, Bond JH, Church TR, Snover DC, Bradley GM, Schuman LM, et al. Reducing mortality from colorectal cancer by screening for fecal occult blood. New England Journal of Medicine 328 (1993): 1365-71.
- Kronborg O, Fenger C, Olsen J, Jørgensen OD, Søndergaard O. Randomised study of screening for colorectal cancer with faecal-occult-blood test. The Lancet 348 (1996): 1467-71.
- Maisonneuve P, Botteri E, Lowenfels AB. Screening and surveillance for the early detection of colorectal cancer and adenomatous polyps. Gastroenterology 135 (2008): 710.
- Byers T, Levin B, Rothenberger D, Dodd GD, Smith RA, American Cancer Society Detection and Treatment Advisory Group on Colorectal Cancer). American Cancer Society guidelines for screening and surveillance for early detection of colorectal polyps and cancer: update 1997. CA: a cancer journal for clinicians 47 (1997): 154-60.
- Selby JV, Friedman GD, Quesenberry Jr CP, Weiss NS. A case–control study of screening sigmoidoscopy and mortality from colorectal cancer. New England Journal of Medicine 326 (1992): 653-7.
- Rabeneck L, Chiu HM, Senore C. International perspective on the burden of colorectal cancer and public health effects. Gastroenterology 158 (2020): 447-52.
- Onyoh EF, Hsu WF, Chang LC, Lee YC, Wu MS, Chiu HM. The rise of colorectal cancer in Asia: epidemiology, screening, and management. Current gastroenterology reports 21 (2019): 1-0.
- Sung JJ, Chiu HM, Lieberman D, Kuipers EJ, Rutter MD, Macrae F, et al. Third Asia-Pacific consensus recommendations on colorectal cancer screening and postpolypectomy surveillance. Gut 71 (2022): 2152-66.
- Stewart DB. Updated USPSTF guidelines for colorectal cancer screening: the earlier the better. JAMA surgery 156 (2021): 708-9.
- Siegel RL, Jakubowski CD, Fedewa SA, Davis A, Azad NS. Colorectal cancer in the young: epidemiology, prevention, management. American Society of Clinical Oncology Educational Book 40 (2020): e75-88.
- https://eur- lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2003:327:0034:0038:EN:PDF
- Gogenur I, Qvortrup C. Colorectal cancer screening in Europe: what are the next steps?. The Lancet. Oncology 22 (2021): 898-9.
- https://ec.europa.eu/commission/presscorner/detail/en/ip_22_7548
- https://cancer-code-europe.iarc.fr/index.php/en/ecac-12-ways/screening-recommandation/bowel-cancer screening/221-when-should-i-participate-in-bowel-cancer- screening
- Cardoso R, Guo F, Heisser T, Hackl M, Ihle P, De Schutter H, et al. Colorectal cancer incidence, mortality, and stage distribution in European countries in the colorectal cancer screening era: an international population-based study. The Lancet Oncology 22 (2021): 1002-13.
- Kwakye G, Dally CK. Colorectal cancer screening in sub-Saharan Africa. The Lancet Global Health 10 (2022): e938-9.
- Carroll MR, Seaman HE, Halloran SP. Tests and investigations for colorectal cancer screening. Clinical biochemistry 47 (2014): 921-39.
- Issa IA, Noureddine M. Colorectal cancer screening: An updated review of the available options. World journal of gastroenterology 23 (2017): 5086.
- Senore C, Inadomi J, Segnan N, Bellisario C, Hassan C. Optimising colorectal cancer screening acceptance: a review. Gut 64 (2015): 1158-77.
- Hoff G, Grotmol T, Skovlund E, Bretthauer M. Risk of colorectal cancer seven years after flexible sigmoidoscopy screening: randomised controlled trial. Bmj 338 (2009).
- Kastenberg D, Bertiger G, Brogadir S. Bowel preparation quality scales for colonoscopy. World journal of gastroenterology 24 (2018): 2833.
- Millien VO, Mansour NM. Bowel preparation for colonoscopy in 2020: a look at the past, present, and future. Current Gastroenterology Reports 22 (2020): 1-9.
- Pavlidis E, Gkizas I, Mavromati O, Milonakis N, Syrianos K. Splenic injury following elective colonoscopy: a rare complication. Journal of Surgical Case Reports 21 (2016): rjw214.
- Reumkens A, Rondagh EJ, Bakker MC, Winkens B, Masclee AA, Sanduleanu S. Post- colonoscopy complications: a systematic review, time trends, and meta-analysis of population-based studies. Official journal of the American College of Gastroenterology| ACG 111 (2016): 1092-101.
- Ullah W, Rashid MU, Mehmood A, Zafar Y, Hussain I, Sarvepalli D, Hasan MK. Splenic injuries secondary to colonoscopy: Rare but serious complication. World Journal of Gastrointestinal Surgery 12 (2020) Feb 27;12(2):55.
- Rutter MD, Beintaris I, Valori R, Chiu HM, Corley DA, Cuatrecasas M, Dekker E, Forsberg A, Gore-Booth J, Haug U, Kaminski MF. World Endoscopy Organization consensus statements on post-colonoscopy and post-imaging colorectal cancer. Gastroenterology 155 (2018): 909-25.
- Ertem FU, Ladabaum U, Mehrotra A, Tehranian S, Shi Z, Saul M, Morris M, Crockett SD, Schoen RE. Incidence of interval colorectal cancer attributable to an endoscopist in clinical practice. Gastrointestinal endoscopy 88 (2018): 705-11.
- Lin JS, Piper MA, Perdue LA, Rutter C, Webber EM, O’Connor E, Smith N, Whitlock EP. Screening for colorectal cancer: a systematic review for the US Preventive Services Task Force.
- Zapka J, Klabunde CN, Taplin S, Yuan G, Ransohoff D, Kobrin S. Screening colonoscopy in the US: attitudes and practices of primary care physicians. Journal of general internal medicine 27 (2012): 1150-8.
- Akerkar GA, Yee J, Hung R, McQuaid K. Patient experience and preferences toward colon cancer screening: a comparison of virtual colonoscopy and conventional colonoscopy. Gastrointestinal endoscopy 54 (2001): 310-5.
- Thomeer M, Bielen D, Vanbeckevoort D, Dymarkowski S, Gevers A, Rutgeerts P, et al. Patient acceptance for CT colonography: what is the real issue?. European radiology 12 (2002): 1410-5.
- Svensson MH, Svensson E, Lasson A, Hellström M. Patient acceptance of CT colonography and conventional colonoscopy: prospective comparative study in patients with or suspected of having colorectal disease. Radiology 222 (2002): 337-45.
- Senore C, Correale L, Regge D, Hassan C, Iussich G, Silvani M, et al. Flexible sigmoidoscopy and CT colonography screening: patients’ experience with and factors for undergoing screening-insight from the PROTEUS colon trial.
- Obaro AE, Burling DN, Plumb AA. Colon cancer screening with CT colonography: logistics, cost-effectiveness, efficiency and progress. The British journal of radiology 91 (2018): 20180307.
- Neri E, Giusti P, Battolla L, Vagli P, Boraschi P, Lencioni R, et al. Colorectal cancer: role of CT colonography in preoperative evaluation after incomplete colonoscopy. Radiology 223 (2002): 615-9.
- Morrin MM, Farrell RJ, Raptopoulos V, McGee JB, Bleday R, Kruskal JB. Role of virtual computed tomographic colonography in patients with colorectal cancers and obstructing colorectal lesions. Diseases of the colon & rectum 43 (2000): 303-11.
- Fenlon HM, McAneny DB, Nunes DP, Clarke PD, Ferrucci JT. Occlusive colon carcinoma: virtual colonoscopy in the preoperative evaluation of the proximal colon. Radiology 210 (1999): 423-8.
- Schreyer AG, Herfarth H, Kikinis R, Seitz J, Schölmerich J, Geissler A, Feuerbach S. 3D modeling and virtual endoscopy of the small bowel based on magnetic resonance imaging in patients with inflammatory bowel disease. Investigative radiology 37 (2002): 528-33.
- Gluecker TM, Johnson CD, Wilson LA, MacCarty RL, Welch TJ, Vanness DJ, Ahlquist DA. Extracolonic findings at CT colonography: evaluation of prevalence and cost in a screening population. Gastroenterology 124 (2003): 911-6.
- Laghi A, Iannaccone R, Bria E, Carbone I, Trasatti L, Piacentini F, et al. Contrast-enhanced computed tomographic colonography in the follow-up of colorectal cancer patients: a feasibility study. European radiology 13 (2003): 883- 9.
- Winawer S, Fletcher R, Rex D, Bond J, Burt R, Ferrucci J, et al. Colorectal cancer screening and surveillance: clinical guidelines and rationale—update based on new evidence. Gastroenterology 124 (2003): 544-60.
- Colorectal cancer screening
- Rex DK, Adler SN, Aisenberg J, Burch Jr WC, Carretero C, Chowers Y, et al. Accuracy of capsule colonoscopy in detecting colorectal polyps in a screening population. Gastroenterology 148 (2015): 948-57.
- Kobaek-Larsen M, Kroijer R, Dyrvig AK, Buijs MM, Steele RJ, Qvist N, et al. Back-to-back colon capsule endoscopy and optical colonoscopy in colorectal cancer screening individuals. Colorectal Disease 20 (2018): 479-85.
- Cash BD, Fleisher MR, Fern S, Rajan E, Haithcock R, Kastenberg DM, et al. 479 A multicenter, prospective, randomized study comparing the diagnostic yield of colon capsule endoscopy versus computed tomographic colonography in a screening population. Results of the TOPAZ study. Gastrointestinal Endoscopy 89 (2019): AB87-8.
- Van Dam L, Bretthauer M. Ethical issues in colorectal cancer screening. Best Practice & Research Clinical Gastroenterology 28 (2014): 315-26.
- Bibbins-Domingo K, Grossman DC, Curry SJ, Davidson KW, Epling JW, García FA, et al. Screening for colorectal cancer: US Preventive Services Task Force recommendation statement. Jama 315 (2016): 2564-75.
- Chido-Amajuoyi OG, Sharma A, Talluri R, Tami-Maury I, Shete S. Physician-office vs home uptake of colorectal cancer screening using FOBT/FIT among screening-eligible US adults. Cancer medicine 8 (2019): 7408-18.
- Li JN, Yuan SY. Fecal occult blood test in colorectal cancer screening. Journal of digestive diseases 20 (2019): 62-4.
- Fraser CG, Allison JE, Halloran SP, Young GP. Expert working group on fecal immunochemical tests for hemoglobin, colorectal cancer screening committee, world endoscopy organization. A proposal to standardize reporting units for fecal immunochemical tests for hemoglobin. J Natl Cancer Inst 104 (2012): 810-4.
- Akram A, Juang D, Bustamante R, Liu L, Earles A, Ho SB, et al. Replacing the guaiac fecal occult blood test with the fecal immunochemical test increases proportion of individuals screened in a large healthcare setting. Clinical Gastroenterology and Hepatology 15 (2017): 1265-70.
- Zhu MM, Xu XT, Nie F, Tong JL, Xiao SD, Ran ZH. Comparison of immunochemical and guaiac-based fecal occult blood test in screening and surveillance for advanced colorectal neoplasms: a meta-analysis. Journal of digestive diseases 11 (2010): 148- 60.
- Vart G, Banzi R, Minozzi S. Comparing participation rates between immunochemical and guaiac faecal occult blood tests: a systematic review and meta-analysis. Preventive medicine 55 (2012): 87-92.
- Wu D, Luo HQ, Zhou WX, Qian JM, Li JN. The performance of three-sample qualitative immunochemical fecal test to detect colorectal adenoma and cancer in gastrointestinal outpatients: an observational study. PloS one 9 (2014): e106648.
- Rozen P, Comaneshter D, Levi Z, Hazazi R, Vilkin A, Maoz E, et al. Cumulative evaluation of a quantitative immunochemical fecal occult blood test to determine its optimal clinical use. Cancer 116 (2010): 2115-25.
- Levi Z, Rozen P, Hazazi R, Vilkin A, Waked A, Maoz E, et al. A quantitative immunochemical fecal occult blood test for colorectal neoplasia. Annals of internal medicine 146 (2007): 244-55.
- Zorzi M, Fedeli U, Schievano E, Bovo E, Guzzinati S, Baracco S, et al. Impact on colorectal cancer mortality of screening programmes based on the faecal immunochemical test. Gut 64 (2015): 784-90.
- Clebak KT, Nickolich S, Mendez-Miller M. Multitarget Stool DNA Testing (Cologuard) for Colorectal Cancer Screening. American family physician 105 (2022): 198-200.
- Imperiale TF, Ransohoff DF, Itzkowitz SH, Levin TR, Lavin P, Lidgard GP, Ahlquist DA, Berger BM. Multitarget stool DNA testing for colorectal-cancer screening. New England Journal of Medicine 370 (2014): 1287-97.
- Huang CS, Lal SK, Farraye FA. Colorectal cancer screening in average risk individuals. Cancer Causes & Control 16 (2005): 171-88.
- Ladabaum U, Mannalithara A. Comparative effectiveness and cost effectiveness of a multitarget stool DNA test to screen for colorectal neoplasia. Gastroenterology 151 (2016): 427-39.
- Green BB, Coronado GD, Devoe JE, Allison J. Navigating the murky waters of colorectal cancer screening and health reform. American journal of public health 104 (2014): 982-6.
- Lin JS, Piper MA, Perdue LA, Rutter CM, Webber EM, O’Connor E, Smith N, Whitlock EP. Screening for colorectal cancer: updated evidence report and systematic review for the US Preventive Services Task Force. Jama 315 (2016): 2576-94.
- Mostowy S, Cossart P. Septins: the fourth component of the cytoskeleton. Nature reviews Molecular cell biology 13 (2012): 183-94.
- Bertin A, McMurray MA, Thai L, Garcia III G, Votin V, Grob P, et al. Phosphatidylinositol-4, 5-bisphosphate promotes budding yeast septin filament assembly and organization. Journal of molecular biology 404 (2010): 711-31.
- Dolat L, Hu Q, Spiliotis ET. Septin functions in organ system physiology and pathology. Biological chemistry 395 (2014): 123-41.
- Tóth K, Galamb O, Spisák S, Wichmann B, Sipos F, Valcz G, Leiszter K, Molnár B, Tulassay Z. The influence of methylated septin 9 gene on RNA and protein level in colorectal cancer. Pathology & Oncology Research 17 (2011): 503-9.
- Yang X, Lay F, Han H, Jones PA. Targeting DNA methylation for epigenetic therapy. Trends in pharmacological sciences 31 (2010): 536-46.
- Krokowski S, Mostowy S. Bacterial cell division is recognized by the septin cytoskeleton for restriction by autophagy. Autophagy 15 (2019): 937-9.
- Grützmann R, Molnar B, Pilarsky C, Habermann JK, Schlag PM, Saeger HD, et al. Sensitive detection of colorectal cancer in peripheral blood by septin 9 DNA methylation assay. PloS one 3 (2008): e3759.
- Füchtbauer A, Lassen LB, Jensen AB, Howard J, Quiroga AD, Warming S, et al. Septin9 is involved in septin filament formation and cellular stability.
- Song L, Li Y. SEPT9: a specific circulating biomarker for colorectal cancer. advances in clinical chemistry 72 (2015): 171-204.
- Sun J, Fei F, Zhang M, Li Y, Zhang X, Zhu S, Zhang S. The role of mSEPT9 in screening, diagnosis, and recurrence monitoring of colorectal cancer. BMC cancer 19 (2019): 1-0.
- Song L, Yu H, Jia J, Li Y. A systematic review of the performance of the SEPT9 gene methylation assay in colorectal cancer screening, monitoring, diagnosis and prognosis. Cancer Biomarkers 18 (2017): 425-32.
- Ma Z, Williams M, Cheng YY, Leung WK. Roles of methylated DNA biomarkers in patients with colorectal cancer. Disease markers 3 (2019).
- Xu F, Yu S, Han J, Zong M, Tan Q, Zeng X, Fan L. Detection of Circulating Tumor DNA Methylation in Diagnosis of Colorectal Cancer. Clinical and Translational Gastroenterology 8 (2021).
- Chan AT, Arber N, Burn J, Chia WK, Elwood P, Hull MA, Logan RF, Rothwell PM, Schrör K, Baron JA. Aspirin in the Chemoprevention of Colorectal Neoplasia: An OverviewAspirin in Chemoprevention of Colorectal Neoplasia. Cancer prevention research 5 (2012): 164-78.
- Wang D, DuBois RN. Eicosanoids and cancer. Nature Reviews Cancer 10 (2010): 181-93.
- Gala MK, Chan AT. Molecular Pathways: Aspirin and Wnt Signaling—A Molecularly Targeted Approach to Cancer Prevention and TreatmentAspirin and Wnt Signaling. Clinical Cancer Research 21 (2015): 1543-8.
- Zumwalt TJ, Wodarz D, Komarova NL, Toden S, Turner J, Cardenas J, Burn J, Chan AT, Boland CR, Goel A. Aspirin-Induced Chemoprevention and Response Kinetics Are Enhanced by PIK3CA Mutations in Colorectal Cancer CellsAspirin Sensitivity in PIK3CA- Mutant Colorectal Cancer Cells. Cancer Prevention Research 10 (2017): 208-18.
- Pan MR, Chang HC, Hung WC. Non-steroidal anti-inflammatory drugs suppress the ERK signaling pathway via block of Ras/c-Raf interaction and activation of MAP kinase phosphatases. Cellular signalling 20 (2008): 1134-41.
- Ying J, Zhou HY, Liu P, You Q, Kuang F, Shen YN, Hu ZQ. Aspirin inhibited the metastasis of colon cancer cells by inhibiting the expression of toll-like receptor 4. Cell & bioscience 8 (2018): 1-9.
- Din FV, Valanciute A, Houde VP, Zibrova D, Green KA, Sakamoto K, et al. Aspirin inhibits mTOR signaling, activates AMP-activated protein kinase, and induces autophagy in colorectal cancer cells. Gastroenterology 142 (2012): 1504- 15.
- Gu Q, Wang JD, Xia HH, Lin MC, He H, Zou B, et al. Activation of the caspase-8/Bid and Bax pathways in aspirin-induced apoptosis in gastric cancer. Carcinogenesis 26 (2005): 541-6.
- Wang Y, Du C, Zhang N, Li M, Liu Y, Zhao M, et al. TGF-β1 mediates the effects of aspirin on colonic tumor cell proliferation and apoptosis. Oncology letters 15 (2018): 5903-9.
- Patrono C. The multifaceted clinical readouts of platelet inhibition by low-dose aspirin. Journal of the American College of Cardiology 66 (2015): 74-85.
- Mizuno R, Kawada K, Sakai Y. Prostaglandin E2/EP signaling in the tumor microenvironment of colorectal cancer. International Journal of Molecular Sciences 20 (2019): 6254.
- Flossmann E, Rothwell PM. Effect of aspirin on long-term risk of colorectal cancer: consistent evidence from randomised and observational studies. The Lancet 369 (2007): 1603-13.
- Thun MJ, Namboodiri MM, Heath Jr CW. Aspirin use and reduced risk of fatal colon cancer. New England Journal of Medicine 325 (1991): 1593-6.
- Jacobs EJ, Thun MJ, Bain EB, Rodriguez C, Henley SJ, Calle EE. A large cohort study of long-term daily use of adult-strength aspirin and cancer incidence. Journal of the National Cancer Institute 99 (2007): 608-15.
- Chan AT, Giovannucci EL, Meyerhardt JA, Schernhammer ES, Wu K, Fuchs CS. Aspirin dose and duration of use and risk of colorectal cancer in men. Gastroenterology 134 (2008): 21-8.
- Chan AT, Giovannucci EL, Meyerhardt JA, Schernhammer ES, Curhan GC, Fuchs CS. Long-term use of aspirin and nonsteroidal anti-inflammatory drugs and risk of colorectal cancer. Jama 294 (2005): 914-23.
- Rothwell PM, Fowkes FG, Belch JF, Ogawa H, Warlow CP, Meade TW. Effect of daily aspirin on long-term risk of death due to cancer: analysis of individual patient data from randomised trials. The Lancet 377 (2011): 31-41.
- Ridker PM, Cook NR, Lee IM, Gordon D, Gaziano JM, Manson JE, et al. A randomized trial of low-dose aspirin in the primary prevention of cardiovascular disease in women. New England Journal of Medicine 352 (2005): 1293-304.
- Benamouzig R, Deyra J, Martin A, Girard B, Jullian E, Piednoir B, Couturier D, Coste T, Little J, Chaussade S. Daily soluble aspirin and prevention of colorectal adenoma recurrence: one-year results of the APACC trial. Gastroenterology 125 (2003): 328-36.
- Logan RF, Grainge MJ, Shepherd VC, Armitage NC, Muir KR. Uk CAP Trial Group. Aspirin and folic acid for the prevention of recurrent colorectal adenomas. Gastroenterology 134 (2008): 29-38.
- Sandler RS, Halabi S, Baron JA, Budinger S, Paskett E, Keresztes R, et al. A randomized trial of aspirin to prevent colorectal adenomas in patients with previous colorectal cancer. New England Journal of Medicine 348 (2003): 883-90.
- Baron JA, Cole BF, Sandler RS, Haile RW, Ahnen D, Bresalier R, et al. A randomized trial of aspirin to prevent colorectal adenomas. New England Journal of Medicine 348 (2003): 891- 9.
- Cole BF, Logan RF, Halabi S, Benamouzig R, Sandler RS, Grainge MJ, et al. Aspirin for the chemoprevention of colorectal adenomas: meta-analysis of the randomized trials. JNCI: Journal of the National Cancer Institute 101 (2009): 256- 66.
- Tsoi KK, Chan FC, Hirai HW, Sung JJ. Risk of gastrointestinal bleeding and benefit from colorectal cancer reduction from long-term use of low-dose aspirin: A retrospective study of 612 509 patients. Journal of Gastroenterology and Hepatology 33 (2018): 1728- 36.
- Lanas A, Wu P, Medin J, Mills EJ. Low doses of acetylsalicylic acid increase risk of gastrointestinal bleeding in a meta-analysis. Clinical Gastroenterology and Hepatology 9 (2011): 762-8.
- Brenner H, Calderazzo S, Seufferlein T, Ludwig L, Dikopoulos N, Mangold J, et al. Effect of a single aspirin dose prior to fecal immunochemical testing on test sensitivity for detecting advanced colorectal neoplasms: a randomized clinical trial. Jama 321 (2019): 1686-92.
- Randel KR, Botteri E, Romstad KM, Frigstad SO, Bretthauer M, Hoff G, et al. Effects of oral anticoagulants and aspirin on performance of fecal immunochemical tests in colorectal cancer screening. Gastroenterology 156 (2019): 1642-9.
- Nieuwenburg SA, Vuik FE, Kruip MJ, Kuipers EJ, Spaander MC. Effect of anticoagulants and NSAIDs on accuracy of faecal immunochemical tests (FITs) in colorectal cancer screening: a systematic review and meta-analysis. Gut 68 (2019): 866-72.
- Burn J, Sheth H, Elliott F, Reed L, Macrae F, Mecklin JP, et al. Cancer prevention with aspirin in hereditary colorectal cancer (Lynch syndrome), 10-year follow-up and registry-based 20-year data in the CAPP2 study: a double-blind, randomised, placebo-controlled trial. The Lancet 395 (2020): 1855-63.
- Grancher A, Michel P, Di Fiore F, Sefrioui D. Colorectal cancer chemoprevention: is aspirin still in the game?. Cancer Biology & Therapy 23 (2022): 446-61.
- Aspirin Use to Prevent Cardiovascular Disease: Preventive Medication
- Siegel RL, Jemal A. Percentage of colorectal cancer diagnosed in adults aged younger than 50 years. Cancer 122 (2016): 1462-3.
- Meester RG, Peterse EF, Knudsen AB, de Weerdt AC, Chen JC, Lietz AP, et al. Optimizing colorectal cancer screening by race and sex: microsimulation analysis II to inform the American Cancer Society colorectal cancer screening guideline. Cancer 124 (2018): 2974-85.
- Peterse EF, Meester RG, Siegel RL, Chen JC, Dwyer A, Ahnen DJ, et al. The impact of the rising colorectal cancer incidence in young adults on the optimal age to start screening: microsimulation analysis I to inform the American Cancer Society colorectal cancer screening guideline. Cancer 124 (2018): 2964-73.
- Wolf AM, Fontham ET, Church TR, Flowers CR, Guerra CE, LaMonte SJ, et al. Colorectal cancer screening for average-risk adults: 2018 guideline update from the American Cancer Society. CA: a cancer journal for clinicians 68 (2018): 250-81.
- Siegel RL, Fedewa SA, Anderson WF, Miller KD, Ma J, Rosenberg PS, Jemal A. Colorectal cancer incidence patterns in the United States, 1974–2013. JNCI: Journal of the National Cancer Institute 109 (2017): djw322.
- Chung RY, Tsoi KK, Kyaw MH, Lui AR, Lai FT, Sung JJ. A population-based age- period-cohort study of colorectal cancer incidence comparing Asia against the West. Cancer epidemiology 59 (2019): 29-36.
- Yang Y, Land KC. Age-period-cohort analysis: New models, methods, and empirical applications. Taylor & Francis (2013).
- Whatley Z, Daram SR, Yousuf S, Tang SJ. Gastrointestinal cancers in Mississippi. Southern Medical Journal 107 (2014): 229-34.
- Stoffel EM, Murphy CC. Epidemiology and mechanisms of the increasing incidence of colon and rectal cancers in young adults. Gastroenterology 158 (2020): 341-53.
- Murphy CC, Wallace K, Sandler RS, Baron JA. Racial disparities in incidence of young- onset colorectal cancer and patient survival. Gastroenterology 156 (2019): 958-65.
- Vilar E, Stoffel EM. Universal genetic testing for younger patients with colorectal cancer. JAMA oncology 3 (2017): 448-9.
- Stoffel EM. Colorectal Cancer in Young Individuals: Opportunities for Prevention. Journal of Clinical Oncology: Official Journal of the American Society of Clinical Oncology 33 (2015): 3525-7.
- Klabunde CN, Schenck AP, Davis WW. Barriers to colorectal cancer screening among Medicare consumers. American journal of preventive medicine 30 (2006): 313-9.
- Dharni N, Armstrong D, Chung-Faye G, Wright AJ. Factors influencing participation in colorectal cancer screening—a qualitative study in an ethnic and socio-economically diverse inner city population. Health Expectations 20 (2017): 608-17.
- Gimeno Garcia AZ. Factors influencing colorectal cancer screening participation. Gastroenterology research and practice (2012).
- Viguier J, Morere JF, Pivot X, Cortot AB, Blay JY, Touboul C, et al. Factors influencing colorectal cancer screening participation rates in 2016. Annals of Oncology 28 (2017): iii90-1.
- de Bekker-Grob EW, Donkers B, Veldwijk J, Jonker MF, Buis S, Huisman J, et al. What factors influence non-participation most in colorectal cancer screening? A discrete choice experiment. The Patient-Patient-Centered Outcomes Research 14 (2021): 269- 81.
- Zhu X, Weiser E, Griffin JM, Limburg PJ, Rutten LJ. Factors influencing colorectal cancer screening decision-making among average-risk US adults. Preventive Medicine.
- Del Vecchio Blanco G, Dwairi R, Giannelli M, Palmieri G, Formica V, Portarena I, et al. Clinical care pathway program versus open-access system: a study on appropriateness, quality, and efficiency in the delivery of colonoscopy in the colorectal cancer. Internal and Emergency Medicine 16 (2021): 1197-206.
- Schreuders EH, Ruco A, Rabeneck L, Schoen RE, Sung JJ, Young GP, Kuipers EJ. Colorectal cancer screening: a global overview of existing programmes. Gut 64 (2015): 1637-49.
- Doubeni CA, Corley DA, Levin TR. Time to diagnostic testing after a positive colorectal cancer screening test. JAMA 318 (2017): 483.
- Corley DA, Jensen CD, Quinn VP, Doubeni CA, Zauber AG, Lee JK, et al. Association between time to colonoscopy after a positive fecal test result and risk of colorectal cancer and cancer stage at diagnosis. Jama 317 (2017): 1631-41.
- Lin JS, Perdue LA, Henrikson NB, Bean SI, Blasi PR. Screening for Colorectal Cancer: An Evidence Update for the US Preventive Services Task Force.
- Halm EA, Beaber EF, McLerran D, Chubak J, Corley DA, Rutter CM, et al. Association between primary care visits and colorectal cancer screening outcomes in the era of population health outreach. Journal of general internal medicine 31 (2016): 1190-7.
- Chubak J, Garcia MP, Burnett-Hartman AN, Zheng Y, Corley DA, Halm EA, et al. Time to Colonoscopy after Positive Fecal Blood Test in Four US Health Care SystemsTime to Colonoscopy Follow-Up. Cancer Epidemiology, Biomarkers & Prevention 25 (2016): 344-50.
- May FP, Yano EM, Provenzale D, Brunner J, Yu C, Phan J, et al. Barriers to follow-up colonoscopies for patients with positive results from fecal immunochemical tests during colorectal cancer screening. Clinical Gastroenterology and Hepatology 17 (2019): 469-76.
- Idigoras I, Arrospide A, Portillo I, Arana-Arri E, Martínez-Indart L, Mar J, et al. Evaluation of the colorectal cancer screening Programme in the Basque Country (Spain) and its effectiveness based on the Miscan-colon model. BMC Public Health 18 (2018): 1-2.
- Kapidzic A, van der Meulen MP, Hol L, van Roon AH, Looman CW, Lansdorp-Vogelaar I, et al. Gender differences in fecal immunochemical test performance for early detection of colorectal neoplasia. Clinical Gastroenterology and Hepatology 13 (2015): 1464-71.
- Rutten LJ, Jacobson DJ, Jenkins GD, Fan C, Weiser E, Parks P, et al. Colorectal cancer screening completion: an examination of differences by screening modality. Preventive medicine reports 20 (2020): 101202.
- Eckmann JD, Ebner DW, Bering J, Kahn A, Rodriguez E, Devens ME, et al. Multitarget stool DNA screening in clinical practice: high positive predictive value for colorectal neoplasia regardless of exposure to previous colonoscopy. The American journal of gastroenterology 115 (2020): 608.
- Meester RG, Zauber AG, Doubeni CA, Jensen CD, Quinn VP, Helfand M, et al. Consequences of increasing time to colonoscopy examination after positive result from fecal colorectal cancer screening test. Clinical Gastroenterology and Hepatology 14 (2016): 1445-51.
- Paterson WG, Depew WT, Pare P, Petrunia D, Switzer C, van Zanten SJ, Daniels S. Canadian Association of Gastroenterology Wait Time Consensus Group. Canadian consensus on medically acceptable wait times for digestive health care. Canadian Journal of Gastroenterology 20 (2006): 411-23.
- Levin TR, Corley DA, Jensen CD, Schottinger JE, Quinn VP, Zauber AG, et al. Effects of organized colorectal cancer screening on cancer incidence and mortality in a large community-based population. Gastroenterology 155 (2018): 1383-91.
- Sahay TB, Gray RE, Fitch M. A qualitative study of patient perspectives on colorectal cancer. Cancer practice 8 (2000): 38-44.
- Bachman AS, Cohen EL, Collins T, Hatcher J, Crosby R, Vanderpool RC. Identifying communication barriers to colorectal cancer screening adherence among Appalachian Kentuckians. Health communication 33 (2018): 1284-92.
- Johnson DA, Wilson K, Lucero KS, Maeglin JM, Dermer S, Rex D. S0327 Patient Perspectives and Experience with Colorectal Cancer Screening. Official journal of the American College of Gastroenterology| ACG 115 (2020): S159-60.
- Brewer T, Sattari M. Colorectal cancer screening: understanding the patients' perspective. InANNALS OF ONCOLOGY. GREAT CLARENDON ST, OXFORD OX2 6DP, ENGLAND: OXFORD UNIV PRESS 27 (2016): 69-69.
- Gede N, Kívés ZH, Vajda R, Pakai A, Boncz I, Gyuró M, Kiss I. Factors Influencing Attitudes to Colorectal Cancer Screening. Value in Health 19 (2016): A749-50.
- Nelson HD, Cantor A, Wagner J, Jungbauer R, Fu R, Kondo K, Stillman L, Quiñones A. Effectiveness of patient navigation to increase cancer screening in populations adversely affected by health disparities: a meta-analysis. Journal of general internal medicine 35 (2020): 3026-35.
- Escoffery C, Fernandez ME, Vernon SW, Liang S, Maxwell AE, Allen JD, et al. Patient navigation in a colorectal cancer screening program. Journal of public health management and practice: JPHMP 21 (2015): 433.
- Percac-Lima S, Ashburner JM, Zai AH, Chang Y, Oo SA, Guimaraes E, Atlas SJ. Patient navigation for comprehensive cancer screening in high-risk patients using a population- based health information technology system: a randomized clinical trial. JAMA internal medicine 176 (2016): 930-7.
- Ladabaum, Uri, et al. "Strategies for colorectal cancer screening. "Gastroenterology 158.2 (2020): 418-432.
- Dachman AH, Yoshida H. Virtual colonoscopy: past, present, and future. Radiologic Clinics 41 (2003): 377-93.
- Chang KJ, Yee J. Dose reduction methods for CT colonography. Abdominal imaging 38 (2013): 224-32.
- Nagata K, Fujiwara M, Kanazawa H, Mogi T, Iida N, Mitsushima T, Lefor AT, Sugimoto H. Evaluation of dose reduction and image quality in CT colonography: comparison of low-dose CT with iterative reconstruction and routine-dose CT with filtered back projection. European radiology 25 (2015): 221-9.
- Song L, Jia J, Yu H, Peng X, Xiao W, Gong Y, Zhou G, Han X, Li Y. The performance of the mSEPT9 assay is influenced by algorithm, cancer stage and age, but not sex and cancer location. Journal of cancer research and clinical oncology 143 (2017): 1093- 101.
- Mead R, Duku M, Bhandari P, Cree IA. Circulating tumour markers can define patients with normal colons, benign polyps, and cancers. British journal of cancer 105 (2011): 239-45.
- Wu JC, Tseng PY, Tsai WS, Liao MY, Lu SH, Frank CW, et al. Antibody conjugated supported lipid bilayer for capturing and purification of viable tumor cells in blood for subsequent cell culture. Biomaterials 34 (2013): 5191-9.
- Tsai WS, You JF, Hung HY, Hsieh PS, Hsieh B, Lenz HJ, et al. Novel circulating tumor cell assay for detection of colorectal adenomas and cancer. Clinical and translational gastroenterology (2019).
- Chao S, Pilcz T, Stamatiou D, Ying J, Burakoff R. Stability of the ColonSentry Colon Cancer Risk Stratification Test. Int. J Dis Markers (2019).
- Deng L, Fang H, Tso VK, Sun Y, Foshaug RR, Krahn SC, et al. Clinical validation of a novel urine-based metabolomic test for the detection of colonic polyps on Chinese population. International journal of colorectal disease 32 (2017): 741-3.
- Deng L, Ismond K, Liu Z, Constable J, Wang H, Alatise OI, et al. Urinary Metabolomics to Identify a Unique Biomarker Panel for Detecting Colorectal Cancer: A Multicenter StudyUrinary Biomarker Panel for Detecting Colorectal Cancer. Cancer Epidemiology, Biomarkers & Prevention 28 (2019): 1283-91.
- Wang H, Tso V, Wong C, Sadowski D, Fedorak RN. Development and validation of a highly sensitive urine-based test to identify patients with colonic adenomatous polyps. Clinical and Translational Gastroenterology 5 (2014): e54.
- De Klaver W, Wisse PH, van Wifferen F, Bosch LJ, Jimenez CR, van der Hulst RW, et al. Clinical validation of a multitarget fecal immunochemical test for colorectal cancer screening: a diagnostic test accuracy study. Annals of Internal Medicine 174 (2021): 1224-31.
- Marcus PM, Pashayan N, Church TR, Doria-Rose VP, Gould MK, Hubbard RA, et al. Population-Based Precision Cancer Screening: A Symposium on Evidence, Epidemiology, and Next Steps Population- Based Precision Cancer Screening. Cancer Epidemiology, Biomarkers & Prevention 25 (2016): 1449-55.
- Jeon J, Du M, Schoen RE, Hoffmeister M, Newcomb PA, Berndt SI, et al. Determining risk of colorectal cancer and starting age of screening based on lifestyle, environmental, and genetic factors. Gastroenterology 154 (2018): 2152-64.
- Robertson DJ, Ladabaum U. Opportunities and challenges in moving from current guidelines to personalized colorectal cancer screening. Gastroenterology 156 (2019): 904-17.
- CDC colon cancer statistics
- Sabatino SA, White MC, Thompson TD, Klabunde CN. Cancer screening test use— United States, 2013. Morbidity and Mortality Weekly Report 64 (2015): 464.
- American cancer society guidelines
- Ladabaum U, Mannalithara A, Meester RG, Gupta S, Schoen RE. Cost-effectiveness and national effects of initiating colorectal cancer screening for average-risk persons at age 45 years instead of 50 years. Gastroenterology 157 (2019): 137-48.
- ACS colon cancer treatment
- Abdel-Rahman O. ECOG performance score 0 versus 1: impact on efficacy and safety of first-line 5-FU-based chemotherapy among patients with metastatic colorectal cancer included in five randomized trials. International journal of colorectal disease 34 (2019): 2143-50.
- Selby K, Baumgartner C, Levin TR, Doubeni CA, Zauber AG, Schottinger J, Jensen CD, Lee JK, Corley DA. Interventions to improve follow-up of positive results on fecal blood tests: a systematic review. Annals of internal medicine 167 (2017): 565-575.
- https://www.thecommunityguide.org/media/pdf/Cancer-Screening-Multicomponent- Colorectal.pdf


 Impact Factor: * 5.3
Impact Factor: * 5.3 Acceptance Rate: 75.63%
Acceptance Rate: 75.63%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 
