Craniofacial Fibrous Dysplasia with Orbital Involvement: Two Rare Case Reports and Comprehensive Review
Harikrishnan Marappan1*, Raja Ayakutty Muni2
1Department of Ophthalmology, Karuna Institute of Medical Sciences, Vilayodi-Vembra Rd, Chittur-Thathamangalam, Perumatty, Kerala
2Department of Ophthalmology, All India Institute of Medical Sciences, Madurai, Tamil Nadu, India
*Corresponding Author: Harikrishnan Marappan, Department of Ophthalmology, Karuna Institute of Medical Sciences, Vilayodi-Vembra Rd, Chittur-Thathamangalam, Perumatty, Kerala.
Received: 05 December 2024; Accepted: 12 December 2024; Published: 20 December 2024
Article Information
Citation: Harikrishnan Marappan, Raja Ayakutty Muni. Craniofacial Fibrous Dysplasia with Orbital Involvement: Two Rare Case Reports and Comprehensive Review. Archives of Clinical and Medical Case Reports. 8 (2024): 234-238.
View / Download Pdf Share at FacebookAbstract
Fibrous dysplasia (FD) is a rare, non-heritable bone disorder where normal bone is replaced by fibro-osseous tissue, leading to structural instability and deformities. This report discusses two cases of craniofacial fibrous dysplasia (CFD) with orbital involvement, presenting the clinical findings, diagnostic challenges, and management strategies. Additionally, a comprehensive literature review is provided to offer insights into the pathophysiology, clinical manifestations, and treatment options for this condition.
Keywords
Fibrous dysplasia; Craniofacial; Orbit; Proptosis; Ground glass
Article Details
1. Introduction
Fibrous dysplasia (FD) is a skeletal disorder characterized by the replacement of normal bone with fibrous connective tissue. When FD involves the craniofacial bones, it is referred to as craniofacial fibrous dysplasia (CFD). Fibrous dysplasia, a rare bone disorder, particularly affects the craniofacial region, where its proximity to critical structures like the orbit presents unique clinical complexities. Site-specific considerations are essential because orbital involvement can lead to significant functional impairments, such as vision loss and facial deformities, necessitating precise diagnosis and tailored intervention strategies [1,2]. Additionally, management challenges, including the risks associated with surgery and the need for long-term monitoring, underscore the importance of a comprehensive, multidisciplinary approach. Highlighting these aspects offers insight into the intricacies of treating CFD and the necessity for individualized patient care. This case report details two patients with CFD and orbital involvement, followed by a literature review to contextualize the clinical approach and outcomes.
2. Case Reports
2.1 Case 1:
2.1.1 Patient presentation
A 41-year-old male presented with a progressive onset of asymmetry in his facial features, protrusion of the left eye (proptosis), and sporadic occurrences of double vision (diplopia) over three years. The patient reported no significant medical history or previous traumas.
2.1.2 Clinical examination
During the physical examination, we observed asymmetry on the left side of the forehead and left-sided proptosis, with the left eye protruding 22 mm compared to 17 mm for the right eye, as measured by Hertel exophthalmometry (Figure 1). The patient reported no vision loss but did experience occasional discomfort and double vision. Colour vision was normal in both eyes on Ishihara test. The patient displayed normal eye movements and no other abnormalities during the eye examination. Upon thorough examination, no other bones were found to be involved beyond the affected craniofacial region. The lesion remained localized, consistent with a diagnosis of monostotic fibrous dysplasia. Additionally, no hyperpigmented spots, such as café-au-lait macules, were noted, further supporting the absence of systemic involvement). Particular attention was given to regions of the skeleton commonly affected by fibrous dysplasia, such as the proximal femur, ribs, and vertebrae, and no lesions were identified in these areas (Suggestive of Monostotic FD).
2.1.3 Diagnostic workup
Computed tomography (CT) imaging showed expansive bony lesions affecting the left frontal bone, left squamous part of the temporal bone, and left greater wing of the sphenoid, leading to complete obliteration of the left frontal sinus. These lesions displayed a distinctive "ground-glass" appearance on CT, characteristic of FD (Figure 2).
Figure 2: Computed tomography (CT) imaging revealed expansile bony lesions involving the left frontal bone, left squamous part of the temporal bone and left greater wing of sphenoid with complete obliteration of left frontal sinus. The lesions exhibited a characteristic "ground-glass" appearance (2A, 2B – Bony window, 2C – Soft tissue window).
2. Case 2:
2.2.1 Patient presentation
A 65-year-old male presented with progressive facial asymmetry, ptosis of the upper eyelid, and proptosis of the right eye over two years. The patient's medical history revealed no significant conditions or prior trauma.
2.2.2 Clinical examination
During the physical examination, it was observed that the midface was asymmetric, with the right-side showing eccentric (down and out) proptosis (measuring 20 mm, compared to 18 mm on the left according to Hertel exophthalmometry) and severe ptosis (Figure 3). Additionally, systemic evaluation revealed multiple bony lesions on both forearms (Suggestive of Polyostotic FD) (Figure 4). Although there was no evidence of vision loss, the patient reported discomfort and occasional diplopia. Colour vision was normal in both eyes, according to Ishihara’s chart. No impairments in extraocular movement were found, and no other abnormalities were noted during the ophthalmic examination. Furthermore, no other cranial nerve deficits were observed.
2.2.3 Diagnostic workup
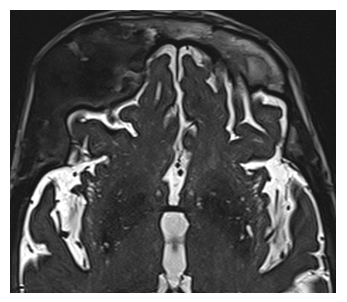
MRI imaging revealed an expanding abnormal growth impacting the upper part of the right eye socket and the right sphenoid bone, showing restricted diffusion. Altered signals were also detected in the sphenoid bone (Figure 5A). Furthermore, CT scans indicated an enlargement of the diploic space with a predominantly ground glass appearance, suggesting fibrous dysplasia changes (Figure 5B).
3. Treatment
Both patients received a comprehensive evaluation involving ophthalmology, otolaryngology, and neurosurgery. Due to the significant impact on vision caused by the severity of orbital involvement, it was recommended that they undergo regular follow-up to monitor the progression and be open to the possibility of surgical intervention in the future, if necessary. However, both patients declined further investigations and interventions due to concerns about surgical risks and the absence of subjective or objective visual decline since their initial presentation. The diagnosis was based solely on clinical and radiologic findings, as no histologic diagnosis was made because the patients were unwilling to proceed with additional tests.
4. Comprehensive Review
4.1 Pathophysiology
FD is caused by a post-zygotic mutation in the GNAS gene, leading to abnormal proliferation of fibro-osseous tissue. This mutation affects the cAMP signaling pathway, causing increased osteoblastic activity and the replacement of normal bone with fibrous tissue [1-3]. The craniofacial bones are frequently involved due to their developmental complexity and continuous remodelling [4,5].
Fibrous dysplasia comprises monostotic and less common polyostotic forms. Monostotic fibrous dysplasia involves a single focus, while the polyostotic form encompasses multiple dysplastic foci. Approximately 20% to 30% of fibrous dysplasia cases are polyostotic [6]. About 3% of cases with the polyostotic form manifest as McCune-Albright syndrome, characterized by a triad of polyostotic fibrous dysplasia, hyperfunctional endocrinopathies (including precocious puberty), and café-au-lait skin pigmentation [2,6].
5. Clinical Manifestations
CFD can present with a variety of symptoms depending on the bones involved. Common features include facial asymmetry, pain, and functional impairment such as vision changes, hearing loss, and dental abnormalities [4]. Orbital bone involvement in the frontal, sphenoid, and ethmoid regions can commonly lead to symptoms such as proptosis, dystopia, and hypertelorism. Less frequently, it may cause diplopia, extraocular muscle palsies, epiphora, trigeminal neuralgia, headaches, periorbital and retro-orbital pain, and visual loss. The frequency of these presentations has been reported in various orders [7-9].
5.1 Diagnosis
The diagnosis of CFD relies on clinical examination, imaging studies, and histopathological confirmation. Radiographic features, particularly on CT scans, typically show a "ground-glass" appearance. MRI can provide detailed information on soft tissue involvement and the extent of the lesion [9,10]. It’s important to note that other lesions exhibiting a ground-glass appearance on imaging include osteosarcoma, chondrosarcoma, and Paget's disease. However, fibrous dysplasia can be differentiated from these lesions on MRI by its characteristic signal intensity, which typically shows a homogeneous, low-signal intensity on T1-weighted images and a variable signal intensity on T2-weighted images, in contrast to the often heterogeneous appearance of malignant tumors. Additionally, the presence of a typical "cortical sparing" pattern and the absence of soft tissue masses further support the diagnosis of fibrous dysplasia over other lesions [11]. Biopsy remains the gold standard for definitive diagnosis. When there is uncertainty surrounding the diagnosis, it is advisable to perform a bone biopsy. However, in cases where obtaining a biopsy is deemed to pose unacceptable risks, a diagnosis can often be established through a combination of patient history, clinical examination, and radiographic findings. This approach is particularly useful when the lesion is located in a site that cannot be easily biopsied [12].
5.2 Management
Treatment of CFD with orbital involvement is tailored to the severity of symptoms and the extent of the disease [12]. Options include conservative management with regular monitoring for asymptomatic cases, and surgical intervention for symptomatic cases. Conservative management involves regularly evaluating the patient's ophthalmologic condition, conducting visual field testing, and monitoring disease progression through radiologic follow-up, including CT scans [13]. Surgery aims to relieve symptoms, improve function, and address cosmetic concerns. Techniques may include debulking, contouring, and, in severe cases, reconstructive surgery [12,14]. Pharmacological treatments, such as bisphosphonates, have been explored to reduce bone turnover, but their efficacy remains uncertain [15]. Recent advancements in the management of craniofacial fibrous dysplasia have introduced several promising alternatives beyond surgery and bisphosphonates. Immunotherapy, focusing on agents that inhibit osteoclast activity and bone turnover, has shown potential in slowing disease progression. For example, therapies targeting the RANKL (receptor activator of nuclear factor kappa-Β ligand) pathway, such as denosumab, are being studied for their ability to reduce abnormal bone resorption in fibrous dysplasia cases. Additionally, radiotherapy, especially stereotactic radiosurgery, is being increasingly considered in cases where surgery is contraindicated, offering precise lesion targeting with minimal impact on surrounding structures. This approach has been used successfully in cases where patients present with optic nerve compression or other significant orbital involvement, providing symptomatic relief without surgical intervention. However, further long-term studies are necessary to fully establish the safety and efficacy of these newer therapies [16].
5.3 Prognosis
The prognosis for CFD varies based on the extent and location of the disease. Orbital involvement poses a risk for significant morbidity due to potential vision impairment [7,9].
Malignant degeneration of fibrous dysplasia occurs in 0.5-1% of cases, with increased risk in polyostotic disease, especially McCune-Albright patients and after radiation. Transformation is more common in craniofacial bones and the femur. The primary malignancy is osteogenic sarcoma, followed by fibrosarcoma and chondrosarcoma. The average time from diagnosis to malignancy is 13.5 years, with signs such as rapid lesion growth, pain, necrosis, and elevated serum alkaline phosphatase [17]. Regular follow-up and monitoring are crucial for early detection of complications and timely intervention.
6. Conclusion
Craniofacial fibrous dysplasia with orbital involvement presents a unique challenge due to its complex presentation and potential for significant functional impairment. A multidisciplinary approach is essential for optimal management, combining surgical and non-surgical strategies tailored to individual patient needs. Further research is needed to better understand the disease mechanisms and improve treatment outcomes.
References
- DiCaprio MR, Enneking WF. Fibrous dysplasia. Pathophysiology, evaluation, and treatment. J Bone Joint Surg Am 87 (2005): 1848-1864.
- Boyce AM, Collins MT. Fibrous Dysplasia/McCune-Albright Syndrome: A Rare, Mosaic Disease of Gαs Activation. Endocr Rev 41 (2020): 345-370.
- Riddle ND, Bui MM. Fibrous dysplasia. Archives of Pathology & Laboratory Medicine 137 (2013): 134-8.
- Tabrizi R, Özkan BT. Craniofacial fibrous dysplasia of orbit. Journal of Craniofacial Surgery 19 (2008): 1532-7.
- Selva D, White VA, O'Connell JX, et al. Primary bone tumors of the orbit. Survey of ophthalmology 49 (2004): 328-42.
- Cruz AA, Constanzi M, De Castro FA, et al. Apical involvement with fibrous dysplasia: implications for vision. Ophthalmic Plastic and Reconstructive Surgery 23 (2007): 450-4.
- Liakos GM, Walker CB, Carruth JA. Ocular complications in craniofacial fibrous dysplasia. British Journal of Ophthalmology 63 (1979): 611-6.
- Sammut SJ, Kandasamy J, Newman W, et al. Relief of severe retro-orbital pain and vision improvement after optic-nerve decompression in polyostotic fibrous dysplasia: case report and review of the literature. Child's Nervous System 24 (2008): 515-20.
- Ricalde P, Horswell BB. Craniofacial fibrous dysplasia of the fronto-orbital region: a case series and literature review. Journal of Oral and Maxillofacial Surgery. 2001 Feb 1;59(2):157-67.
- Cai M, Ma L, Xu G, et al. Clinical and radiological observation in a surgical series of 36 cases of fibrous dysplasia of the skull. Clinical Neurology and Neurosurgery 114 (2012 ): 254-9.
- Singh G, Bell D, Sharma R, et al. Fibrous dysplasia. Reference article, Radiopaedia.org
- Lee JS, FitzGibbon EJ, Chen YR, et al. Clinical guidelines for the management of craniofacial fibrous dysplasia. InOrphanet Journal of Rare Diseases 7 (2012).
- Amit M, Collins MT, FitzGibbon EJ, et al. Surgery versus watchful waiting in patients with craniofacial fibrous dysplasia–a meta-analysis. PloS One 6 (2011): e25179.
- Edgerton MT, Persing JA, Jane JA. The surgical treatment of fibrous dysplasia. Annals of surgery 202 (1985): 459-79.
- Mäkitie AA, Törnwall J, Mäkitie O. Bisphosphonate treatment in craniofacial fibrous dysplasia—a case report and review of the literature. Clinical Rheumatology 27 (2008): 809-12.
- Javaid MK, Boyce A, Appelman-Dijkstra N, et al. Best practice management guidelines for fibrous dysplasia/McCune-Albright syndrome: a consensus statement from the FD/MAS international consortium. Orphanet journal of rare diseases 14 (2019): 1-7.
- Parekh SG, Donthineni-Rao R, Ricchetti E, et al. Fibrous Dysplasia. J Am Acad Orthop Surg 12 (2004): 305-13.







 Impact Factor: * 5.3
Impact Factor: * 5.3 Acceptance Rate: 75.63%
Acceptance Rate: 75.63%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 
