Gait Kinematics and Spatiotemporal Variables after Enriched, Task-Specific Therapy in the Chronic Phase after Stroke. A Single-Subject Experimental Design Study
Sara Vive, RPT, MSc1,2*, Roland Zügner RPT, PhD3, Roy Tranberg C.P.O, PhD3, Lina Bunketorp-Käll, RPT, PhD1,2
1Section for Health and Rehabilitation, Institute of Neuroscience and Physiology, The Sahlgrenska Academy at the University of Gothenburg, Gothenburg, Sweden
2Neurocampus, Sophiahemmet Hospital, Stockholm, Sweden
3Department of Orthopedics, Institute of Clinical Sciences, Sahlgrenska Academy, University of Gothenburg, SE 413 45 Göteborg, Sweden
4Centre for Advanced Reconstruction of Extremities (C.A.R.E.), Sahlgrenska University Hospital, Mölndal, Sweden
*Corresponding Author: Sara Vive Neurocampus, Sophiahemmet Hospital, Stockholm, Sweden.
Received: 10 February 2021; Accepted: 02 March 2021; Published: 22 March 2021
Article Information
Citation: Sara Vive, Roland Zügner, Roy Tranberg, Lina Bunketorp-Käll. Gait Kinematics and Spatiotemporal Variables after Enriched, Task-Specific Therapy in the Chronic Phase after Stroke. A Single-Subject Experimental Design Study. Archives of Clinical and Medical Case Reports 5 (2021): 325-341.
View / Download Pdf Share at FacebookAbstract
Objectives: In this single-subject experimental design study, we investigated the effect of late-phase stroke rehabilitation on gait pattern.
Methods: A subgroup from a previous clinical study, four men in the chronic phase after stroke, received 3 weeks of enriched and task-specific therapy (ETT) consisting of task-specific exercise, socialization, sensory and cognitive stimulation in a Mediterranean climate. Spatiotemporal gait variables, kinematics and symmetry were measured before and after the intervention in an advanced gait laboratory. Statistical significance was determined with the two-standard deviation band method.
Results: Subject 1 had kinematic improvements (increased knee flexion during swing and dorsiflexion during stance) and spatiotemporal gains (increased speed, double-limb support, stride length and cadence) after ETT. Subject 2 had improved swing time symmetry ratio but no spatiotemporal or kinematic improvements. Subject 3 had gains in speed, stride width and length, and knee flexion during swing. Subject 4 had a change in cadence but no gain in kinematics, nor symmetry.
Conclusions: Two of the four participants had significant improvements in gait kinematics, symmetry, and spatiotemporal variables after the intervention. Future research should consider the potential effects of ETT with the aim of validating the conclusions that can be drawn from this study.
Keywords
<p>Stroke; Rehabilitation; Enriched environment; Recovery of function; Gait analysis</p>
Article Details
1. Introduction
Hemiplegia after stroke contributes significantly to reduced mobility as a result of impaired balance and dyscoordinated gait. Stroke survivors are often sedentary, have impaired postural control, and are at increased risk for falls and difficulties with daily activities [1-4]. Mobility restrictions limit activity and participation [5], which may increase social isolation and loneliness. Walking dysfunction is present in 80% of stroke survivors 3 months after stroke onset [6]. Among patients discharged from stroke rehabilitation units, only 7% are community ambulatory, which implies having the ability to walk continuously for 500 meters at a speed commensurate with crossing a road safely [7].
Restoring functions after stroke is a complex process involving both the spontaneous recovery usually seen within the first 3 months and the effects of therapeutic interventions, which are most beneficial when applied intensively and early after stroke onset [6, 8]. Most return of function occurs early, and recovery plateaus at 3–6 months [9]. Among community-dwelling individuals with stroke, most falls occur while walking [10], and up to 70% of all stroke-related falls occur during the first year. Thus, regaining walking ability is one of the main objectives in rehabilitation after stroke [11].
One of the most promising and clinically feasible multimodal interventions is enriched rehabilitation, which combines environmental enrichment and task-specific therapy [12]. In rodents, an enriched environment that enhances motor, cognitive, sensory, and social stimulation profoundly affects neuroplasticity [12, 13]. This supports the idea that such enrichments can aid recovery from stroke-related deficits. The combination of different modalities in multimodal interventions is thus expected to have a synergistic effect on neuroplasticity in rehabilitation after stroke in humans [12-15]. A priority for human stroke research is to translate promising basic research to clinical settings and to evaluate enriched combinational therapy programs that appear to be more effective than single interventions [12, 13]. In a recent clinical exploratory study, we evaluated the effectiveness of an enriched intense and task-oriented therapy program in enhancing motor function in patients with moderate to moderately severe hemiplegia after stroke [16].
The biomechanics of normal gait are rather reproducible [17]. The major requirements are stability in stance, foot clearance and appropriate prepositioning during the swing phase, adequate step length, and energy conservation [18]. Poststroke hemiplegic gait reflects deviations and compensatory motions imposed by residual and impaired motor functions [19], resulting in pronounced gait asymmetry [20]. The spatiotemporal characteristics of a hemiplegic gait include a decreased stance phase and prolonged swing phase on the affected side, slower walking speed, and shorter stride length [19, 21]. The lack of active dorsiflexion and the impaired knee flexion reduces heel strike and often causes hyperextension of the knee at loading response [22]. These deficiencies along with reduced hip extension make it difficult to move body mass forward over the foot [23]. Lack of control of the abductor muscles of the affected hip joint often causes the pelvis to shift laterally [24]. Lack of knee flexion and ankle plantar-flexion may further compromise effective preparation for the swing phase. The limited knee flexion in early and mid-swing together with limited dorsiflexion often causes circumduction of the leg during the swing phase, which along with limited knee extension at terminal swing compromises weight-acceptance at initial contact [19]. Such asymmetries may increase metabolic cost [25, 26]. The step length asymmetry is related to slower self-selected speed [27].
The difference between true stroke recovery and compensatory movement patterns due to motor impairment is increasingly highlighted, and the quality of the gait pattern needs to be observed and examined in more detail [28]. Measuring gait speed in isolation does not inform about whether the task (walking) is performed by using compensatory strategies – or by using alternative movement patterns [28]. Advanced gait analyses is a valuable tool to evaluate the effectiveness of treatment in patients affected by conditions that alter their gait performance. Quantitative three-dimensional gait analysis is the gold standard for measuring spatiotemporal variables, joint kinetics, and kinematics [29]. Although poststroke gait dysfunction is among the most investigated gait disorders, the therapeutic benefits of rehabilitation on gait pattern during the chronic phase (defined as >6 months after stroke) are not fully understood.
In this study, we investigated three-dimensional spatiotemporal gait variables and kinematic data in a subgroup of stroke patients who underwent 3 weeks of enriched and task-specific therapy (ETT) [16]. The ETT program included task-specific exercise, socialization, sensory and cognitive stimulation in a climate suitable for both indoor and outdoor activities. The program provided durable benefits across a wide spectrum of motor deficits and impairments after stroke, including significant gains in gait capacity and speed [16]. In this study, we determined whether the ETT intervention improves any spatiotemporal variables or kinematic features of the participants’ gait.
2. Methods
2.1 Study design and subjects
The subjects were four participants in our previous exploratory clinical study [16]. The eligibility criteria are listed in Table 1. The study was done with a single-subject ABA experimental design [30], with a single follow-up 6 months after the intervention (Figure 1). The initial phase (A1) was a 2-week baseline period before the intervention. During this phase, three to five analyses were done at least one day apart [31]. The subjects were not offered any new rehabilitation activities but were allowed to continue their regular treatment for a maximum of 3 hours per week. The B-phase was the 3-week intervention period. Immediately after the intervention, the A-phase was repeated (A2 phase). A single follow-up was done 6 months after the intervention. This report follows The Single-Case Reporting Guideline in Behavioural Interventions (SCRIBE) [32].
|
At least 6 months and a maximum 10 years after the onset of stroke |
|
Disability grade 2–4 on the Modified Rankin Scalea Baseline motor deficit defined as less than a full score on the primary outcome measure (M-MAS UASb) |
|
No other injury, illness, making the individual unsuitable for participation, including exercise-induced epilepsy, assessed by the referring or prescribing physician. |
|
Cognitive and speech ability that enables instruction, intervention, and evaluation |
|
Ability and willingness to travel to the place of evaluation |
|
Able to perform sit-to-stand and stand-to-sit transfers independently or with assistance, without assistive technology such as mechanical lifts |
|
Not having participated in a similar high-dose rehabilitation program (other than post-stroke acute and subacute rehabilitation) within the previous 6 months |
|
Not scheduled for other treatment focused on intensive high-dose training during the study period Expanded eligibility criteria for the present study Affected/assymetric gait pattern Ability to walk independently 10 m indoors without assistive devices Live near Gothenburg, able to do repetitive measures in the gait lab according to the ABA design |
aAn ordinal disability rating scale, scored 0–6 (0 = no symptoms); bModified Motor Assessment Scale developed at Uppsala University Hospital in 1999.
Table 1: Eligibility criteria.
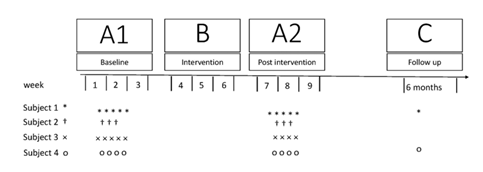
Figure 1: Design and flow of the study. Symbols indicate the numbers of assessments for each subject.
2.2 Enriched task-specific therapy (ETT)
In this context, “enriched” refers to environmental enrichment—an intervention to increase motor, sensory, cognitive, and social activity by providing a stimulating environment in a climate suitable for both indoor and outdoor activities. “Task-specific” refers to repetitive functional training in everyday tasks, meaningful for the individual. The ETT program started immediately after the baseline period (A1 phase) and took place at two rehabilitation facilities near Marbella and Malaga in Spain. The ETT was individually tailored, supervised by registered physical therapists, and performed in groups of four to nine patients. The schedule included rehabilitation activities for 3 weeks, lasting 5.5 hours on weekdays and 3.5 hours on Saturdays. The therapy was combined with social activities, such as scheduled coffee breaks and lunches. Participants with speech impairments received individual treatment with a speech therapist for a maximum of 2 hours per day. The physical therapy interventions were characterized by repetitive massed practice and based on noncompensatory strategies [33, 34]. The participants were encouraged to physically engage in the challenging outdoor environment. The various components of the enriched environment are described in detail [16] in Appendix A.
2.3 Gait analysis method
Gait patterns and components of gait were analyzed with an instrumental gait analysis system (Qualisys, Göteborg, Sweden) at the Orthopaedic Research Unit at Sahlgrenska University Hospital, Gothenburg, Sweden. The subject was asked to wear shorts or underwear and was instructed to walk independently without shoes, orthoses, or assistive walking devices until 3–10 trials were registered. For analysis of spatiotemporal gait variables and lower limb kinematics [35-40], 35 reflective markers were placed at well-defined anatomical landmarks on the skin with double-sided adhesive tape. The participant was asked to walk at a comfortable speed approximately 10 meters straight across the calibrated volume. Movements were recorded with 12 cameras (Oqus 4). Spatiotemporal variables and kinematics were analyzed with Visual 3D software (C-Motion, Germatown, MD, USA) [35-40].
2.4 Outcome measures
Spatiotemporal gait variables, including gait speed, stride time, step time, step length, step width, double limb support (DLS) time, stance time, swing time, and cadence (steps per minute for affected and nonaffected limbs), were registered. DLS time and stance were calculated as a percentage of the gait cycle time. From the spatiotemporal variables, gait symmetry ratios were calculated by dividing the paretic swing time or swing length (V) by that of the nonparetic leg (symmetry ratio = Vparetic/Vnon-paretic). Further, joint kinematics in the sagittal plane of the lower extremities, including maximum dorsiflexion during stance and plantarflexion during swing, maximum knee flexion during swing, and maximum hip extension during stance, were determined for both the affected and nonaffected side.
2.5 Statistics
For the kinematic data, maximum and minimum joint angles were determined for each gait cycle. The means of all maximum and minimum values were calculated for each time point. Excel was used to summarize data and calculate maximum and minimum values. SPSS v. 22.0 (IBM, Armonk, NY, USA) was used for statistical analyses. At all testing points for each patient, quantile-quantile plots and histograms as well as skewness and kurtosis were used to determine whether the outcome variables approximated a normal distribution. Since the plots were found satisfactory, we used a semi-statistical approach, the two standard deviation band (TSDB) method, in which significance changes are identified by visual inspection of graphs. Treatment effects were considered to be obvious from visual inspection of the data [41]. Horizontal bands representing 2 SD of the baseline data (phase A1) were drawn on the graphs. A change was defined as significant if at least two successive observations in one phase were outside the 2 SD range from the previous phase [42]. Since this method is not suitable for single assessments, the results of the follow-up assessment for each subject are simply presented in the text and plotted graphic. Values are reported as mean ± SD.
2.6 Ethics
The study was approved by the Regional Ethical Review Board in Gothenburg, Sweden (Ref number: 549-12) and done in accordance with relevant ethical guidelines. All participants received detailed study information, signed a written informed consent form, and were told they could withdraw from the study at any time. This study complies with the Declaration of Helsinki.
2.7 Role of the funding source
The funders played no role in the design, conduct, or reporting of this study.
3. Results
Between May 1, 2015, and November 30, 2016, four men were included in the study. The mean age was 62 years (range 57–65 years), and the mean time since stroke was 36 months (range 11–88 months). The degree of disability or dependence in daily activities was 2–3 on the Modified Rankin Scale.43 Demographic and clinical characteristics of the subjects are presented in Table 2. Since a reflective marker was sometimes obscured by the affected upper limb, there was some data variability between subjects. Two subjects did not participate in the 6-month follow-up, owing to a lack of time and the long journey it would have entailed (proximity to Gothenburg was an inclusion criterion).
|
Category |
Subject 1 |
Subject 2 |
Subject 3 |
Subject 4 |
|
Age (years) |
62 |
63 |
65 |
57 |
|
Gender (M/F) |
M |
M |
M |
M |
|
Affected side |
Left |
Left |
Right |
Right |
|
Modified Rankin Scale score |
2 |
3 |
3 |
3 |
|
Time since stroke (months) |
32 |
11 |
88 |
14 |
|
Stroke type (infarction/bleeding) |
I |
B |
I |
I |
|
Spatiotemporal gait variables |
X |
X |
X |
X |
|
Kinematics |
X |
X |
X |
X |
|
No. of analyses (no. of gait trials) |
||||
|
A1 phase |
5 (16) |
3 (7) |
5 (20) |
4 (19) |
|
A2 phase |
5 (13) |
3 (7) |
4 (13) |
4 (9) |
|
6-month follow-up |
X (3) |
- |
- |
X (5) |
Values in parentheses are numbers of gait trials
X= satisfactory data collected
- = data not analyzable or not collected.
Table 2: Characteristics of the study subjects and analyzable data.
3.1 Kinematics
The results of the kinematic analyses are presented in Table 3. Subject 1 had statistically significant changes in three of the collected gait variables. Specifically, he had increased dorsiflexion during stance (from -1.9° in phase A1 to 9.4° in phase B, and to 17.1° at 6-month follow-up) and increased knee flexion during swing in both the affected leg (from 48.7° to 52.3° and 53.1° at 6 months) and the nonaffected leg (from 42.4° to 45.6°, and 46.2° at 6 months) (Figure 2). Subject 3 had no significant kinematic changes on the affected side and increased maximum knee-flexion during swing on the nonaffected side (from 26.8° to 33.6°). Subjects 2 and 4 had no significant kinematic changes. However, subject 4 had small but not significant increases of maximum knee flexion angles during swing on both the affected and nonaffected side.
Values are mean (SD) of the maximum sagittal gait movement angles; *Significant difference vs. A1 (2SD band method).
Table 3: Kinematics.
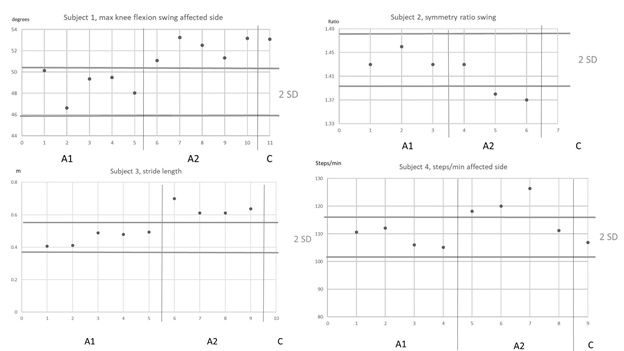
Figure 2: Selection of the graphical interpretation of the standard deviation (SD) band method. Horizontal lines indicate the 2 SD bands. A1, A2 and C indicates the phases.
3.2 Spatiotemporal gait variables
The results of the spatiotemporal gait analysis are presented in Table 4. After the intervention, subject 1 had a significant increase in gait speed (from 0.77 to 1.0 m/s) and a decreased in DLS time (0.25 to 0.22 s). The increase in gait speed was sustained at 6 months (1.0 m/s), but the reduction in DLS time was not. Subject 1 also had an increase in stride length (0.77 m in phase A1 to 0.94 m in phase B), which was sustained at the 6-month follow-up (0.93 m), and increased cadence in both the affected leg (from 120 to 125 steps/min) and the unaffected leg (from 123 to 130 steps/min). At 6 months, these gains were sustained (130 steps/min in both legs). Subject 2 had no changes in spatiotemporal gait variables. Subject 3 had a significant increase in gait speed (0.19 to 0.35 m/s), a significantly narrower stride width (from 0.20 to 0.19 m), and increased stride length (from 0.46 to 0.64 m). Subject 4 had a significant increase of cadence in the affected limb (108 to 118 steps/min) that was not sustained at the 6-month follow-up (107 steps/min). Subject 4 also had increased walking speed (0.77 to 0.81 m/s); however, the increase was not significant as only one observation in phase B exceeded the mean by 2 SD, and the speed at 6 months was less than the baseline mean.
3.3 Gait symmetry ratios
The gait symmetry ratios are presented in Appendix B. Gait was considered more symmetric as the gait symmetry ratio approached 1. After the intervention, the variables shown to be more symmetrical were the stance and swing time of subjects 3 and 4, step time of subject 3, and step length of subject 4. However, only step length for subject 1 and swing time for subject 2 was significantly more symmetrical after the intervention.
Values are mean (SD); *Significant difference vs. A1 (2SD band method)
Table 4: Spatiotemporal Gait Variables.
4. Discussion
This study provides information on the pattern of changes in gait after ETT in subjects with moderate to moderately severe disability and low to moderate gait speed in the chronic phase of stroke. All four subjects had asymmetric spatiotemporal gait deviations, such as decreased speed, asymmetric step length, and prolonged swing time on the affected side [44], consistent with previous reports [24]. In two subjects, several spatiotemporal gait variables improved significantly after ETT, and cadence improved in a third. The significant gains in kinematic gait variables shown for the two subjects might point towards possible true recovery, rather than being induced by compensatory gait mechanisms. Jonkers et al. have shown that stroke individuals with low function were not able to improve their gait kinematics and kinetics to increase walking speed because they had already maxed their power output at their comfortable gait speed [45]. These results does not seem applicable on our small study cohort where the two individuals who did not improve in terms of gait kinematics, both were above the limit by Jonkers definition to low functioning, and one of the subjects that did improve the gait kinematics could be considered an individual in the lower functioning group [45]. Our findings are more in line with the findings of Jonsdottir et al. meaning that every individual is functionally different and adopts an individual strategy to increase their gait speed [46].
The gait speeds of our study subjects were 0.19–0.77 m/s at baseline and 0.35–1.0 m/s after the intervention. The acquired speed for walking in the community is around 0.8 m/s [47]. The minimal detectable change (MDC) in comfortable gait speed for late-phase stroke survivors increases with increasing baseline gait speed, categorized as low (<0.4 m/s), moderate (0.4–0.8 m/s), or high (>0.8 m/s); the corresponding MDCs are 0.10, 0.15, and 0.18 m/s, respectively [48]. Two subjects had statisically significant increases in gait speed that would be considered clinically significant: 0.23 m/s in subject 1, who had moderate gait speed, and 0.16 m/s in subject 3, who had low gait speed. Gait speed was unchanged in subject 2 and increased in subject 4; however, the increase (0.04 m/s) was not statistically or clinically significant [48].
To assess gait symmetry, we used equations to calculate symmetry ratios for step length, swing time, and stance time. This method has been recommended as easier to interpret than a symmetry index [49]. The swing time symmetry ratio corresponds well with single limb stance time, which is often shorter in the paretic limb [50]. However, despite the correlation between the ratios, symmetry in one ratio but not the others has been reported in some subjects [49]. All four of our subjects had large changes in their symmetry ratios; however, in most instances, the differences within the phases were too large to indicate a consistent change in symmetry. The only statistically significant changes were in the swing time ratio of subject 2 and the step length ratio of subject 1.
Reducing asymmetry caused by hemiplegia is a common goal in rehabilitation after stroke [51]. However, it has been debated whether increased symmetry always is beneficial. According to one report [52], asymmetrical step lengths may not necessarily limit self-selected walking speed. Indeed, an asymmetric gait may be considered beneficial if gait speed is the primary performance criterion, and in some cases may facilitate gait. However, increased asymmetry in step length [53] and timing [54] cause higher energy costs.
Normally, the hip joint has a vital role in stabilizing and supporting the leg and helps maintain a stable trunk, while allowing forward progression of the body. During forward progression, the hip moves from a flexed angle of about 20° to an extended angle of about 10°, contributing to normal contralateral step length [55]. The hemiplegic gait often has a decreased stance in the affected limb, and a more flexed hip during stance, contributing to a shorter step length on the nonaffected side [19, 55, 56]. All four of our subjects had a variation in hip extension during stance at baseline. Hip extension was assessed in two subjects after the intervention. Neither had an improvement in extension in the stance phase of the affected limb, and one also had no improvement in extension in the nonaffected limb. Apparently, the ETT intervention did not improve the ability to extend the hip joint during gait.
The mean knee flexion during the swing phase of gait in a normal population is 60° [44]. Hemiplegia often causes stiffness of the knee, resulting in compensatory circumduction of the leg [55]. In this study, the maximum knee flexion during swing exceed 40° in three of four subjects. Maximum knee flexion during swing in subject 3, who had the greatest deviation from normal in this variable, increased from 26.8° (A1) to 33.6° after the ETT intervention. This improvement of 6.8° is within the range of the MDC of maximum knee flexion during swing in chronic stroke (6.54–7.61°) [57], but not exceeding the MCID estimates of 8.48° according to Guzic et al. [58]. In subject 1 maximum knee flexion increased on both sides after the ETT intervention, which may be associated with the demonstrated gain in stride length [59]. Nevertheless, these improvements did not reach the previously reported figures on MCD or MCID for sagittal knee flexion change [57, 58].
One of the most important prerequisites of gait is foot clearance during swing [18]. Several stroke-related ankle joint impairments cause inadequate dorsiflexion control during gait, including weakness of dorsiflexors, spasticity of plantar flexors, passive stiffness of the plantar flexors, and abnormal muscle coactivation [60]. In normal gait, when the heel rises during the last part of the stance phase, only the forefoot is in contact with the floor, while the ankle moves from 15° of dorsiflexion to a brief plantarflexion of 20–30° [44]. Three of our four subjects had at least 10° of dorsiflexion during stance before the intervention and no significant change of ankle kinematics afterward. In subject 1, however, maximum dorsiflexion on the affected side during stance increased from -1.9° to 9.4° after the intervention. This change of 11.3° exceeds the MDC (4.99–5.65°) [57].
Unsuccessful foot clearance during the swing phase and tripping of the affected foot can contribute to falls and injury after stroke [1]. Unsuccessful foot clearance is related to lack of both knee and hip flexion during swing, and also to ankle dorsiflexion [55]. In a kinematic study of joint mobility during gait late after stroke [61], the degree of ankle plantar flexion at toe-off contributed to a less successful foot clearance and presumably a higher risk for falls. More specifically, ankle plantar flexion at toe-off was 1.0° greater for trip steps than non-trip steps on a treadmill [61]. In our study, only one participant had significantly lower plantarflexion after the intervention during initial swing of the affected leg (from 18.7° to 12.1°). This degree of improvement in joint kinematics ought to reduce the risk of tripping after stroke.
4.1 Limitations
Our study had certain limitations. We used a single-subject experimental design and used the TSDB method to assess the significance of therapeutic changes that could be ascribed to the three-week long ETT intervention [16]. Although the TSDB method has a high agreement with the C statistic method [62], it is very sensitive to extreme values—one outlier can largely influence the variance in a small set of data and produce misleading results—and it considers only mean changes, and not the clinical importance of results. Another limitation of our study is that energy cost during gait was not measured.
5. Conclusion
Our findings suggest that ETT may have beneficial effects on spatiotemporal and kinematic gait variables in the chronic phase of stroke. Two of four study participants had significant improvements in the symmetry, kinematic and spatiotemporal variables of gait after ETT, pointing towards true motor recovery rather than compensatory gait mechanistic factors. Our findings are encouraging in that treatments can be refined and further developed and may eventually produce large clinical effects [62]. The procedures used in this study should be replicated in other studies to allow a broader generalization of the findings to varied clinical settings. Future research should consider the potential effects of ETT with the aim of validating the conclusions that can be drawn from this study.
References
- Weerdesteyn V, de Niet M, van Duijnhoven HJ, et al. Falls in individuals with stroke. J Rehabil Res Dev 45 (2008): 1195-1213.
- Ikai T, Kamikubo T, Takehara I, et al. Dynamic postural control in patients with hemiparesis. Am J Phys Med Rehabi 82 (2003): 463-469.
- Sunnerhagen KS, Olver J and Francisco GE. Assessing and treating functional impairment in poststroke spasticity. Neurology 80 (2013): S35-S44.
- Batchelor FA, Mackintosh SF, Said CM, et al. Falls after stroke. Int J Stroke 7 (2012): 482-490.
- Andrenelli E, Ippoliti E, Coccia M, et al. Features and predictors of activity limitations and participation restriction 2 years after intensive rehabilitation following first-ever stroke. Eur J Phys Rehabil Med 51 (2015): 575-585.
- Duncan PW, Zorowitz R, Bates B, et al. Management of Adult Stroke Rehabilitation Care: a clinical practice guideline. Stroke 36 (2005): e100-143.
- Hill K, Ellis P, Bernhardt J, et al. Balance and mobility outcomes for stroke patients: a comprehensive audit. Aust J Physiother 43 (1997): 173-180.
- Kwakkel G, van Peppen R, Wagenaar RC, et al. Effects of augmented exercise therapy time after stroke: a meta-analysis. Stroke 35 (2004): 2529-2539.
- Duncan PW, Goldstein LB, Matchar D, et al. Measurement of motor recovery after stroke. Outcome assessment and sample size requirements. Stroke 23 (1992): 1084-1089.
- Hyndman D, Ashburn A, Stack E. Fall events among people with stroke living in the community: circumstances of falls and characteristics of fallers. Arch Phys Med Rehabil 83 (2002): 165-170.
- Dobkin BH. Clinical practice. Rehabilitation after stroke. N Engl J Med 352 (2005): 1677-1684.
- Corbett D, Nguemeni C, Gomez-Smith M. How can you mend a broken brain? Neurorestorative approaches to stroke recovery. Cerebrovasc Dis 38 (2014): 233-239.
- Corbett D, Jeffers M, Nguemeni C, et al. Lost in translation: rethinking approaches to stroke recovery. Prog Brain Res 218 (2015): 413-434.
- Livingston-Thomas J, Nelson P, Karthikeyan S, et al. Exercise and Environmental Enrichment as Enablers of Task-Specific Neuroplasticity and Stroke Recovery. Neurotherapeutics 13 (2016): 395-402.
- Venna VR, Xu Y, Doran SJ, et al. Social interaction plays a critical role in neurogenesis and recovery after stroke. Translational psychiatry 4 (2014): e351.
- Vive S, Af Geijerstam J-L, Kuhn HG, et al. Enriched, Task-Specific Therapy in the Chronic Phase After Stroke: An Exploratory Study. Journal of Neurologic Physical Therapy 44 (2020): 145-155.
- Winter DA. Biomechanics and motor control of human movement. (3 Edn.) Hoboken, N.J. Hoboken N.J, Wiley (2005).
- Perry J, Davids JR. Gait analysis: normal and pathological function. Journal of Pediatric Orthopaedics 12 (1992): 815.
- Beyaert C, Vasa R, Frykberg GE. Gait post-stroke: Pathophysiology and rehabilitation strategies. Neurophysiol Clin 45 (2015): 335-355.
- Olney SJ, Richards C. Hemiparetic gait following stroke. Part I: Characteristics. Gait & Posture 4 (1996): 136-148.
- Allen JL, Kautz SA, Neptune RR. Step length asymmetry is representative of compensatory mechanisms used in post-stroke hemiparetic walking. Gait Posture 33 (2011): 538-543.
- De Quervain IA, Simon SR, Leurgans S, et al. Gait pattern in the early recovery period after stroke. J Bone Joint Surg Am 78 (1996): 1506-1514.
- Cruz TH, Lewek MD, Dhaher YY. Biomechanical impairments and gait adaptations post-stroke: multi-factorial associations. J Biomech 42 (2009): 1673-1677.
- Chen G, Patten C, Kothari DH, et al. Gait differences between individuals with post-stroke hemiparesis and non-disabled controls at matched speeds. Gait Posture 22 (2005): 51-56.
- Platts MM, Rafferty D, Paul L. Metabolic Cost of Overground Gait in Younger Stroke Patients and Healthy Controls. Medicine & Science in Sports & Exercise 38 (2006).
- Stoquart G, Detrembleur C, Lejeune TM. The reasons why stroke patients expend so much energy to walk slowly. Gait & Posture 36 (2012): 409-413.
- Hall AL, Bowden MG, Kautz SA, et al. Biomechanical variables related to walking performance 6-months following post-stroke rehabilitation. Clin Biomech (Bristol, Avon) 27 (2012): 1017-1022.
- Levin MF, Kleim JA, Wolf SL. What do motor "recovery" and "compensation" mean in patients following stroke? Neurorehabil Neural Repair 23 (2009): 313-319.
- McGinley JL, Baker R, Wolfe R, et al. The reliability of three-dimensional kinematic gait measurements: a systematic review. Gait Posture 29 (2009): 360-369.
- Barlow DH, Hersen M. Single-Case Experimental Designs: Uses in Applied Clinical Research. Archives of General Psychiatry 29 (1973): 319-325.
- Zhan S, Ottenbacher KJ. Single subject research designs for disability research. Disability and rehabilitation 23 (2001): 1-8.
- Tate R, Perdices M, Rosenkoetter U, et al. The Single-Case Reporting Guideline In BEhavioural Interventions (SCRIBE) 2016: Explanation and elaboration. Archives of Scientific Psychology 4 (2016): 10-31.
- Kwakkel G, Veerbeek JM, van Wegen EE, et al. Constraint-induced movement therapy after stroke. The Lancet Neurology 14 (2015): 224-234.
- Wolf SL, Winstein CJ, Miller JP, et al. Effect of constraint-induced movement therapy on upper extremity function 3 to 9 months after stroke: the EXCITE randomized clinical trial. Jama 296 (2006): 2095-2104.
- Bell AL, Brand RA. Roentgenographic changes in proximal femoral dimensions due to hip rotation. Clin Orthop Relat Res (1989):194-199.
- Bell AL, Pedersen DR, Brand RA. A comparison of the accuracy of several hip center location prediction methods. J Biomech 23 (1990): 617-621.
- Tranberg R, Saari T, Zugner R, et al. Simultaneous measurements of knee motion using an optical tracking system and radiostereometric analysis (RSA). Acta Orthop 82 (2011): 171-176.
- Weidow J, Tranberg R, Saari T, et al. Hip and knee joint rotations differ between patients with medial and lateral knee osteoarthritis: gait analysis of 30 patients and 15 controls. J Orthop Res 24 (2006): 1890-1899.
- Zugner R, Tranberg R, Lisovskaja V, et al. Validation of gait analysis with dynamic radiostereometric analysis (RSA) in patients operated with total hip arthroplasty. J Orthop Res 35 (2017): 1515-1522.
- Zugner R, Tranberg R, Lisovskaja V, et al. Different reliability of instrumented gait analysis between patients with unilateral hip osteoarthritis, unilateral hip prosthesis and healthy controls. BMC Musculoskelet Disord 19 (2018): 224.
- Ottenbacher KJ. Evaluating clinical change: strategies for occupational and physical therapists / Kenneth J. Ottenbacher. Baltimore, MD, U.S.A: Williams & Wilkins, (1986).
- Kazdin AE. Single-case experimental designs. Evaluating interventions in research and clinical practice. Behav Res Ther 117 (2019): 3-17.
- Rankin J. Cerebral vascular accidents in patients over the age of 60. III. Diagnosis and treatment. Scott Med J 2 (1957): 254-268.
- Bonnefoy-Mazure A, Armand S. Normal gait. Orthopedic Management of Children With Cerebral Palsy: A Comprehensive Approach Nova Science Publishers Inc, (2015), pp.199-214.
- Jonkers I, Delp S, Patten C. Capacity to increase walking speed is limited by impaired hip and ankle power generation in lower functioning persons post-stroke. Gait & posture 29 (2009): 129-137.
- Jonsdottir J, Recalcati M, Rabuffetti M, et al. Functional resources to increase gait speed in people with stroke: strategies adopted compared to healthy controls. Gait & posture 29 (2009): 355-359.
- Perry J, Garrett M, Gronley JK, et al. Classification of walking handicap in the stroke population. Stroke; 26 (1995): 982-989.
- Lewek MD, Sykes R, 3rd. Minimal Detectable Change for Gait Speed Depends on Baseline Speed in Individuals with Chronic Stroke. J Neurol Phys Ther 43 (2019): 122-127.
- Patterson KK, Gage WH, Brooks D, et al. Evaluation of gait symmetry after stroke: A comparison of current methods and recommendations for standardization. Gait & Posture 31 (2010): 241-246.
- Brandstater ME, de Bruin H, Gowland C, et al. Hemiplegic gait: analysis of temporal variables. Arch Phys Med Rehabil 64 (1983): 583-587.
- Wall JC, Turnbull GI. Gait asymmetries in residual hemiplegia. Arch Phys Med Rehabil 67 (1986): 550-553.
- Balasubramanian CK, Bowden MG, Neptune RR, et al. Relationship between step length asymmetry and walking performance in subjects with chronic hemiparesis. Arch Phys Med Rehabil 88 (2007): 43-49.
- Finley JM, Bastian AJ, Gottschall JS. Learning to be economical: the energy cost of walking tracks motor adaptation. J Physiol 591 (2013): 1081-1095.
- Ellis RG, Howard KC, Kram R. The metabolic and mechanical costs of step time asymmetry in walking. Proc Biol Sci 280 (2013): 20122784.
- Balaban B, Tok F. Gait disturbances in patients with stroke. PM R 6 (2014): 635-642.
- Sheffler LR, Chae J. Hemiparetic Gait. Phys Med Rehabil Clin N Am 26 (2015): 611-623.
- Geiger M, Supiot A, Pradon D, et al. Minimal detectable change of kinematic and spatiotemporal parameters in patients with chronic stroke across three sessions of gait analysis. Hum Mov Sci 64 (2019): 101-107.
- Guzik A, Druzbicki M, Wolan-Nieroda A, et al. Estimating Minimal Clinically Important Differences for Knee Range of Motion after Stroke. Journal of Clinical Medicine 9 (2020): 3305.
- Kirtley C, Whittle MW, Jefferson RJ. Influence of walking speed on gait parameters. J Biomed Eng 7 (1985): 282-288.
- Lamontagne A, Malouin F, Richards CL, et al. Mechanisms of disturbed motor control in ankle weakness during gait after stroke. Gait Posture15 (2002): 244-255.
- Burpee JL, Lewek MD. Biomechanical gait characteristics of naturally occurring unsuccessful foot clearance during swing in individuals with chronic stroke. Clin Biomech (Bristol, Avon) 30 (2015): 1102-1107.
- Nourbakhsh MR, Ottenbacher KJ. The statistical analysis of single-subject data: a comparative examination. Physical therapy 74 (1994): 768-776.


 Impact Factor: * 5.3
Impact Factor: * 5.3 Acceptance Rate: 75.63%
Acceptance Rate: 75.63%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 
