Intrapancreatic Accessory Spleen: A Single Center Experience and A Literature Review
Stepanova Yu.A.1*, Ionkin D.A.2, Alimurzaeva M.Z.3, Glotov A.V.4, Akhtanin E.A.5
1Academic Secretary, A.V. Vishnevsky National Medical Research Center of Surgery, Bolshaya Serpukhovskaya st., 27, Moscow, 115093, Russia
2Associate Professor of Educational Department, Surgeon of Abdominal Surgery Department, A.V. Vishnevsky National Medical Research Center of Surgery, st. Bolshaya Serpukhovskaya, 27, 115093, Moscow
3Assistant at Department of Radiology Diagnostics and Radiation Therapy with a course in Ultrasound Diagnostics, Dagestan State Medical University; 367000, Republic of Dagestan, Makhachkala, Lenina sq., 1
4Pathologist of pathological department, A.V. Vishnevsky National Medical Research Center of Surgery, Bolshaya Serpukhovskaya st., 27, Moscow, 115093, Russia
5Head of the 1st Surgical Department of "S.S. Yudin City Clinical Hospital of the Moscow City Health Department", Kolomensky proezd, 4, Moscow, 115446, Russia
*Corresponding Author: Stepanova Yulia Aleksandrovna, Academic Secretary, A.V. Vishnevsky National Medical Research Center of Surgery, st. B. Serpukhovskaya, 27, 115093, Moscow, Russia
Received: 10 May 2025; Accepted: 04 June 2025; Published: 19 June 2025.
Article Information
Citation: Stepanova Yu.A., Ionkin D.A., Alimurzaeva M.Z., Glotov A.V., Akhtanin E.A. Intrapancreatic Accessory Spleen: A Single Center Experience and A Literature Review. Archives of Clinical and Medical Case Reports. 9 (2025): 117-124.
View / Download Pdf Share at FacebookAbstract
An accessory spleen (splenuncle) is an extrasplenic accumulation of splenic tissue that has its own blood supply, innervation, capsule, and serous membrane. Although an accessory spleen is not an unusual phenomenon, intrapancreatic localization of such masses is rare. Currently, 247 cases of accessory spleen in the structure of the pancreas have been published in Pubmed database since 2000 (for 25 years).
During the period 2012-2024, 3 patients with intrapancreatic accessory spleen (IAS) were identified at A.V. Vishnevsky National Medical Research Center of Surgery (1 man and 2 women aged 38 to 61 years). The presented clinical cases demonstrate the difficulties of ectopic pancreatic spleen differential diagnosis. Despite the obviousness of the radiology picture similar to the visualization of the orthotopic spleen, a similar picture is also characteristic for such focal lesions, as NETs, solid pseudopapillary tumor and kidney cancer metastasis. Nevertheless, a thorough analysis of ultrasound, MSCT and MRI data of the detected lesion using appropriate visualization methods can confirm the diagnosis of IAS without invasive procedures or surgery. This is extremely important for the patient, since the accessory spleen is essentially a benign mass and surgery is not required in the vast majority of cases. Focal lesions can develop in IAS, as in the orthotopic spleen. Currently, Pubmed database contains 66 such lesions, which is 26.4% of all cases of such ectopia (N = 250, also taking into account our 3 cases).
Thus, IAS is a diagnosis that should be kept in mind as a differential diagnosis when considering a case with a focal lesion, since this will avoid unnecessary intervention due to erroneous diagnosis.
Keywords
<p>Accessory spleen; Intrapancreatic accessory spleen; Lesions in intrapancreatic accessory spleen; Frequency of occurrence; Pathogenesis; Diagnostics; Tactics</p>
Article Details
1. Introduction
An accessory spleen (splenuncle) is an extrasplenic accumulation of splenic tissue that has its own blood supply, innervation, capsule, and serous membrane. The structure of the accessory spleen is completely consistent with the splenic tissue. An accessory spleen is one of the common congenital malformations of the organ, detected in 11-16% of patients undergoing examination for abdominal diseases and in 10-30% of autopsy cases. Usually, accessory spleen have sizes from 4-5 to 25 mm, but there are known examples of nodes up to 10 cm. One accessory spleen is determined in 63% of those examined, two - in 20%, three or more - in 17%. Sometimes a cluster of several small spleens replaces the main organ. In 75% of cases, the additional spleen is localized in the hilum of the normal spleen, almost 20% - in or near the tail of the pancreas, less often in the gastrosplenic, splenic-gastric, gastrocolic, splenic-renal ligaments, along the splenic artery, in the lesser and greater omentum, mesentery of the small intestine, Douglas's pouch in women, the epididymis and near the left testicular artery in men, and retroperitoneal tissue [1-4].
Ectopia of splenic tissue occurs, as a rule, in two cases: as a result of splenectomy and subsequent autotransplantation of the organ and congenital migration of splenic cells [5, 6]. The latter option, according to different authors, occurs in the population with a frequency of 10 to 40%, while the number of accessory spleens can vary [7-9]. The development of accessory lobes of the spleen occurs with the laying of the spleen on the 4th-5th week of embryogenesis in the thickness of the mesenchyme of the dorsal mesentery, when some of the mesenchymal cells of the future organ penetrate into neighboring organs and structures [10]. In a study of various years (Ambrosius-Diener K. et al. [11] and Abdullina R.F. et al. [12]), an increase in the incidence of accessory spleen in the presence of other congenital malformations is noted. In case of intrapancreatic location of splenic tissue, its structure may contain ducts and acinar complexes of the exocrine apparatus of the pancreas [12]. There is evidence that the probability of occurrence of ectopia of the spleen in pancreatic tissue increases in patients with a history of splenectomy [13, 14].
At the turn of the 20th century, researchers were already trying to analyze the frequency of accessory spleens. Thus, M. Jolly in 1895 described 25% of accessory spleens in 80 operated children under 17 years of age [15]. In 1909, J.G. Adami and A.G. Nichols noted the presence of accessory spleens in 11% of the autopsies they performed [16]. M. Morrison, M. Lederer and W.Z. Fradkin in 1928, after analyzing autopsy data, published on 35% of accessory spleens [17]. G.M. Curtis and P.L. White claimed that they found an accessory spleen in 7 (20%) of 35 splenectomies [18]. In 1946, G.M. Curtis and D. Movitz, having analyzed the data of 56 patients with 131 accessory spleens after surgery, published a large analytical article in which they examined the issues of diseases that were the reason for surgery, the number of accessory spleens, sizes, localization, age and sex of the patients [1]. B. Halpert and F. Gyorkey in 1959 described lesions found in accessory spleens in 311 patients [7]. In 1964, B. Halpert already with Z.A. Alden studied the frequency and localization of ectopia of splenic tissue based on data from 2700 autopsies [2]. In 1965, F. Tischendorf, based on the results of the study, concluded that the spleen and accessory spleen have a similar reaction [19]. In 1973-1974, J. Kaude [20] and B. Menanteau et al. [21] described accessory spleens identified by abdominal angiography, indicating that the accessory spleen has its own blood supply.
Currently, 247 cases of intrapancreatic accessory spleen (IAS) have been published in the Pubmed database since 2000 (over 25 years). It is difficult to evaluate earlier publications, since there are few of them and almost all of them are presented only by the output data (even abstracts revealing the essence of the publication are not presented). This is most likely not the true frequency of occurrence of such spleens; the authors consider the lesions of IAS the rare cases and of interest to colleagues, while the ectopia itself cause less interest now. In reviews, you can only find analytical data of that time, IAS was detected in about 17% of patients during 3000 autopsies at the Veterans Administration Hospital between 1949 and 1958 (cited from Spencer L.A. et al., 2010 [22]). After 2000, most publications provide only single clinical cases and only a few authors analyze several of their own cases: S.H. Kim et al. (2006) - 7 cases [23, 24]; H.S. Hwang et al. (2011) - 12 cases [25]; A.D. Tatsas et al. (2012) - 6 cases [26]; A.B. Conway et al. (2013) - 5 cases [27]; N. Bhutiani et al. (2017) - 7 cases [28]; Q. Ding et al. (2018) - 8 cases [29]. Literature reviews are also published. In the vast majority of the cases, the accessory spleen is localized only in pancreatic tail, however, in 1970, G.A. Patrushev described the development of intestinal obstruction caused by an IAS localized in pancreatic head [30]. In 2020, H.J. Ko et al. described an epidermoid cyst of the accessory intrapancreatic spleen in the head of the gland [31].
As a rule, an accessory spleen is asymptomatic and is an incidental finding during examination for concomitant diseases. The exception is patients with idiopathic thrombocytopenic purpura, who have characteristic hematological symptoms. In the absence of clinical manifestations, an accessory spleen does not require not only surgery, but also dynamic observation, but the main difficulties lie in the accuracy of diagnosis. Given the hypervascular nature of the IAS, its localization in the tail of the pancreas, the differential diagnostic series should include non-functioning neuroendocrine tumors (NETs), solid pseudopapillary tumor, as well as metastases of renal cell carcinoma to the pancreas [32]. We present the experience of A.V. Vishnevsky National Medical Research Center of Surgery in diagnosing and treating patients with IAS. During the period from 2012 to 2024, 3 such patients were identified (1 man and 2 women aged 38 to 61 years).
2. Clinical case 1
Female M., 38 years old, was hospitalized on 27.08.12 with a preliminary diagnosis of the pancreatic tail tumor, which was detected on an outpatient basis during an ultrasound. The patient underwent contrast-enhanced MSCT, which revealed the lesion of a heterogeneous structure with clear uneven contours up to 10 × 18 mm in size along the posterior surface of the distal parts of the pancreatic tail, with a density of up to 71 HU and 108 HU, which is similar to spleen tissue. In order to clarify the diagnosis, an MRI was performed, which confirmed the presence of an accessory spleen. The patient was recommended dynamic observation, performing an MRI of the abdominal cavity after 6 months. During the control examination, according to the MRI data from 2018, the existing lesion of the pancreatic tail was without any dynamics.
3. Clinical case 2
Female V., 43 years old, was admitted in October 2017. No complaints at the time of hospitalization. It is known from the anamnesis that in December 2010, a laparoscopic right-sided nephrectomy was performed due to T1bN0M0 right kidney cancer. During a routine dispensary examination, MSCT with intravenous contrast was performed, according to which a hypervascular round lesion in the pancreatic tail was detected.
Upon admission, the patient's condition is satisfactory. There is no hyperthermia. The skin and visible mucous membranes are of normal color. There is no peripheral edema. The lymph nodes are not enlarged. Vesicular breathing. No wheezing. Heart sounds are clear, rhythmic, no pathological sounds or noises are heard. The second heart sound is accentuated on the aorta. Hemodynamics are stable. Blood pressure in both arms is 120/80 mm Hg. The pulse is of satisfactory filling and tension, the frequency is 86 per minute. There is no pulse deficit. The tongue is moist and clean. The abdomen is not distended, soft on palpation, painful in the epigastrium. Physiological functions are normal.
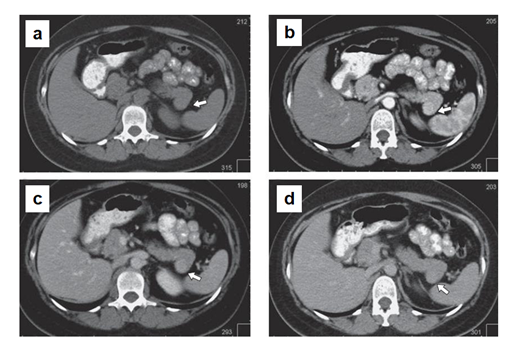
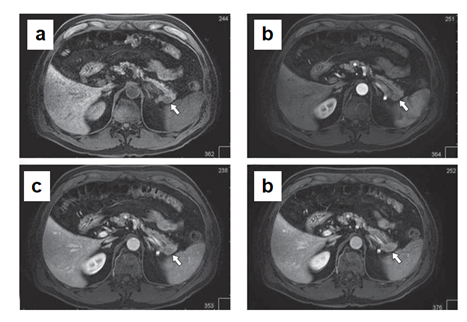
In MSCT (Figure 1), the pancreas is lobular in structure with clear, even contours. A hypervascular lesion of a round shape up to 18 mm in size is determined in the tail of the gland. The main pancreatic duct is visualized along its entire length, not dilated. The parapancreatic tissue is unchanged. The parapancreatic lymph nodes are not enlarged. The spleen is located normally, not enlarged in size (splenic index up to 400, N < 440). An additional lobule up to 7 mm is determined along the upper contour. No areas of pathological density or pathological accumulation of contrast agent in the parenchyma were detected. Conclusion: hypervascular lesion of pasncreatic tail, differentiate between renal cell carcinoma metastasis and neuroendocrine tumor (Grade 2). Additional spleen lobule.
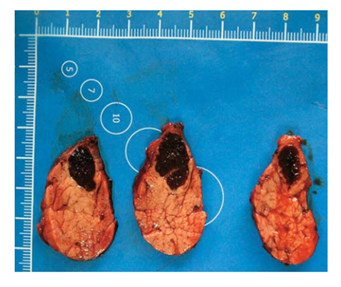
The patient underwent robot-assisted distal pancreatectomy (Figure 2).
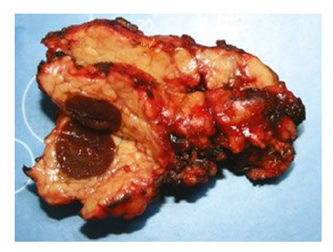
According to the histology, the lesion in the pancreatic tissue is represented by the structures of the spleen - white and red pulp, trabeculae, thin fibrous capsule. The surrounding pancreatic tissue is of normal structure. The morphological picture corresponds to IAS.
4. Clinical case 3
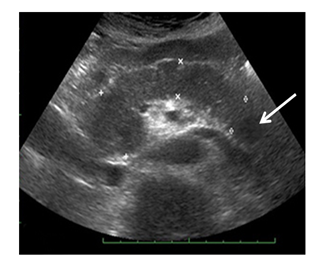
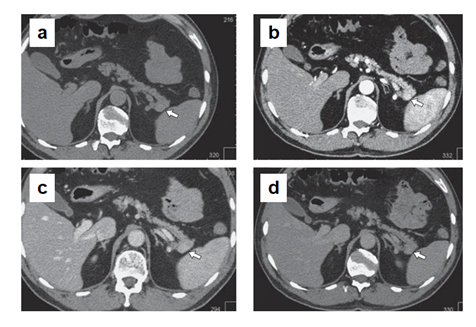
Male Ch., 61 years old, was admitted in December 2017. It is known from the anamnesis that a year ago, during a routine dispensary examination, a round tumor up to 20 mm in diameter was detected in the pancreatic tail during an ultrasound. According to the ultrasound, the pancreas is not enlarged. Its contours are smooth and clear, the structure is heterogeneous, with increased echogenicity. The lesion of slightly reduced echogenicity, about 20 mm in size, is determined in the pancreatic tail (Figure 3). According to MSCT (Figure 4), an ovoid lesion measuring 28×18 mm, a solid structure, is visualized in the pancreatic tail, accumulating the contrast agent to the maximum in the venous phase of scanning. The splenic vein is tightly adjacent to the lesion, its contour is smooth, clear, without signs of invasion. The main pancreatic duct is visualized along its entire length, not dilated. The parapancreatic tissue is unchanged. The parapancreatic lymph nodes are not enlarged. The spleen is of normal shape, with smooth, clear contours, the splenic index is about 417, the parenchyma density is unchanged (45 HU). The parenchyma is homogeneous, without pathological foci, and usually contrasts. Conclusion: The lesion in the pancreatic tail most likely corresponds to NETs (Grade 2). According to MRI (Figure 5), an oval-shaped lesion of a solid structure with clear contours, measuring 16×13.5 mm, is visualized in the pancreatic tail, maximally accumulating the contrast agent in the venous phase. The main pancreatic duct is not dilated. The parapancreatic tissue is homogeneous, unchanged. The spleen is usually located, not enlarged in size, no pathological lesions were found in its parenchyma. Conclusion: the pancreatic tail lesion (probably NETs).
Thus, the preoperative diagnosis was NEN of the pancreas.
The patient underwent robot-assisted distal pancreatic resection (Figure 6).
According to the histology, the node in the pancreatic tissue is represented by the spleen tissue with a thin fibrous capsule; the structures of the white and red pulp are determined. The surrounding pancreatic tissue has a preserved acini structure. According to the microscopy, the node in the thickness of the pancreatic tissue is represented by the white and red pulp of the spleen with a thin fibrous capsule. In one of the small ducts along the periphery of the node, foci of low-grade intraepithelial neoplasia (PanIN-I) are determined.
The presented clinical cases demonstrate the difficulties of differential diagnosis of the ectopic spleen in the pancreas. Despite the obviousness of the radiology picture similar to the visualization of the orthotopic spleen, a similar picture is also characteristic for focal pancreatic lesions, such as NETs, solid pseudopapillary tumor and kidney cancer metastasis.
For more accurate diagnostics, it is advisable to use additional methods of various radiology methods of diagnostics. In ultrasound, it is advisable to use contrast enhancement. In this case, improved visualization of the vascular pedicle is noted in the arterial phase, which is a characteristic sign of an accessory spleen in the pancreatic parenchyma, as well as heterogeneous contrast enhancement of the accessory spleen pulp. In the venous and delayed phases, the contrast agent is retained in the cells of the reticuloendothelial system with the lesion of a "mosaic" pattern, which is typical for the spleen [24, 33]. Further, at MSCT during the arterial phase, a pronounced accumulation of the contrast agent is noted, with large lobule sizes - "tiger" contrasting, similar to the contrasting of the main spleen. In the venous and delayed contrast enhancement phases, the accessory lobule is maximally clearly delineated from the pancreatic parenchyma, since the contrast agent is already washed out of the latter, but remains in the spleen tissue [23, 34, 35]. In MRI, high-field MR-tomographs allow the use of diffusion-weighted imaging in the differential diagnosis of NETs and intrapancreatic spleen with subsequent construction of maps of the measured diffusion coefficient (MDC). For NETs, the MDC is lower than that of ectopic splenic tissue. The MDC values for the accessory spleen are close to the MDC of the main spleen. The property of the spleen to limit diffusion can serve as a key to differential diagnosis [23, 36, 37].
It should be noted that focal lesions can develop in IAS, as well as in the orthotopic spleen. Currently, the Pubmed database contains 66 cases of focal intrapancreatic spleen lesions, which is 26.4% of all cases of such ectopia (N = 250, also taking into account our 3 cases). Table 1 presents the morphological forms of lesions found in IAS and their number in Pubmed database as of 2024, a total of 9 types of these forms.
Table 1: Morphological forms of the lesions found in IAS and their number in Pubmed database (2000-2024)
|
No |
Morphology |
Number of published cases |
|
|
1 |
Cyst |
Epidermoid cyst [38-41] |
47 (71,3%) |
|
2 |
Epithelial cyst [42, 43] |
11 (16,7%) |
|
|
3 |
Serous cyst [44] |
1 (1,5%) |
|
|
4 |
Vascular lesions |
Cavernous hemangioma [45] |
1 (1,5%) |
|
5 |
Hemangiomatosis [46] |
1 (1,5%) |
|
|
6 |
Lithorium cell angioma [47, 48] |
2 (3,0%) |
|
|
7 |
B-cell lymphoma [49] |
1 (1,5%) |
|
|
8 |
Inflammatory pseudotumor [50] |
1 (1,5%) |
|
|
9 |
Amyloidosis [51] |
1 (1,5%) |
|
The most common (71.3%) is an epidermoid cyst (EC) of the ectopic spleen in the pancreas. Such case was first published by E.D. Davidson et al. in 1980 [52]. The exact histogenesis of EC in the ectopic pancreatic spleen is not entirely clear. When generalizing the results of the literature, three main theories were proposed. The first is based on similar studies of the histogenesis of EC in the normal spleen, suggesting invagination of the capsular mesothelium with subsequent cystic lesion and metaplastic changes [53, 54]. The second, based on the presence of a keratokines profile of the splenic cyst, claimed that EC are of teratomatous origin or are the result of inclusion of fetal squamous epithelium [55]. The third, based on immunohistochemical data, suggests that EC in the ectopic pancreatic spleen may arise either from aberrant embryonic inclusion of the pancreatic duct epithelium [56] or from protrusion of the pancreatic duct into the ectopic pancreatic spleen [57]. The latter is questionable, since macroscopically, H. Yokomizo et al. [58] and Y. Iwasaki et al. [59] using retrograde pancreatography, and A. Urakami et al. [60] using magnetic resonance cholangiopancreatography, did not find any connection between the pancreatic duct and EC in the ectopic pancreatic spleen. It should be noted that there is one publication of an epidermoid cyst of ectopic pancreatic spleen, which describes malignant transformation of the lesion [61].
Of note is the published case of adenocarcinoma in the IAS, where after distal pancreatectomy of a vascularized tumor of the pancreatic tail, histology revealed invasive growth of adenocarcinoma in a structure identical to the spleen. The results of both radiology and histology studies suggested that the tumor arose from the IAS. Extended examinations didn’t reveal any other malignant neoplasms, on the basis of which the authors concluded that the adenocarcinoma was primary [62]. Most likely, this is explained by the fact that the structure of the pancreatic tail did not contain an additional spleen, similar to an orthotopic one and having a fibrous capsule, but splenosis - a non-encapsulated lesion containing altered splenic tissue with ducts and acinar complexes of the exocrine apparatus of the pancreas [12].
Thus, IAS represents a diagnostic challenge in imaging and is often misdiagnosed as a pancreatic tumor [32], which may lead to unnecessary surgery or biopsy. Accurate diagnosis of the IAS may save the patient from unnecessary intervention. The possible presence of focal lesions in these spleens also emphasizes the need for correct diagnosis.
Conclusion
Although ectopic splenic tissue (accessory spleen) is not an uncommon phenomenon, intrapancreatic localization of such masses is rare. They represent a diagnostic challenge when detected by radiology methods, since their characteristics resemble a malignant tumor. However, careful analysis of ultrasound, MSCT and MRI data of the detected mass using appropriate imaging methods can confirm the diagnosis of IAS without invasive procedures or surgery. This is extremely important for the patient, since the accessory spleen is essentially a benign mass and surgery is not required in the vast majority of cases. An exception is focal lesions of IAS. Thus, accessory spleen within the pancreatic parenchyma is a diagnosis that should be kept in mind as a differential diagnosis when considering a case with a focal mass, since this will avoid unnecessary intervention due to an erroneous diagnosis.
References
- Curtis G M, Movitz D. The surgical significance of the accessory spleen. Annals of Surgery 123 (1946): 276-298.
- Halpert B, Alden Z A. Accessory spleens in or at the tail of the pancreas. A survey of 2.700 additional necropsies. Archives of Pathology 77 (19646): 652-654.
- Barta I. Spleen (anatomy, physiology, pathology and clinical features). – Budapest: Publishing House of the Hungarian Academy of Sciences 264 (1976).
- Sica G T, Reed M F. Case 27: intrapancreatic accessory spleen. Radiology 217 (2000): 134-137.
- Movitz D. Accessory spleens and experimental splenosis. Principles of growth. Chic Med Sch Q. Winter 26 (1967): 183-187.
- Beltrame V, Merigliano S, Sperti C. Splenosis. Presenting as Pancreatic Neoplasm: Report of Two Cases. Austin J. Gastroenterol 1 (2014): 1015.
- Halpert B, Gyorkey F. Lesions observed in accessory spleens of 311 patients. Am J Clin Pathol 32 (1959): 165-168.
- Wacha M. Laparascopic resection of an accessory spleen in a patient with chronic lower abdominal pain. Surg. Endosc 16 (2002): 1242-1243.
- Belik O V, Katerynyuk I M, Spinei L V, et al. On the accessory spleen. Clinical anatomy and operative surgery 9 (2010): 31-35.
- Afanasyev Yu I, Yurina N A, Kotovsky E F. Histology, embryology, cytology (2012): 800 p.
- Ambrosius-Diener K, López-Varela V. Asplenia, polisplenia y bazos accesorios. Frecuencia y asociación con otras malformaciones congénitas [Asplenia, polysplenia and accessory spleens. Incidence and association with other congenital malformations]. Bol Med Hosp Infant Mex 40 (1983): 560-565.
- Abdullin R F, Kondratenko E G, Koshik E A, et al. Morphological characteristics of the intrapancreatic accessory spleen in newborns and children of the first year of life. Modern pediatrics 2 (2014): 95-99.
- Georgin-Lavialle S, Gossot D, Galicier L, et al. Accessory spleens after splenectomy in a patient with common variable immunodeficiency. Rev. Méd. Interne 31 (2010): 41-45.
- Li B Q, Xu X Q, Guo J C. Intrapancreatic accessory spleen: a diagnostic dilemma. HPB (Oxford) 20 (2018): 1004-1011.
- Jolly M. Rates surnumeraires chez l’enfant. Soc. Anatom. de Paris 5 (1895): 745.
- Adami J G, Nichols A G. Principles of Pathology. Lea and Febiger II (1909): 222.
- Morrison M, Lederer M, Fradkin W Z. Accessory Spleen: Their Significance in Essential Thrombocytopenic Purpura Hemorrhagica. Am. Jr. Med. Sci 176 (1928): 672-681.
- Curtis G M, White P L. The surgical significance of the accessory spleen. Trans. West. Surg. Assn 46 (1937): 364-376.
- Tischendorf F. Uber die gleichsinnige reaktion von haupt- und nebenmilz. Ein beitrag zur kenntnis der menschlichen nebenmilz und zum Banti-problem [On the similar reaction of the spleen and accessory spleen. A contribution to data on the human accessory spleen and on the problem of Banti's disease]. Z Anat Entwicklungsgesch 124 (1965): 335-352.
- Kaude J. Accessory spleens as demonstrated by celiac angiography. Radiologe 13 (1973): 53-56.
- Menanteau B, Maffioli C, Levasseur J C, et al. Aspect angiographique d'une ectopie de la rate [Proceedings: Angiographic aspect of the ectopic spleen]. J Radiol Electrol Med Nucl 55 (1974): 352-353.
- Spencer L A, Spizarny D L, Williams T R. Imaging features of intrapancreatic accessory spleen. Br J Radiol 83 (2010): 668-673.
- Kim S H, Lee J M, Han J K, et al. MDCT and superparamagnetic iron oxide (SPIO)-enhanced MR findings of intrapancreatic accessory spleen in seven patients. Eur Radiol 16 (2006): 1887-1897.
- Kim S H, Lee J M, Lee J Y, et al. Contrast-enhanced sonography of intrapancreatic accessory spleen in six patients. Am J Roentgenol 188 (2007): 422-428.
- Hwang H S, Lee S S, Kim S C, et al. Intrapancreatic accessory spleen: clinicopathologic analysis of 12 cases. Pancreas 40 (2011): 956-965.
- Tatsas A D, Owens C L, Siddiqui M T, et al. Fine-needle aspiration of intrapancreatic accessory spleen: cytomorphologic features and differential diagnosis. Cancer Cytopathol 120 (2012): 261-268.
- Conway A B, Cook S M, Samad A, et al. Large platelet aggregates in endoscopic ultrasound-guided fine-needle aspiration of the pancreas and peripancreatic region: a clue for the diagnosis of intrapancreatic or accessory spleen. Diagn Cytopathol 41 (2013): 661-672.
- Bhutiani N, Egger M E, Doughtie C A, et al. Intrapancreatic accessory spleen (IPAS): A single-institution experience and review of the literature. Am J Surg 213 (2017): 816-820.
- Ding Q, Ren Z, Wang J, et al. Intrapancreatic accessory spleen: Evaluation with CT and MRI. Exp Ther Med 16 (2018): 3623-3631.
- Patrushev GA. Intestinal obstruction caused by accessory spleen. Khirurgiia (Mosk) 46 (1970): 134-135.
- Ko H J, Shim J R, Lee T B, et al. Epidermoid cyst in an intrapancreatic accessory spleen in the pancreas head: a case report. BMC Gastroenterol 20 (2020): 392.
- Matthaei H, Schmelzle M, Braunstein S, et al. Pancreatic incidentalomas: a growing clinical challenge exemplified by an intrapancreatic accessory spleen. Wien Klin Wochenschr 123 (2011): 186-188.
- von Herbay A, Vogt C, Häussinger D. Das Ultraschallkontrastmittel Levovist hilft bei der Differenzierung von Nebenmilzen und Lymphknoten im Milzhilus: eine Pilotstudie [The ultrasound contrast agent levovist helps with the differentiation between accessory spleen and lymph nodes in the splenic hilum: a pilot study]. Z Gastroenterol 42 (2004): 1109-1115.
- Coquia S F, Kawamoto S, Zaheer A, et al. Intrapancreatic accessory spleen: possibilities of computed tomography in differentiation from nonfunctioning pancreatic neuroendocrine tumor. J. Computer Assis. Tomogr 38 (2014): 874-878.
- Tyunibabyan A I, Blokhin I A, Chernina V Yu, et al. Errors in the diagnosis of pancreatic neoplasms: intrapancreatic lobule of the spleen. Medical Visualization 22 (2018): 70-80.
- Kang B K, Kim J H, Byun J H, et al. Diffusion-weighted MRI: usefulness for differentiating intrapancreatic accessory spleen and small hypervascular neuroendocrine tumor of the pancreas. Eur. Radiol 28 (2018): 1560-1567.
- Pandey A, Pandey P, Ghasabeh M A, et al. Accuracy of apparent diffusion coefficient in differentiating pancreatic neuroendocrine tumour from intrapancreatic accessory spleen. Eur. Radiol 28 (2018): 1560-1567.
- Servais E L, Sarkaria I S, Solomon G J, et al. Giant epidermoid cyst within an intrapancreatic accessory spleen mimicking a cystic neoplasm of the pancreas: case report and review of the literature. Pancreas 36 (2008): 98-100.
- Kadota K, Kushida Y, Miyai Y, et al. Epidermoid cyst in an intrapancreatic accessory spleen: three case reports and review of the literatures. Pathol Oncol Res 16 (2010): 435-442.
- Fujii M, Yoshioka M, Shiode J. Two Cases of an Epidermoid Cyst Developing in an Intrapancreatic Accessory Spleen Identified during Laparoscopic Distal Pancreatectomy. Intern Med 55 (2016): 3137-3141.
- Minami R, Nakahodo J, Tabata H, et al. Epidermoid cyst in an intrapancreatic accessory spleen diagnosed by contrast-enhanced EUS and EUS-FNA (with video). Endosc Ultrasound 13 (2024): 49-51.
- Wakasugi M, Tori M, Akamatsu H, et al. Laparoscopic distal pancreatectomy for multiple epithelial cysts in an intrapancreatic accessory spleen. A case report and review of literature. JOP 14 (2013): 636-641.
- Hiroi S, Hamaoka M, Yamamoto R, et al. A lymphoepithelial cyst in the pancreatic accessory spleen: A case report. Clin Case Rep 9 (2021): e04241.
- Hori S, Nara S, Shimada K, et al. Serous cystic neoplasm in an intrapancreatic accessory spleen. Pathol Int 60 (2010): 681-684.
- Huang J Y, Yang R, Li J W, et al. Cavernous hemangioma of an intrapancreatic accessory spleen mimicking a pancreatic tumor: A case report. World J Clin Cases 10 (2022): 1973-1980.
- Makino I, Tajima H, Kitagawa H, et al. A rare case of hemangiomatosis of the spleen and intrapancreatic accessory spleen. Abdom Imaging 39 (2014): 1169-1174.
- Pilz J B, Sperschneider T, Lutz T, et al. Littoral cell angioma in main and accessory intrapancreatic spleen presenting as splenic rupture. Am J Surg 201 (2011): 15-17.
- Zhixiong H, Daguang T, Runlin F, et al. Littoral cell angioma in the main and intrapancreatic accessory spleen: A case report. Asian J Surg 47 (2024): 1101-1103.
- Kansoun A, Julliard O, Imperiale A, et al. B Cell Lymphoma in an Intrapancreatic Accessory Spleen. J Gastrointest Surg 26 (2022): 261-262.
- Okura N, Mori K, Morishita Y, et al. Inflammatory pseudotumor of the intrapancreatic accessory spleen: computed tomography and magnetic resonance imaging findings. Jpn J Radiol 30 (2012): 171-175.
- Manuel-Vázquez A, Rodrigues Figueira Y, Ramia J M. Intrapancreatic accessory spleen with amyloidosis: Exceptional finding for a solid pancreatic lesion. Med Clin (Barc) 152 (2019): e45-e46.
- Davidson E D, Campbell W G, Hersh T. Epidermoid splenic cyst occurring in an intrapancreatic accessory spleen. Dig Dis Sci 25 (1980): 964-967.
- Ough Y D, Nah H R, Wood D A. Mesothelial cysts of the spleen with squamous metaplasia. Am J Clin Pathol 76 (1981): 666-669.
- Bürrig K F. Epithelial (true) splenic cysts. Pathogenesis of the mesothelial and so-called epidermoid cyst of the spleen. Am J Surg Pathol 12 (1988): 275-281.
- Lifschitz-Mercer B, Open M, Kushnir I, et al. Epidermoid cyst of the spleen: a cytokeratin profile with comparison to other squamous epithelia. Virchows Arh 424 (1994): 213-216.
- Morohoshi T, Hamamoto T, Kunimura T, et al. Epidermoid cyst derived from an accessory spleen in the pancreas: a case report with review survey. Acta Pathol Jpn 41 (1991): 916-921.
- Tateyama H, Tada T, Murase T, et al. Lymphoepithelial cyst and epidermoid cyst of the accessory spleen in the pancreas. Mod Pathol 11 (1998): 1171-1177.
- Yokomizo H, Hifumi M, Yamane T, et al. Epidermoid cyst of an accessory spleen at the pancreatic tail: the diagnostic value of MRI. Abdom Imaging 27 (2002): 557-559.
- Iwasaki Y, Tagaya N, Nakagawa A, et al. Laparoscopic resection of epidermoid cyst arising from an intrapancreatic accessory spleen: a case report with review of the literature. Surg Laparosc Endosc Percutan Tech 21 (2011): 275-279.
- Urakami A, Yoshida K, Hirabayashi Y, et al. Laparoscopic-assisted spleen preserving pancreatic resection for epidermoid cyst in an intrapancreatic accessory spleen. Asian J End Surg 4 (2011): 185-188.
- Wang J, Kang W J, Cho H. Malignant Transformation of an Epidermoid Cyst in an Intrapancreatic Accessory Spleen: A Case Report. Nucl Med Mol Imaging 54 (2020): 58-60.
- Yamaoka R, Nishihira T, Shimada T, et al. Adenocarcinoma in an intrapancreatic accessory spleen: report of a case. Surg Today 41 (2011): 1552-1555.


 Impact Factor: * 5.3
Impact Factor: * 5.3 Acceptance Rate: 75.63%
Acceptance Rate: 75.63%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 
